Abstract
A sedentary lifestyle is associated with overweight/obesity, which involves excessive fat body accumulation, triggering structural and functional changes in tissues, organs, and body systems. Research shows that this fat accumulation is responsible for several comorbidities, including cardiovascular, gastrointestinal, and metabolic dysfunctions, as well as pathological pain behaviors. These health concerns are related to the crosstalk between adipose tissue and body systems, leading to pathophysiological changes to the latter. To deal with these health issues, it has been suggested that physical exercise may reverse part of these obesity-related pathologies by modulating the cross talk between the adipose tissue and body systems. In this context, this review was carried out to provide knowledge about (i) the structural and functional changes in tissues, organs, and body systems from accumulation of fat in obesity, emphasizing the crosstalk between fat and body tissues; (ii) the crosstalk between fat and body tissues triggering pain; and (iii) the effects of physical exercise on body tissues and organs in obese and non-obese subjects, and their impact on pathological pain. This information may help one to better understand this crosstalk and the factors involved, and it could be useful in designing more specific training interventions (according to the nature of the comorbidity).
1. Introduction
A sedentary lifestyle may trigger obesity, since the lack of physical activity or inactivity could lead to weight gain, and a sedentary lifestyle is highly associated with abdominal and visceral fat excess [1,2,3,4,5,6]. In the United States (U.S.), about 75% of adults older than 20 years meet the criteria for being overweight or obese; 42% are obese, and 9% have morbid or severe obesity [7]. As for the European Union (EU), about 52.7% of the population aged 18 and over are overweight and about 20% are obese [8]. In the U.S., it is estimated that the medical costs of obesity exceed USD 275 billion annually [9]. Considering that the U.S. total health care expenditure reached USD 3.3 trillion in 2016 [10], the obesity-related medical costs represented 8.3% of the total health budget. In Europe, the situation is not much better. It is estimated that, in the EU, the health expenditure associated with overweight and obesity in the period 2020–2050 will be approximately 5% [11]. Hence, these available data indicate that between 20 and 40% of industrialized western populations would be obese, and the related medical costs may represent between 5 and 8% of health budgets.
The World Health Organization (WHO, Geneva, Switzerland) defines obesity as an “abnormal or excessive fat accumulation that presents a risk to health”, commonly classified by the body mass index (BMI). The fundamental cause of obesity is an energy imbalance between calories consumed and calories expended, due to consumption of foods rich in energy, which mainly contain sugars and fats, as well as an increase in physical inactivity due to the increasingly sedentary nature of many forms of work, changing modes of transportation, and increasing urbanization [12]. The excess fat accumulated in different tissues, organs, or body systems, leads to structural and functional human body changes. Several studies suggest that the fat distribution pattern in the human body is associated with the risk of suffering from various obesity-related pathologies. In men, obesity associates with fat accumulation in the abdomen and in the upper part of the body, whereas in women, it affects the gluteofemoral region and the lower part of the body, although as the latter gain weight, they become more likely to develop both upper-body and abdominal fat. [13]. The accumulation of abdominal fat is associated with the development of various disorders, such as atherogenic profile, arterial stiffness, elevated fibrinogen levels, hypertension, insulin resistance, hyperinsulinemia, glucose intolerance, arthritis, irregular menstruation, and gallbladder disorders [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Research shows that physical exercise can reverse part of these obesity-related pathologies by decreasing the availability of free fatty acids and improving the insulin sensitivity of glucose metabolism [29], reducing hypertension [30], decreasing arterial stiffness [31], reducing both fibrinogen and fibrinolytic biomarkers [32], relieving arthritis [33,34], and improving menstrual issues [35]. In addition, exercise could lead to lower body fat and weight [36,37,38,39,40,41,42,43,44,45], which, consequently, could help one deal with the above-mentioned obesity comorbidities.
