Effect of Simulated Gastrointestinal Tract Conditions on Survivability of Probiotic Bacteria Present in Commercial Preparations
Abstract
1. Introduction
2. Materials and Methods
2.1. Commercial Probiotic Preparations
2.2. Growth Media and Solutions
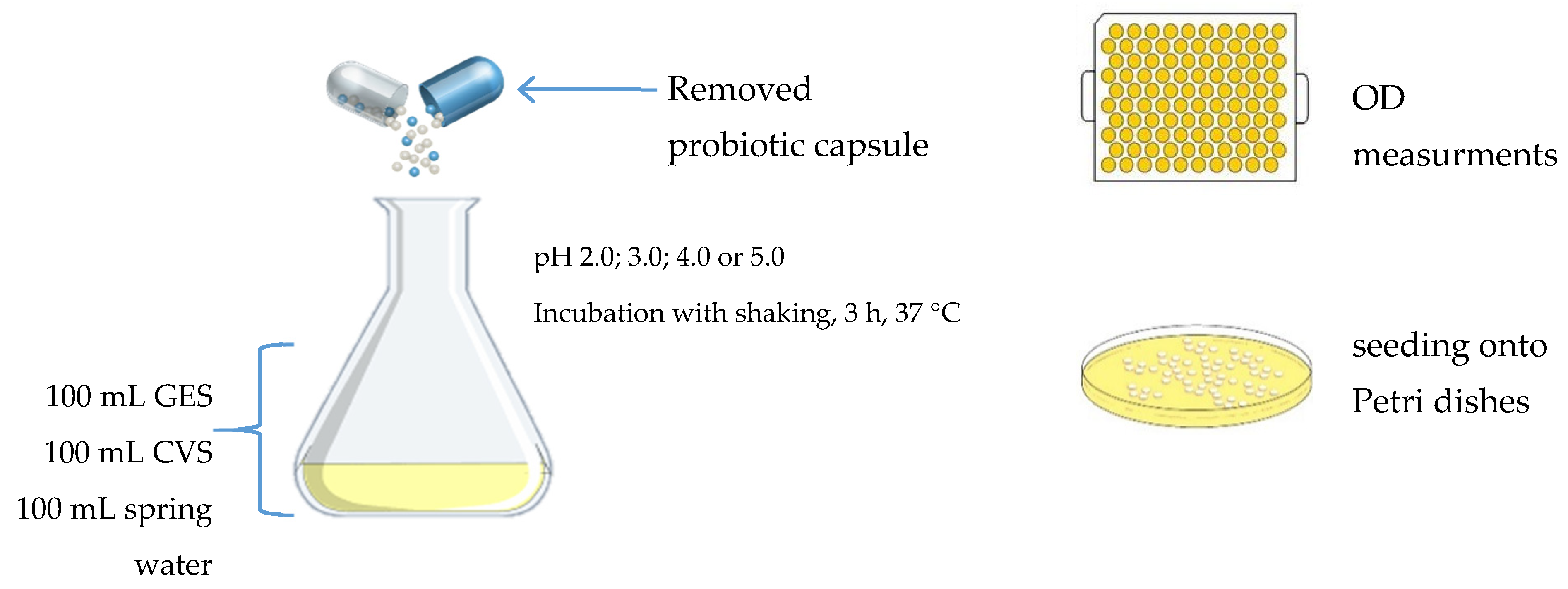
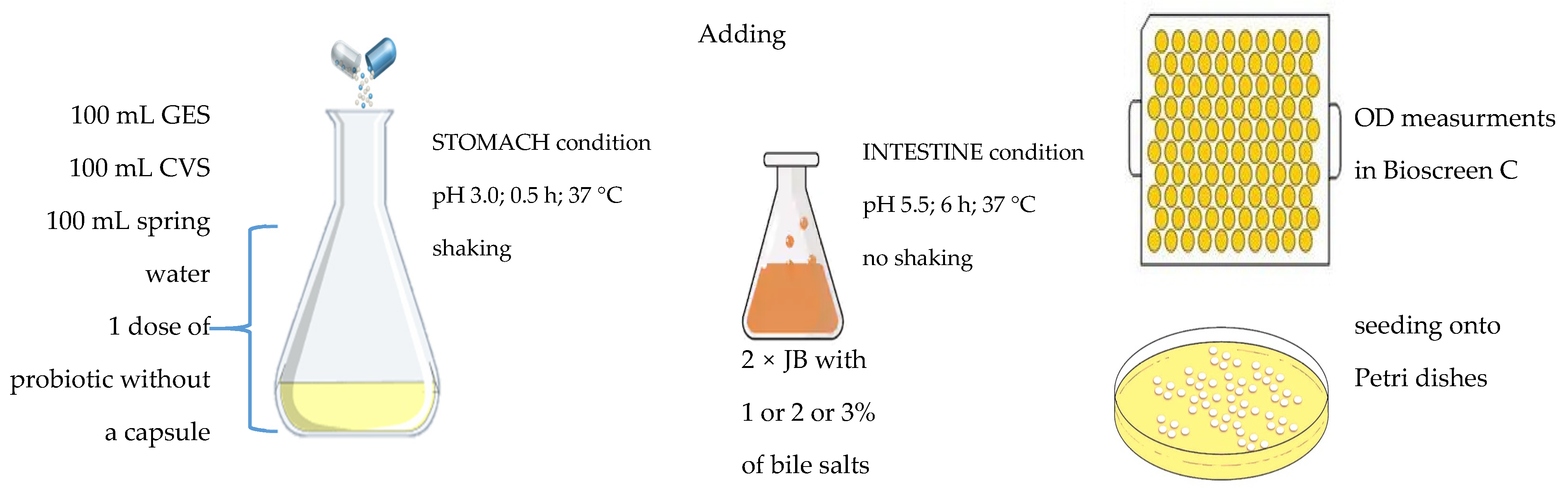
2.3. Study Design and Culture Conditions
2.4. Calculation of Coefficient of Specific Growth Rate
2.5. Statistical Analysis
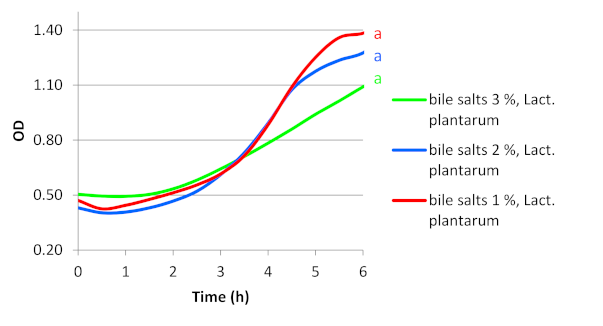
3. Results
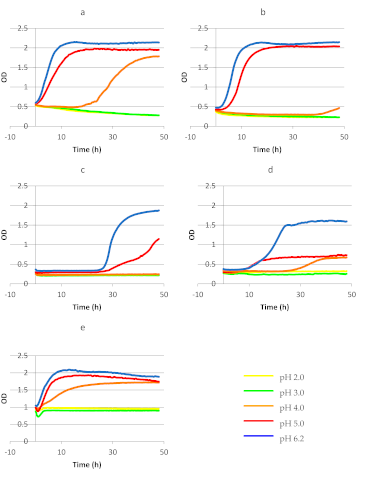
3.1. Survivability and Growth of Bacteria Present in Commercial Probiotic Preparations in MRS Broth
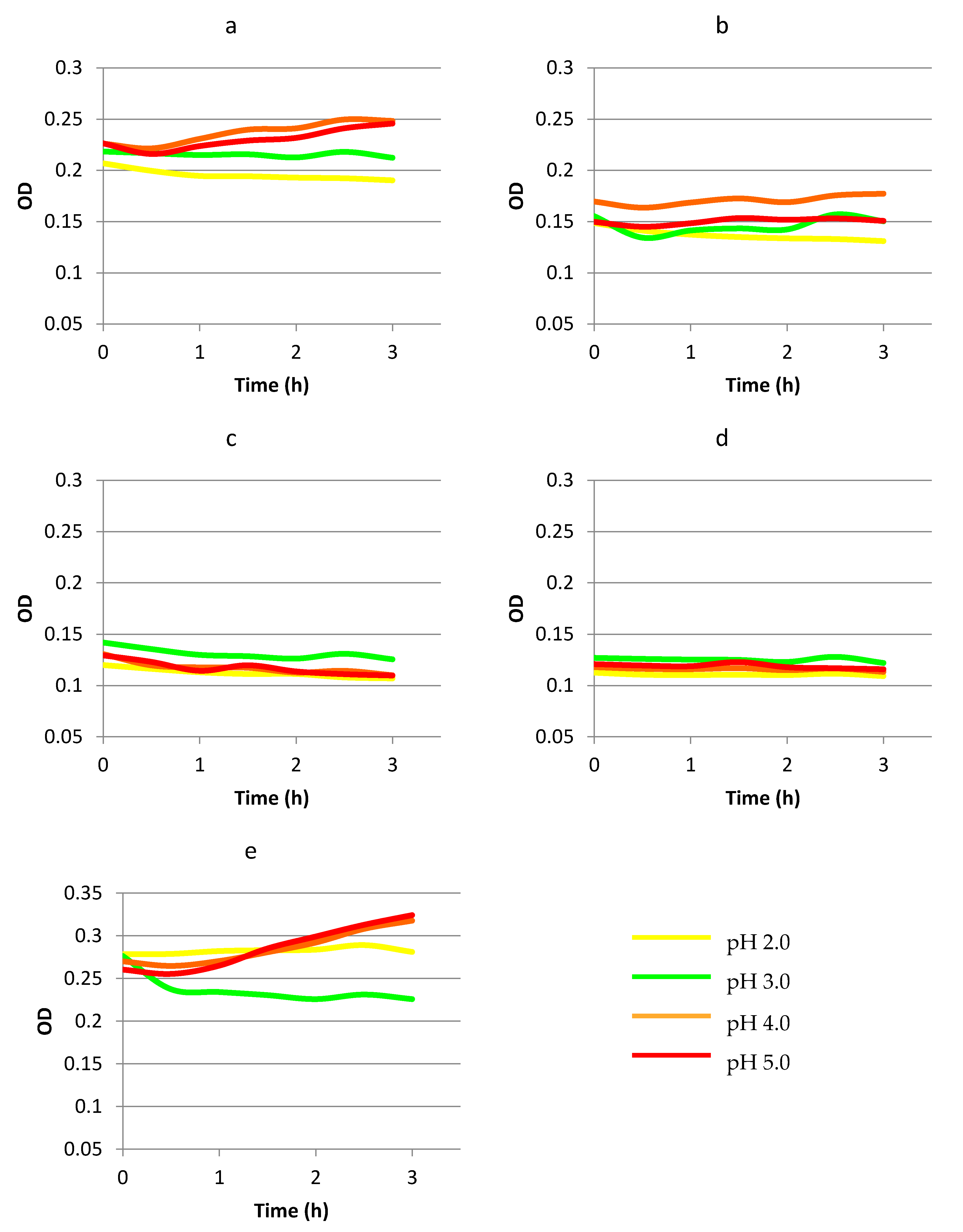
3.2. Survivability and Growth of Bacteria Present in Commercial Probiotic Preparations in a Food Matrix Simulating Gastric Passage
3.3. Survivability of Commercial Probiotic Strains in a Food Matrix Simulating Gastrointestinal Passage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Kligler, B.; Cohrssen, A. Probiotics. Am. Fam. Physician 2008, 78, 1073–1078. [Google Scholar]
- Gomes, D.O.V.S.; Morais, M.B. Gut microbiota and the use of probiotics in constipation in children and adolescents: Systematic review. Rev. Paul. Pediatr. 2020, 38. [Google Scholar] [CrossRef]
- Vandenplas, Y. Probiotics and prebiotics in infectious gastroenteritis. Best. Pract. Res. Clin. Gastroenterol. 2016, 30, 49–53. [Google Scholar] [CrossRef]
- Khare, A.; Gaur, S. Cholesterol-Lowering Effects of Lactobacillus Species. Curr. Microbiol. 2020, 1–7. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.M.; Dequenne, I.; Timay, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef]
- Pratap, K.; Taki, A.C.; Johnston, E.B.; Lopata, A.L.; Kamath, S.D. A Comprehensive Review on Natural Bioactive Compounds and Probiotics as Potential Therapeutics in Food Allergy Treatment. Front. Immunol. 2020, 11, 996. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.; Savva, G.M.; Clapuci, R.; Jones, J.; Maimouni, H.; Brown, E.; Minocha, A.; Hall, L.J.; Clarke, P. Incidence of necrotising enterocolitis before and after introducing routine prophylactic Lactobacillus and Bifidobacterium probiotics. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. Safe and efficacious probiotics: What are they? Trends Microbiol. 2006, 14, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Soccol, C.R.; Vandenberghe, L.P.S.; Spier, M.R.; Medeiros, A.B.P.; Yamaguishi, C.T.; Lindner, J.D.D.; Pandey, A.; Thomaz-Soccol, V. The Potential of Probiotics. Food Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Rui, X.; Ma, S.X.A. Retrospective study of probiotics for the treatment of children with antibiotic-associated diarrhea. Medicine 2020, 99, e20631. [Google Scholar] [CrossRef] [PubMed]