In addition to obesity-induced disorders, clinical evidence suggests that pain conditions development in obese subjects, such as low back pain [46,47,48,49,50,51,52,53,54,55,56], musculoskeletal pain [57,58,59,60,61,62,63], osteoarthritis pain [64,65,66], knee pain [67,68,69,70], hip pain [71], foot pain [72], spine-related pain [73], migraine and/or headache [74,75,76,77,78,79,80], orofacial pain [81,82], and postsurgical pain [83]. Research shows that physical exercise may relieve obesity-induced pain in affected subjects [84,85,86,87,88,89].
Overall, obesity is a health concern characterized by excessive body fat accumulation and by a set of comorbidities, which may be associated with structural and functional changes in tissues, organs, and body systems. Physical exercise, which reduces body fat, alleviates some of these obesity-related comorbidities, including pathological pain. In this context, the objectives of this work are to review: (i) the structural and functional changes suffered by tissues, organs, and body systems, due to the accumulation of fat in obesity, emphasizing the crosstalk between fat and body tissues; (ii) the crosstalk between fat and body tissues triggering pain exacerbation; and (iii) the effects of physical exercise on body tissues and organs in obese and non-obese subjects, and their impact on pathological pain relief.
2. Obesity-Induced Changes in Tissues, Organs, and Body Systems: Crosstalk between Fat and Body Tissues
Before describing the effects of physical exercise on tissue crosstalk (in a “fat excess” context), in this section, we review preclinical and clinical evidence of the accumulation of fat in different tissues, organs, and body systems in obese subjects, the humoral factors synthesized and released by these adipose tissues, and how these factors influence the physiology of the tissues, organs, and systems where they accumulate. At the end of this section, we review the deposit of body adipose tissue, and how this deposit of fat confers to an increased risk of metabolic diseases in obese subjects, such as metabolic syndrome, metabolically healthy obesity, and metabolically unhealthy obesity, indicating, for each of them, the characteristics of their adipose tissue.
2.1. Skeletal Muscle
Obesity causes an accumulation of fat in skeletal muscles, mainly inside muscle cells occupying a central region in the muscle fiber. This accumulation of lipid drops in obese subjects affects the three types of muscle fibers (fibers I, IIA, and IIB) [90,91]. Obesity causes dysregulation of the metabolism of these accumulated lipids in skeletal muscle cells, which leads to a significant decrease in the degree of lipid unsaturation [92]. This lipid unsaturation reduction in muscle cells of obese subjects may result from increased lipid peroxidation. Moreover, in the Otsuka Long-Evans Tokushima fatty (OLETF) rat, an animal model of obesity, the levels of lipid peroxidation in skeletal muscle fibers were significantly higher in sedentary—rather than runner—animals, suggesting that muscle lipid peroxidation increases in sedentary obese subjects [93]. In turn, the accumulation of lipid droplets within skeletal muscle fibers and the consequent increase in lipid peroxidation in these cells may be involved in the development of skeletal muscle insulin resistance in obese sedentary subjects [94]. Under physiological conditions, insulin secreted by an increase of glucose in the blood, causes upregulation of glucose transporter type 4 (GLUT4) expression in muscle cells via activation insulin–tyrosine–kinase receptors, phosphorylation of insulin receptor substrates 1 and 2 (IRS1, and IRS2), activation of phosphatidylinositol 3-kinase (PI3K), and atypical protein kinase C (PKCζ) [95]. Overexpression of GLUT4 transporters in the membrane of skeletal muscle fibers promotes glucose reuptake in skeletal muscle fiber, and immediately, glucose is phosphorylated by hexokinase to prevent cell leakage. Phosphorylated glucose can be stored in the form of muscle glycogen or used in oxidative glycolysis for energy production [96]. In contrast, when elevated insulin levels produce an attenuated biological response, this is defined as insulin resistance [97]; this would occur in obese individuals. Under obesity conditions, the accumulated lipids in skeletal muscle cells promotes muscle cells to obtain energy through lipid oxidation, resulting in the production of 4-hydroxy-2-hexenal (4-HHE), a lipid aldehyde that affects insulin-induced IRS1/IRS2 phosphorylation [98]. Moreover, another lipid aldehyde that is produced from lipid peroxidation in skeletal muscle cells is 4-hydroxy-2-nonenal (4-HNE), which is known to causes a significant reduction of IRS1 phosphorylation in skeletal muscle cells, and increases the ROS production promoting lipid peroxidation in these cells [99]. Altogether, under obesity conditions, the accumulation of lipids in skeletal muscle cells favors the use of this energy source for obtaining ATP molecules through the metabolism of lipid oxidation. Two products of this lipid oxidation (4-HHE and 4-HNE) interfere in the phosphorylation of IRS1 and, thus, in the final translocation of GLUT4 transporters in the plasma membrane of the skeletal muscle cell. Hence, in an obese person, after food intake, the insulin plasma increase will not result in proper GLUT4 overexpression in skeletal muscle cells because of the interference of these lipid aldehydes in the signaling pathway responsible for GLUT4 expression, leading to the insulin resistance of the skeletal muscle observed in obese subjects.
In the same context, other physiological changes may explain insulin resistance in obese subjects, such as the increase of fatty acid synthase (FAS) and choline/ethanolamine phosphotransferase 1 (CEPT1) enzymes activity, and/or expression. In mice, it was reported that high-fat diet feeding increases FAS protein expression and enzyme activity in skeletal muscle fibers of obese mice that also show insulin resistance. By using FAS-KO animals that present FAS deficiency in the skeletal muscles, but not in the liver, heart, or pancreas, it was observed that FAS deletion causes AMPK (AMP-activated protein kinase) activation in skeletal muscle cells of mice treated with a high-fat diet. As AMPK promotes skeletal muscle insulin sensitivity, these results suggest that FAS expression inhibits AMPK and, thereby, generates insulin resistance in skeletal muscle fiber [100]. In addition, it is well known that FAS is located in plasmatic membrane and in sarcoplasmic reticulum (SR), promoting SERCA activity, allowing the entry of calcium ions into the SR and, thereby, facilitating the process of muscle relaxation. In FAS-KO mice, it was reported that FAS deficiency decreased SERCA activity by altering the phospholipid composition of the SR, which resulted in increased cytosolic calcium and AMPK activation. FAS-KO mice showed lower insulin resistance than wild type mice, but their skeletal muscles showed higher weakness than wild type animals [100]. These findings suggest that FAS activity is associated with insulin resistance in skeletal muscles. In the same line, a high-fat diet increases choline/ethanolamine phosphotransferase 1 (CEPT1) expression in mouse skeletal muscle, and increases phosphatidylcholine (PC) and phosphatidylethanolamine (PE) abundance in skeletal muscle SR, maintaining the SERCA activity. Similarly, in absence of CEPT1 (CEPT1-KO mice), altered PC:PE content decreased SERCA activity, increased cytosolic calcium, causing muscle weakness, but increased muscle insulin sensitivity, via calcium activation of AMPK [101]. These findings suggest that CEPT1 is also related to insulin resistance.
One of the direct consequences of insulin resistance in muscle fiber is that muscle mitochondria must use free fatty acids (FFA) in plasma as an energy source, to maintain, among other muscle functions, muscle contraction, and muscle strength during exercise and daily activity of the obese subject. However, ATP production by beta-oxidation of FFA is lower than oxidation of glucose, about 14 and 36 ATP molecules, respectively. Therefore, the insulin resistance caused by obesity forces skeletal muscles to use the beta-oxidation of free fatty acids and other lipids with a lower yield of ATP molecule production, and with it, less production of muscle force and muscle fatigue occurs more quickly due to the consumption of muscle glycogen stores [102]. Using a zebrafish model, it was shown that obesity causes a decrease in sustained swimming speed, maximum locomotor capacity, and isolated muscle isometric stress that are not reversed by weight loss, whereas obesity increases resting metabolic rates and decreases maximal metabolic rate that are reversed by weight loss [103].