- Kotowska, M.; Albrecht, P.; Szajewska, H. Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea in children: A randomized double-blind placebo-controlled trial. Aliment. Pharmacol. Therapeut. 2005, 21, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.J.; Ahuja, K.D.K.; Robertson, I.K.; Ball, M.J.; Eri, R.D. Can probiotic yogurt prevent diarrhea in children on antibiotics? A doubleblind, randomised, placebo-controlled study. BMJ Open 2015, 5, e006474. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, S.; Griffin, N.W.; Yori, P.P.; Gehrig, J.L.; Olortegui, M.P.; Salas, M.S.; Trigoso, D.R.; Moulton, L.H.; Houpt, E.R.; Baratt, M.J.; et al. Diarrhea as a potential cause and consequence of reduced gut microbial diversity among undernourished children in Peru. Clin. Infect. Dis. 2020, 71, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Singhi, S.C.; Kumar, S. Probiotics in critically ill children. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Venema, K.; Verhoeven, J.; Beckman, C.; Keller, D. Survival of a probiotic-containing product using capsule-within-capsule technology in an in vitro model of the stomach and small intestine (TIM-1). Benef. Microbes 2020, 1–8. [Google Scholar] [CrossRef]
- Temmerman, R.; Pot, B.; Huys, G.; Swings, J. Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int. J. Food Microbiol. 2003, 81, 1–10. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Saeed, M.; Ahmed, A.; Ateeq, H.; Nadeem, M.T.; Tufail, T. Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal conditions and in pasteurized grape juice. J. Food Proc. Preserv. 2020, 44, e14346. [Google Scholar] [CrossRef]
- Klindt-Toldam, S.; Larsen, S.K.; Saaby, L.; Olsen, L.R.; Svenstrup, G.; Müllertz, A.; Knøchel, S.; Heimdal, H.; Nielsen, D.S.; Zielińska, D. Survival of Lactobacillus acidophilus NCFM® and Bifidobacterium lactis HN019 encapsulated in chocolate during in vitro simulated passage of the upper gastrointestinal tract. LWT Food Sci. Technol. 2016, 74, 404–410. [Google Scholar] [CrossRef]
- Sultana, K.; Godward, G.; Reynolds, N.; Arumugaswamy, R.; Peiris, P.; Kailasapathy, K. Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int. J. Food Microbiol. 2000, 62, 47–55. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Khan, R.A.; Riedl, B.; Lacroix, M. Encapsulation of probiotic bacteria in biopolymeric system. Critic. Rev. Food Sci. Nutr. 2013, 53, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Func. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Cardarelli, H.R.; Saad, S.M.I.; Gibson, G.R.; Vulevic, J. Functional petit-suisse cheese: Measure of the prebiotic effect. Anaerobe 2007, 13, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Carlin, F.; Lairon, D.; Nguyen-Then, C.; Schmitt, P. Survival of Bacillus cereus spores and vegetative cells in acid media simulating human stomach. J Appl. Microbiol. 2004, 97, 214–219. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Pluta, A.; Garbowska, M. The effect of selected factors on the survival of Bacillus cereus in the human gastrointestinal tract. Microb. Pathog. 2015, 82, 7–14. [Google Scholar] [CrossRef]
- Vinderola, C.G.; Reinheimer, J.A. Enumeration of Lactobacillus casei in the presence of L. acidophilus, bifidobacteria and lactic starter bacteria in fermented dairy products. Int. Dairy J. 2000, 271–275. [Google Scholar] [CrossRef]
- Vamanu, E.; Pelinescu, D.; Marin, I.; Vamanu, A. Study of probiotic strains viability from PROBAC product in a single chamber gastrointestinal tract simulator. Food Sci. Biotechnol. 2012, 21, 979–985. [Google Scholar] [CrossRef]
- Stasiak-Różańska, L.; Błażejak, S.; Gientka, I. Effect of glycerol and dihydroxyacetone concentration in the culture medium on the growth of acetic acid bacteria Gluconobacter oxydans ATCC 621. Eur. Res. Technol. 2014, 239, 453–461. [Google Scholar] [CrossRef]
- Mishra, V.; Prasad, D.N. Application of in vitro methods for selection of Lactobacillus casei strains as potential probiotics. Int. J. Food Microbiol. 2005, 103, 109–115. [Google Scholar] [CrossRef]
- De Castro-Cislaghi, F.P.; Silva, C.D.R.E.; Fritzen-Freire, C.B.; Lorentz, J.G.; St.Anna, E.S. Bifidobacterium Bb-12 microencapsulated by spray drying with whey: Survival under simulated gastrointestinal conditions, tolerance to NaCl, and viability during storage. J. Food Eng. 2012, 113, 186–193. [Google Scholar] [CrossRef]
- Cabuk, B.; Harsa, S.T. Protection of Lactobacillus acidophilus NRRL-B 4495 under in vitro gastrointestinal conditions with whey protein/pullulan microcapsules. J. Biosc. Bioeng. 2015, 120, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, D.; Kandylis, P.; Petrovic, T.; Dimitrijevic-Brankovic, S.I.; Levic, S.; Nedovic, V.; Kourkoutas, Y. Survival of spray dried microencapsulated Lactobacillus casei ATCC 393 in simulated gastrointestinal conditions and fermented milk. LWT Food Sci. Technol. 2016, 71, 169–174. [Google Scholar] [CrossRef]
- Dressman, J.B.; Berardi, R.R.; Dermentzoglou, L.C.; Russell, T.L.; Schmaltz, S.P.; Barnett, J.L.; Jarvenpaa, K.M. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm. Res. 1990, 7, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Pitino, I.; Cinzia, L.R.; Giuseppina, M.; LoCurto, A.; Faulks, R.M.; LeMarc, Y.; Bisignano, C.; Caggia, C.; Wickham, M.S.J. Survival of Lactobacillus rhamnosus strains in the upper gastrointestinal tract. Food Microbiol. 2010, 27, 1121–1127. [Google Scholar] [CrossRef]