Finally, intermuscular adipose tissue (IMAT) marbled within skeletal muscles is also related to skeletal muscle insulin resistance. IMAT increases in an obese subject, it is preferent in the lower-extremity muscles [104,105]. Using fat (intermuscular, subcutaneous, and visceral adipose tissues) and muscle biopsies of human subjects, it was reported that factors secreted from IMAT decrease insulin sensitivity in vitro with a potency similar to visceral adipose tissue (VAT), because both adipose tissues induce 1,2-diacylglycerol (DAG) accumulation in the skeletal muscles. The insulin-IMAT resistance is related to a decrease of mitochondrial function and lipid oxidation, which contribute to enhanced release of free fatty acids into interstitial fluid surrounding muscle [106]. In both mice and humans, DAG accumulation in skeletal muscle cells promote the PKC-theta activation and the subsequent decrease in insulin signaling at the level of the insulin receptor substrate (IRS)-1 tyrosine phosphorylation [107,108,109,110,111]. IMAT secretes cytokines and chemokines that contribute to insulin resistance of skeletal muscles, including monocyte chemoattractant protein-1 (MCP-1) [106,112], and contributes to macrophages and lymphocyte infiltration in skeletal muscles of obese subjects [113]. In pigs, it was reported that IMAT secretes several micro-RNAs related to insulin resistance and inflammation of skeletal muscles [114].
2.2. Cardiovascular System
Perivascular adipose tissue (PVAT) is located outside the adventitial layer and surrounds most of the systemic blood vessels, with the exception of the blood vessels of the central nervous system [115,116,117,118]. In small vessels and microvessels, PVAT is integrated in the vascular wall without laminar structures that separate PVAT from the adventitia layer [117,118]. Structurally, PVAT consists of adipocytes, fibroblasts, stem cells, eosinophils, T lymphocytes, and macrophages [119,120]. In large vessels, between PVAT and the vascular wall, a network of elastic and collagen fibers, fibroblasts, vasa vasorum, and sympathetic nerve fibers is observed [121]. PVAT releases several factors that reach medial and endothelial layers of blood vessels by direct diffusion, through the vasa vasorum, and through the network of collagen ducts that connect the media layer with the adventitia. These factors regulate the physiology/pathophysiology of blood vessels, including the vascular tone, inflammation of vascular wall, vascular remodeling, and atherosclerosis [118,119,120,122,123,124].
The factors released by PVAT can be classified into: (i) vasodilator factors, such as hydrogen peroxide (H2O2), hydrogen sulfide (H2S), methyl palmitate, nitric oxide, adiponectin, leptin, apelin, omentin, adrenomedullin [118,120,124,125]; (ii) vasoconstrictor factors including angiotensin-II, chemerin, adiponectin, and apelin [118,120,122,124]; (iii) inducing factors of vascular remodeling, such as micro-RNAs (e.g., miR-221–3p), growth factors (e.g., thrombospondin-1, serpin-E1, TGF-β, PDGF-BB, VEGF, bFGF, hepatocyte growth factor, insulin-like growth factor-binding protein-3), visfatin, and leptin that promote neointima formation, and proliferation of vascular smooth muscle cells after vascular injury and/or atherosclerosis [122,126,127,128]; (iv) inhibitory factors of vascular remodeling including adiponectin, and C1q/TNF-related protein 9 (CTRP-9) [124,129,130]; (v) anti-inflammatory factors secreted under physiological conditions such as adiponectin, IL10, prostacyclin [123]; (vi) pro-inflammatory factors released under vascular injuries (e.g., atherosclerosis, hypertension) including leptin, resistin, visfatin, IL6, TNF-alpha, RANTES, MCP-1/CCL2, CCL3, CCL5, reactive oxygen species (ROS) [123]; (vii) pro-atherogenic factors, such as IL17 [119,131]; (viii) anti-atherogenic agents, including nitric oxide, H2S, adiponectin, vaspin, apelin, omentin, chemerin, leptin, resistin, lipocalin-2 (LCN2), and visfatin [119,120,132,133].
Animals consuming high-fat diets (HFDs) rapidly gain weight and become obese subjects. Obesity causes an increase of PVAT and a hypertrophy of adipocytes around the blood vessels, and an increase of eosinophils, macrophages, T-cells, MCP-1, and TNF-alpha expression, but endothelial nitric oxide synthase expression decreases in these obese animals [132,134,135,136]. In humans, PVAT samples from obese subjects showed that the expression of MCP1/CCL2, CCL8, IL1beta, and IL6 proteins were upregulated, suggesting that obesity triggers upregulation of pro-inflammatory factors secreted by PVAT [137]. In turn, it is well known that CCL2, TNF-alpha, IL1beta, and IL6 are pro-inflammatory factors are involved in atherosclerotic plaque formation; that is, they favor vascular walls lipids deposition [138]. In addition, MCP1/CCL2 secreted by PVAT it is a factor that favors the attraction and recruitment of monocytes/macrophages towards the vascular wall, and monocyte–endothelial cell adhesion plays a key role in the initiation of atherosclerosis. After adhering to the endothelial cells, monocytes penetrate through endothelial cells into the subendothelial space, transforming in macrophages, and participating in scavenging lipoprotein particles and turn into foam cells, which present cytoplasmic and membrane-bound droplets, resulting in more accumulation of lipoproteins in the subendothelial space [139,140]. Several studies suggest that TNF-alpha contributes to eosinophil recruitment [141,142,143], and eosinophils attracted to PVAT-secreted TNF-alpha contribute to atherosclerotic lesion formation by activating the endothelial cell [144]. In addition, eosinophils interact directly with platelets and induce eosinophil activation and the release of eosinophil extracellular traps that stabilizes the thrombus [144]. In summary, obesity causes an increase in perivascular adipose tissue (PVAT) that releases pro-inflammatory factors that interact with the blood vessel walls cells, favoring the recruitment of monocyte–macrophages and eosinophils and the generation of atherosclerotic plaques. That is, the crosstalk between the perivascular adipose tissue and the wall of the blood vessels results in atherosclerotic lesions in the blood vessels of the obese subjects.
On the other hand, epicardial adipose tissue (EAT) is a visceral thoracic fat depot located along the large coronary arteries, on the surface of the ventricles and the apex of the heart. This EAT constitutes about 20% of the total ventricular weight in the human heart. Compared to other fat depots, the number of adipocytes per gram of EAT is higher, but their size is smaller. In comparison with subcutaneous adipose tissue, EAT shows higher levels of saturated fatty acids and lower levels of unsaturated fatty acids, and higher levels of inflammatory mediators, including IL1beta, IL6, TNF-alpha, MCP1/CCL2, CCL3, CCL4, CCL5, CCL11, CCL18, and CCL21. Moreover, EAT express higher levels of resistin, but lower levels of adiponectin and leptin [145,146,147]. It is well known that EAT thickness increases in obese subjects [148,149,150,151,152,153], and in these subjects, the levels of factors secreted by EAT, such as TNF-alpha, IL6, leptin, and free fatty acids, increase in comparison with non-obese subjects [154,155]. As for free fatty acids secreted by EAT in obese subjects, the metabolic signature of EAT is dominated by an increase of phosphatidylglycerol, phosphatidylcholine, and phosphatidylethanolamine [156]. In this context, it is known that TNF-alpha and IL-1beta reduce contractile function and impair cardiac chemomechanical energy transduction in human myocardium [157]. Moreover, activin-A, TNF-alpha, IL6, and MCP1/CCL2 secreted by EAT contributes to myocardial fibrosis [158,159,160]. In addition, elevated free fatty acids levels may impair cardiac function and cardiac dysfunction might result from myocardial insulin resistance with significant changes to PI3K-Akt-GLUT4 and AMPK-eNOS signaling pathways, with increasing free fatty acids levels [161]. Altogether these findings suggest that factors secreted by EAT causes changes in cardiomyocytes, affecting their physiology.