- Villarreal, M.L.M.; Padilha, M.; Vieira, A.D.S.; Franco, B.D.G.M.; Martinez, R.C.R.; Saad, S.M.I. Advantageous direct quantification of viable closely related probiotics in petitsuisse cheeses under in vitro gastrointestinal conditions by propidium monoazide-qPCR. PLoS ONE 2013, 8, e82102. [Google Scholar] [CrossRef]
- Shah, N.P. Health benefits of yogurt and fermented milks. In Manufacturing Yogurt and Fermented Milks, 1st ed.; Chandan, R.C., Ed.; Blackwell Publishing Professional: Minneapolis, MN, USA, 2006; pp. 327–340. ISBN 9780813823041. [Google Scholar]
- Morelli, L. In vitro assessment of probiotic bacteria: From survival to functionality. Int. Dairy J. 2007, 17, 1278–1283. [Google Scholar] [CrossRef]
- Rajam, R.; Karthik, P.; Parthasarathi, S.; Joseph, G.S.; Anandharamakrishnan, C. Effect of whey protein-alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J. Funct. Foods 2012, 4, 891–898. [Google Scholar] [CrossRef]
- Li, S.; Jiang, C.; Chen, X.; Wang, H.; Lin, J. Lactobacillus casei immobilized onto montmorillonite: Survivability in simulated gastrointestinal conditions, refrigeration and yogurt. Food Res. Int. 2014, 64, 822–830. [Google Scholar] [CrossRef]
- Henker, J.; Laass, M.; Blokhin, B.M.; Bolbot, Y.K.; Maydannik, V.G.; Elze, M.; Wolff, C.; Schulze, J. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. Eur. J. Pediatr. 2007, 166, 311–318. [Google Scholar] [CrossRef]
- Kent, R.M.; Doherty, S.B. Probiotic bacteria in infant formula and follow-up formula: Microencapsulation using milk and pea proteins to improve microbiological quality. Food Res. Int. 2004, 64, 567–576. [Google Scholar] [CrossRef]
- Costa-Riberio, H.; Ribeiro, T.C.M.; Mattos, A.P.; Valois, S.S.; Neri, D.A.; Almeida, P.; Cerqueira, C.M.; Ramos, E.; Young, R.J.; Vanderhoof, J.A. Limitations of Probiotic Therapy in Acute, Severe Dehydrating Diarrhea. J. Pediatr. Gastroentero. Nutr. 2003, 36, 112–115. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Elmer, G.W.; McFarland, M. Meta-analysis of probiotics for the prevention and treatment of acute pediatric diarrhea. Int. J. Prob. Preb. 2006, 1, 63–76. [Google Scholar]
- Goldin, B.R.; Gorbach, S.L.; Saxelin, M.; Barakat, S.; Gualtieri, L.; Salminen, S. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Digest. Dis. Sci. 1992, 37, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Korpela, R.; Moilanen, E.; Saxelin, M.; Vapaatalo, H. Lactobacillus rhamnosus GG (ATCC 53103) and platelet aggregation in vitro. Int. J. Food Microbiol. 1997, 37, 83–86. [Google Scholar] [CrossRef]