Pericardial adipose tissue (PAT) covers approximately 80% of the heart and constitutes 20–50% of the heart weight in humans. PAT is located outside the pericardium on the external surface of the parietal pericardium [145]. It was shown that, in obese subjects, the PAT increases [162,163], and in obese Lee-Sung mini pigs, it secretes higher levels of TNF-alpha, TGF-beta, platelet-derived growth factor (PDGF), leptin, and resistin [164]. Moreover, in these mini pigs, the PAT contributes to cardiac fibrosis development [165]. In humans, PAT also contributes to cardiac insulin resistance and left ventricular systolic dysfunction in obese individuals [166,167]. TGF-beta promotes cardiac fibrosis, the formation of cardiac fibroblasts into myofibroblasts. Activated fibroblasts differentiate into myofibroblasts, which increases their ability to produce ECM proteins. This change leads to increased myocardial stiffness and, ultimately, cardiac dysfunction and heart failure [168]. Similarly, PDGF also induces cardiac fibrosis [169], and as mentioned above, TNF-alpha contributes to reduce contractile function of myocardium [157]. Resistin and leptin promote cardiac hypertrophy [170,171,172], whereas leptin is also implicated in the regulation of cardiac contractility via a local nitric oxide dependent mechanism [173]. Hence, these findings suggest that several factors secreted by pericardial adipose tissue also influence the physiology of the heart.
2.3. Accumulation of Fat in Liver and Pancreas
Excessive fat accumulation within the liver is called hepatic steatosis. There is a positive correlation between obesity and hepatic steatosis, and obese people are four times more likely to develop hepatic steatosis [174,175,176]. Hepatic steatosis is one of the characteristics of non-alcoholic fatty liver disease (NAFLD), with >5% of hepatocytes infiltrated with fat [177]; it is well known that a sedentary lifestyle and a high-fat diet (HFD) promotes NAFLD [178,179]. Consequently, NAFLD is strongly linked to obesity, with a reported prevalence as high as 80% in obese patients and only 16% in individuals with a normal body mass index and without metabolic risk factors [175,180,181]. A change in the nomenclature of NAFLD to metabolic (dysfunction)-associated fatty liver disease (MAFLD) was proposed by expert panels, because multiple metabolic factors contributing to the development and progression of NAFLD and, consequently, MAFLD, is used for hepatic disease related to the presence of metabolic causes. According to a new consensus, NAFL, and nonalcoholic steatohepatitis (NASH) is abolished and both phenotypes are placed under the umbrella of MAFLD [182,183,184]. Thus, MAFLD is defined as liver fat accumulation (hepatic steatosis), combined with the presence of overweight/obesity or T2DM, or at least two metabolic risk factors, including (i) waist circumference ≥102 cm in men or ≥88 cm in women (≥90/80 cm in Asian); (ii) triglyceride (TG) levels ≥150 mg/dL or treatment for TGs; (iii) high-density lipoprotein cholesterol levels <40 mg/dL in men and <50 mg/dL in women or treatment for dyslipidemia; (iv) systolic blood pressure ≥130 mm Hg or diastolic pressure ≥85 mm Hg or treatment for arterial hypertension; (v) prediabetes; (vi) homeostasis model assessment of insulin resistance (HOMA-IR) ≥2.5; (vii) high-sensitivity C-reactive protein levels >2 mg/L [183].
A diet consisting of excessive caloric intake composed of fats and carbohydrates, a sedentary lifestyle, and visceral adipose tissue increases are major contributors to the pathogenesis of MAFLD. Insulin resistance also contributes to MAFLD, which promotes lipolysis and de novo lipogenesis. Moreover, dysbiosis of gut microbiota, by increasing gut permeability, leads to increased absorption of fatty acids and bacterial translocation, thus promoting inflammation. It is worth mentioning that genetic predisposition also contributes to the pathogenesis of MAFLD. These factors first result in fat accumulation into the hepatocytes and subsequently trigger oxidative stress, endoplasmic reticulum stress, and inflammation pathways, leading to both hepatocytes apoptosis and fibrosis [185]. Hepatic fat accumulation results from an imbalance between lipid acquisition and lipid disposal, which are regulated through four major pathways: uptake of circulating lipids, de novo lipogenesis (DNL), fatty acid oxidation (FAO), and export of lipids in very low-density lipoproteins (VLDL) [186,187]. The uptake of circulating fatty acids by the liver dependent on fatty acid transporters such as fatty acid transport proteins (e.g., FATP2 and FATP5), a cluster of differentiation 36 (CD36), and caveolins located in the hepatocyte plasma membrane. Following uptake, hydrophobic fatty acids do not diffuse freely in the cytosol and must instead be shuttled between different organelles by specific fatty acid binding proteins (e.g., FABP1). FABP1 facilitates the transportation, storage, and utilization of fatty acids and their acyl-CoA derivatives, and may exert a protective effect against lipotoxicity by binding otherwise cytotoxic free fatty acids, facilitating their oxidation or incorporation into triglycerides [188]. Consequently, FAB1 play an important role in the accumulation of circulating lipids in triglycerides inside hepatic cells, causing hepatic fat accumulation and MAFLD. In addition, hepatic cells synthesize new fatty acids from acetyl-CoA that is converted to malonyl-CoA by acetyl-CoA carboxylase (ACC); malonyl-CoA is then converted to palmitate by fatty acid synthase (FASN). New fatty acid may then undergo a range of desaturation, elongation, and esterification steps before ultimately being stored as triglycerides or exported as VLDL particles. Consequently, increased lipogenesis causes hepatic steatosis and/or hypertriglyceridemia [187,188]. It is well known that hepatic new lipogenesis is regulated by two transcription factors: sterol regulatory element-binding protein 1c (SREBP1c) and carbohydrate regulatory element-binding protein (ChREBP) [189,190,191]; the expressions of both transcription factors are upregulated in patients with MAFLD [192,193,194]. These findings suggest that liver de novo lipogenesis is promoted in MAFLD, causing triglyceride storage in liver cells and the development of hepatic steatosis.
The increase in intrahepatic triglycerides (IHTG) in obese subjects with MAFLD has various functional effects in the liver, such as a reduction in hepatic insulin extraction, and increased triglycerides, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) in plasma [195,196,197]. In addition, it was reported that serum irisin levels reduced gradually with the increase of IHTG contents in obese adults with MAFLD [198]. The level of serum fibroblast growth factor-21 (FGF-21), a hepatokine secreted by liver, increased gradually with the increase of IHTG contents in obese subjects with MAFLD [199]. Irisin inhibits hepatic cholesterol synthesis [200] and their decrease in obese adults with MAFLD suggest that hepatic cholesterol synthesis is potentiated in these patients. On the other hand, hepatocyte cultures treated with FGF-21 showed a reduction of mRNA and protein SREBP1c expression [201], suggesting that lipogenesis in hepatic cells is reduced by FGF-21. However, FGF-21 facilitates fatty acid binding protein (FABP) expression [202]. These findings suggest that diffuse factors secreted by fat liver have paracrine/autocrine effects by enhancing the entry of circulating fatty acids to hepatocytes and promoting the hepatic cholesterol synthesis, thus increasing the accumulation of lipids in the fat liver. In other words, liver fat itself enhances diffusible factors that enhance the accumulation of lipids in hepatocytes.