- Gorbach, S.L.; Goldin, B.R. Lactobacillus Strains and Methods of Selection. US Patent 4839281 A, 1985. [Google Scholar]
- Floch, M.H.; Walker, W.A.; Madsen, K.; Sanders, M.E.; Macfarlane, G.T.; Flint, H.J.; Dieleman, L.A.; Ringel, Y.; Guandalini, S.; Kelly, C.P.; et al. Recommendations for probiotic use-2011 update. J. Clin. Gastroenterol. 2011, 45, S168–S171. [Google Scholar] [CrossRef]
- Hibberd, P.L.; Kleimola, L.; Fiorino, A.M.; Botelho, C.; Haverkamp, M.; Andreyewa, I.; Poutsiaka, D.; Fraser, C.; Solano-Aquilar, G.; Snydman, D.R. No evidence of harms of probiotic Lactobacillus rhamnosus GG ATCC 53103 in healthy elderly—A phase I open label study to assess safety, tolerability and cytokine responses. PLoS ONE 2012, 9, e113456. [Google Scholar] [CrossRef]
- Reale, A.; Di Renzo, T.; Rossi, F.; Zotta, T.; Iacumin, L.; Preziuso, M.; Parente, E.; Sorrentino, E.; Coppola, R. Tolerance of Lactobacillus casei, Lactobacillus paracasei and Lactobacillus rhamnosus strains to stress factors encountered in food processing and in the gastro-intestinal tract. LWT Food Sci. Technol. 2015, 60, 721–728. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Bosnea, L.; Taboukos, S.; Baras, C.; Lambrou, D.; Kanellaki, M. Probiotic cheese production using Lactobacillus casei cells immobilized on fruit pieces. J. Dairy Sci. 2006, 89, 1439–1451. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, Y.; Han, K.S.; You, S.; Oh, S.; Kim, S.H. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett. Appl. Microbiol. 2006, 42, 452–458. [Google Scholar] [CrossRef]
- Lye, H.S.; Rusul, G.; Liong, M.T. Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J. Dairy Sci. 2010, 93, 1383–1392. [Google Scholar] [CrossRef]
- Xu, M.; Gagné-Bourque, F.; Dumont, M.-J.; Jabaji, S. Encapsulation of Lactobacillus casei ATCC 393 cells and evaluation of their survival after freeze-drying, storage and under gastrointestinal conditions. J. Food Eng. 2016, 168, 52–59. [Google Scholar] [CrossRef]
- Gopal, P.K. Lactic Acid Bacteria: Lactobacillus spp.: Lactobacillus acidophilus. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; Volume 3, pp. 91–95. ISBN 978-0-12-374402-9. [Google Scholar]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Upadhyay, A. Investigating the Potential of Plant-Derived Antimicrobials and Probiotic Bacteria for Controlling Listeria Monocytogenes. Doctoral Thesis, University of Connecticut, Storrs, CT, USA, 2014. Available online: http://digitalcommons.uconn.edu/dissertations/326 (accessed on 2 November 2020).
- Apostolidis, E.; Kwon, Y.I.; Shinde, R.; Ghaedian, R.; Shetty, K. Inhibition of Helicobacter pylori by fermented milk and soymilk using select lactic acid bacteria and link to enrichment of lactic acid and phenolic content. Food Biotechnol. 2011, 25, 58–76. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.M.; Tian, F.; Zhang, Q.; Zhang, H.P.; Zhang, H.; Chen, W. Antagonistic Activities of Lactobacilli against Helicobacter pylori Growth and Infection in Human Gastric Epithelial Cells. J. Food Sci. 2012, 77, 9–14. [Google Scholar] [CrossRef]
- Sunanliganon, C.; Thong-Ngam, D.; Tumwasorn, S.; Klaikeaw, N. Lactobacillus plantarum B7 inhibits Helicobacter pylori growth and attenuates gastric inflammation. World J. Gastroenterol. 2012, 18, 2472–8240. [Google Scholar] [CrossRef]
- Ait, S.H.; Bendali, F.; Cudennec, B.; Drider, D. Anti-pathogenic and probiotic attributes of Lactobacillus salivarius and Lactobacillus plantarum strains isolated from feces of Algerian infants and adults. Res. Microbiol. 2017, 168, 244–254. [Google Scholar] [CrossRef]
- Su, P.; Henriksson, A.; Mitchell, H. Prebiotics enhance survival and prolong the retention period of specific probiotic inocula in an in vivo murine model. J. Appl. Microbiol. 2007, 106, 2392–2400. [Google Scholar] [CrossRef]
- Martinez, R.C.R.; Aynaou, A.E.; Albrecht, S.; Schols, H.A.; De Martinis, E.C.P.; Zoetendal, E.G.; Venema, K.; Saad, S.M.I.; Smidt, H. In vitro evaluation of gastrointestinal survival of Lactobacillus amylovorus DSM 16698 alone and combined with galactooligosaccharides, milk and/or Bifidobacterium animalis subsp. lactis Bb-12. Int. J. Food Microbiol. 2011, 149, 152–158. [Google Scholar] [CrossRef]
- Donkor, O.N.; Nilmini, S.L.I.; Stolic, P.; Vasiljevic, T.; Shah, N.P. Survival and activity of selected probiotic organisms in set-type yoghurt during cold storage. Int. Dairy J. 2007, 17, 657–665. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, O.; Muthaiyan, A.; Moreno, F.J.; Montilla, A.; Sanz, M.L.; Ricke, S.C. Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food Microbiol. 2012, 30, 355–361. [Google Scholar] [CrossRef]
- Perrin, S.; Grill, J.P.; Scheneider, F. Effects of fructooligosaccharides and their monomeric components on bile salt resistance in three species of bifidobacteria. J. Appl. Microbiol. 2000, 88, 968–974. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; Pandiella, S.S.; Webb, C. Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. Int. J. Food Microbiol. 2003, 82, 133–141. [Google Scholar] [CrossRef]
- Vandenplas, Y.; De Greef, E.; Hauser, B.; Devreker, T.; Veereman-Wauters, G. Probiotics and prebiotics in pediatric diarrheal disorders. Expert Opin. Pharmacother. 2013, 14, 397–409. [Google Scholar] [CrossRef]
- Freedman, S.B.; Williamson-Urquhart, S.; Farion, K.J.; Gouin, S.; Willan, A.R.; Poonai, N.; Hurley, K.; Sherman, P.M.; Finkelstein, Y.; Lee, B.E. Multicenter trial of a combination probiotic for children with gastroenteritis. N. Engl. J. Med. 2018, 379, 2015–2026. [Google Scholar] [CrossRef]
- Schnadower, D.; Tarr, P.I.; Casper, T.C. Lactobacillus rhamnosus GG versus placebo for acute gastroenteritis in children. N. Engl. J. Med. 2018, 379, 2002–2014. [Google Scholar] [CrossRef]
| pH | Time of Incubation (h) | ||||
|---|---|---|---|---|---|
| 0 | 12 | 24 | 36 | 48 | |
| Lb. rhamnosus GG | |||||
| 2 | 7.76 ± 0.31 | - | - | - | - |
| 3 | 7.79 ± 0.11 | - | - | - | - |
| 4 | 7.79 ± 0.08 | 8.55 ± 0.11 | 8.83 ± 0.05 | 8.68 ± 0.13 | 9.02 ± 0.08 |
| 5 | 7.8 ± 0.15 | 9.59 ± 0.16 | 9.73 ± 0.12 | 9.88 ± 0.05 | 9.14 ± 0.11 |
| 6.2 | 7.81 ± 0.07 | 10.56 ± 0.03 | 10.03 ± 0.14 | 10.74 ± 0.21 | 10.01 ± 0.05 |
| Bifidobacterium BB-12 | |||||
| 2 | 6.74 ± 0.34 | - | - | - | - |
| 3 | 6.82 ± 0.11 | - | - | - | - |
| 4 | 7.13 ± 0.58 | 6.13 ± 0.17 | 5.93 ± 0.08 | 4.24 ± 0.31 | 5.46 ± 0.45 |
| 5 | 6.98 ± 0.27 | 8.61 ± 0.12 | 9.02 ± 0.20 | 9.57 ± 0.16 | 9.38 ± 0.14 |
| 6.2 | 7.23 ± 0.23 | 9.94 ± 0.11 | 9.98 ± 0.01 | 10.16 ± 0.14 | 10.22 ± 0.14 |
| Lb. casei | |||||
| 2 | 6.12 ± 0.16 | - | - | - | - |
| 3 | 6.33 ± 0.19 | 5.16 ± 0.07 | 4.29 ± 0.00 | - | - |
| 4 | 6.14 ± 0.05 | 5.67 ± 0.00 | 5.82 ± 0.25 | 5.55 ± 0.01 | 5.12 ± 0.09 |
| 5 | 6.52 ± 0.08 | 6.41 ± 0.08 | 5.62 ± 0.09 | 6.85 ± 0.11 | 8.08 ± 0.09 |
| 6.2 | 6.42 ± 0.11 | 7.02 ± 0.16 | 7.16 ± 0.05 | 8.71 ± 0.05 | 9.13 ± 0.08 |
| Lb. acidophilus | |||||
| 2 | 6.79 ± 0.13 | - | - | - | - |
| 3 | 6.89 ± 0.08 | - | - | - | - |
| 4 | 6.63 ± 0.11 | 6.51 ± 0.13 | 6.40 ± 0.14 | 6.82 ± 0.14 | 6.99 ± 0.21 |
| 5 | 6.92 ± 0.22 | 6.83 ± 0.02 | 7.94 ± 0.58 | 8.33 ± 0.05 | 8.17 ± 0.16 |
| 6.2 | 7.01 ± 0.22 | 8.64 ± 0.12 | 10.32 ± 0.15 | 10.13 ± 0.04 | 9.97 ± 0.14 |
| Lb. plantarum | |||||
| 2 | 6.13 ± 0.39 | - | - | - | - |
| 3 | 6.29 ± 0.23 | 5.55 ± 0.08 | 4.17 ± 0.08 | - | - |
| 4 | 6.37 ± 0.25 | 7.36 ± 0.08 | 7.98 ± 0.01 | 9.17 ± 0.03 | 9.14 ± 0.24 |
| 5 | 6.22 ± 0.09 | 8.92 ± 0.08 | 9.28 ± 0.12 | 9.91 ± 0.09 | 9.68 ± 0.11 |
| 6.2 | 6.41 ± 0.03 | 9.32 ± 0.13 | 10.16 ± 0.08 | 10.28 ± 0.13 | 9.93 ± 0.03 |
| Strain | Variant of Culture in MRS | Length of Lag Phase (h) | Length of Log Phase (h) | Initial OD600 in Log Phase | Final OD600 in Log Phase | Coefficient of Specific Growth Rate (μ) (h–1) |
|---|---|---|---|---|---|---|
| Lb. rhamnosus GG ATCC 53103 | pH 4.0 pH 5.0 pH 6.2 | 19.0 0.5 0 | 29.0 19.0 16.0 | 0.53 0.59 0.56 | 1.78 1.97 2.15 | 0.042 0.063 0.084 |
| Bifidobacterium BB-12 | pH 5.0 pH 6.2 | 3.0 2.5 | 16.5 12.0 | 0.44 0.54 | 2.05 2.13 | 0.093 0.114 |
| Lb. casei | pH 5.0 pH 6.2 | 24.5 24.5 | 35.0 20.0 | 0.30 0.35 | 1.55 1.85 | 0.047 0.083 |
| Lb. acidophilus | pH 4.0 pH 5.0 pH 6.2 | 25.0 5.5 6.5 | 16.5 49.5 28.0 | 0.33 0.30 0.37 | 0.67 0.78 1.60 | 0.043 0.019 0.053 |
| Lb. plantarum | pH 4.0 pH 5.0 pH 6.2 | 0 0 1.0 | 29.0 17.0 12.0 | 0.98 0.99 1.04 | 1.70 1.93 2.10 | 0.019 0.039 0.058 |
| pH | Time of Incubation (h) | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Lb. rhamnosus GG | ||||
| 2.0 | 6.92 ± 0.17 | 5.19 ± 0.01 | - | - |
| 3.0 | 7.07 ± 0.04 | 5.72 ± 0.08 | 5.55 ± 0.17 | 5.63 ± 0.45 |
| 4.0 | 7.21 ± 0.00 | 6.64 ± 0.05 | 6.78 ± 0.13 | 6.33 ± 0.04 |
| 5.0 | 7.13 ± 0.34 | 6.96 ± 0.17 | 6.82 ± 0.09 | 6.80 ± 0.02 |
| Bifidobacterium BB-12 | ||||
| 2.0 | 5.73 ± 0.28 | 4.62 ± 0.34 | 4.58 ± 0.05 | 4.70 ± 0.11 |
| 3.0 | 6.96 ± 0.00 | 7.13 ± 0.16 | 6.88 ± 0.00 | 6.86 ± 0.00 |
| 4.0 | 7.02 ± 0.13 | 6.32 ± 0.12 | 6.54 ± 0.15 | 6.38 ± 0.31 |
| 5.0 | 7.22 ± 0.13 | 7.16 ± 0.16 | 7.31 ± 0.03 | 7.18 ± 0.21 |
| Lb. casei | ||||
| 2.0 | 5.80 ± 0.08 | - | - | - |
| 3.0 | 6.29 ± 0.11 | - | - | - |
| 4.0 | 6.19 ± 0.54 | 5.37 ± 0.17 | 5.23 ± 0.14 | 4.22 ± 0.14 |
| 5.0 | 6.21 ± 0.35 | 5.98 ± 0.32 | 5.61 ± 0.17 | 4.92 ± 0.12 |
| Lb. acidophilus | ||||
| 2.0 | 6.24 ± 0.12 | 5.70 ± 0.05 | 5.30 ± 0.00 | - |
| 3.0 | 6.78 ± 0.12 | 5.99 ± 0.07 | 5.13 ± 0.05 | - |
| 4.0 | 6.88 ± 0.09 | 6.23 ± 0.13 | 6.19 ± 0.11 | 6.33 ± 0.03 |
| 5.0 | 7.02 ± 0.16 | 6.90 ± 0.12 | 6.96 ± 0.15 | 6.87 ± 0.02 |
| Lb. plantarum | ||||
| 2.0 | 6.68 ± 0.06 | 5.19 ± 0.08 | 5.22 ± 0.02 | 4.97 ± 0.17 |
| 3.0 | 7.18 ± 0.05 | 6.30 ± 0.16 | 6.41 ± 0.01 | 6.43 ± 0.25 |
| 4.0 | 7.20 ± 0.00 | 7.40 ± 0.12 | 7.32 ± 0.05 | 7.38 ± 0.16 |
| 5.0 | 7.31 ± 0.13 | 7.44 ± 0.01 | 7.27 ± 0.03 | 7.45 ± 0.00 |
| Bile Salts (%) | Time of Incubation (h) | |||
|---|---|---|---|---|
| 0 | 2 | 4 | 6 | |
| Lb. rhamnosus GG | ||||
| 1 | 6.13 ± 0.12 | 6.08 ± 0.26 | 5.86 ± 0.03 | 5.80 ± 0.37 |
| 2 | 6.21 ± 0.22 | 5.12 ± 0.00 | 4.87 ± 0.18 | 4.42 ± 0.17 |
| 3 | 6.06 ± 0.01 | 5.02 ± 0.12 | - | - |
| Bifidobacterium BB-12 | ||||
| 1 | 6.32 ± 0.15 | 6.40 ± 0.03 | 6.18 ± 0.28 | 6.16 ± 0.22 |
| 2 | 6.38 ± 0.15 | 6.16 ± 0.2 | 5.97 ± 0.15 | 6.08 ± 0.12 |
| 3 | 6.17 ± 0.05 | 5.93 ± 0.09 | 5.90 ± 0.23 | 5.86 ± 0.06 |
| Lb. casei | ||||
| 1 | 5.30 ± 0.03 | 5.21 ± 0.14 | 4.44 ± 0.01 | - |
| 2 | 5.26 ± 0.12 | 5.07 ± 0.23 | 4.04 ± 0.00 | - |
| 3 | 5.01 ± 0.09 | - | - | - |
| Lb. acidophilus | ||||
| 1 | 6.65 ± 0.05 | 5.37 ± 0.00 | 5.16 ± 0.17 | - |
| 2 | 6.48 ± 0.03 | 5.02 ± 0.12 | 4.86 ± 0.03 | 4.71 ± 0.18 |
| 3 | 6.52 ± 0.22 | 5.12 ± 0.05 | 4.70 ± 0.05 | - |
| Lb. plantarum | ||||
| 1 | 5.63 ± 0.12 | 5.79 ± 0.28 | 5.84 ± 0.00 | 6.47 ± 0.02 |
| 2 | 5.72 ± 0.12 | 5.20 ± 0.02 | 5.63 ± 0.01 | 5.99 ± 0.33 |
| 3 | 5.48 ± 0.10 | 5.53 ± 0.01 | 5.70 ± 0.13 | 5.77 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiak-Różańska, L.; Berthold-Pluta, A.; Pluta, A.S.; Dasiewicz, K.; Garbowska, M. Effect of Simulated Gastrointestinal Tract Conditions on Survivability of Probiotic Bacteria Present in Commercial Preparations. Int. J. Environ. Res. Public Health 2021, 18, 1108. https://doi.org/10.3390/ijerph18031108
Stasiak-Różańska L, Berthold-Pluta A, Pluta AS, Dasiewicz K, Garbowska M. Effect of Simulated Gastrointestinal Tract Conditions on Survivability of Probiotic Bacteria Present in Commercial Preparations. International Journal of Environmental Research and Public Health. 2021; 18(3):1108. https://doi.org/10.3390/ijerph18031108
Chicago/Turabian StyleStasiak-Różańska, Lidia, Anna Berthold-Pluta, Antoni Stanisław Pluta, Krzysztof Dasiewicz, and Monika Garbowska. 2021. "Effect of Simulated Gastrointestinal Tract Conditions on Survivability of Probiotic Bacteria Present in Commercial Preparations" International Journal of Environmental Research and Public Health 18, no. 3: 1108. https://doi.org/10.3390/ijerph18031108
APA StyleStasiak-Różańska, L., Berthold-Pluta, A., Pluta, A. S., Dasiewicz, K., & Garbowska, M. (2021). Effect of Simulated Gastrointestinal Tract Conditions on Survivability of Probiotic Bacteria Present in Commercial Preparations. International Journal of Environmental Research and Public Health, 18(3), 1108. https://doi.org/10.3390/ijerph18031108