In addition to the accumulation of fat in the liver, obese subjects may accumulate it in the pancreas [203,204,205,206]. For instance, the prevalence of a fatty pancreas is about 16–35% in Asian populations [207,208], highly associated with nonalcoholic fatty pancreas disease (NAFPD), which is specifically defined as pancreatic fat accumulation in association with obesity in the absence of significant alcohol consumption [209]. In contrast to the liver, fatty infiltration of the pancreas spares the acini (exocrine function) and islets of Langerhans (endocrine function), and preferentially accumulates within the pancreatic interstitial septa [210]. The death of acinar cells and their substitution by adipocytes (“fatty replacement”) and fatty accumulation (“fatty infiltration”) are the two main mechanisms leading to fatty accumulation in the pancreas [209]. “Fatty replacement” is characterized by the death of pancreatic acinar cells and their respective replacement by adipocytes, which can be caused by several conditions. The main factors for pancreatic cell death include genetic factors, such as fibrosis, or clinical features, such as excessive alcohol consumption, viral infections, iron overload (mainly represented by hemochromatosis), use of medication (corticosteroids), or obstruction of the pancreatic duct (chronic obstructive pancreatitis). ‘‘Fatty infiltration’’ occurs when there is an infiltration of adipocytes in the pancreatic tissue. The infiltration of pancreatic fat initially causes hypertrophy and hyperplasia of the pancreatic cells, resulting in clinical conditions, such as insulin resistance, beta-cell dysfunction, and diabetes mellitus type 2 [211].
Peri-pancreatic adipose tissue releases several factors that modulate beta-cell physiology including CXCL-1, CXCL-2, CXCL-3, CXCL-5/LIX, MIP-1α/CCL3, and VEGF [212]. VEGF secreted by peri-pancreatic adipose tissue plays an important role promoting and maintaining vascularization of pancreatic islets, indispensable to collect insulin secreted by beta-pancreatic cells [213,214]. VEGF also promotes beta-cell proliferation and prevents the development of hyperglycemia [215]. However, increased expression and secretion of VEGF facilitates hypervascularization and infiltration of macrophages in pancreas, which secrete proinflammatory cytokines (e.g., TNF-alpha, IL6) that impair insulin secretion decrease beta-cell mass, and promote hyperglycemia [216]. Increased levels of vascular growth factors (e.g., VEGF, VEGF-C, and VEGF-D) are present in overweight and obese subjects [217]. These findings suggest that obesity causes an overexpression of VEGF that promotes macrophage infiltration in the pancreas and secretion of pro-inflammatory cytokines that affects insulin secretion by beta cells, causing hyperglycemia. It is well known that TNF-alpha inhibits the expression of ATP-binding cassette transporter A1 (ABCA1), a protein related to the insulin secretion by pancreatic beta cells. Thus, TNF-alpha secreted by infiltrated macrophages in the pancreas affect insulin secretion [218]. In contrast, IL6 enhances insulin secretion by increasing glucagon-like peptide-1 (GLP-1) secretion from L cells and alpha cells [219], but the plasma level of IL6 is similar between overweight/obese and non-obese subjects [220]. On the other hand, increased expression and secretion of CXCL-1, -2, -3, and CXCL-5/LIX chemokines by peri-pancreatic adipose tissue do not exert a direct effect on pancreatic β-cell dysfunction [212]. Similar to VEGF, MIP-1α/CCL3 also promotes pancreatic β-cell proliferation [221], as well as the infiltration of macrophages in the pancreas [222], and serum levels of MIP-1α/CCL3 are increased in obese subjects [223]. Dead acinar cells replaced by adipocytes or the infiltration of adipocytes are the two mechanisms of accumulation of pancreatic fat. The peri-pancreatic adipose tissue releases chemokines and VEGF that favor the infiltration of macrophages in the pancreas, immune cells that release cytokines responsible for inhibiting insulin secretion by beta-pancreatic cells and the generation of hyperglycemia in obese subjects.
2.4. Visceral, Abdominal, and Subcutaneous Adipose Tissues
Obesity causes an increase of visceral [224,225], abdominal [226,227,228], and subcutaneous adipose tissues [229]. While the visceral adipose tissue (VAT) is the accumulation of adipocytes around viscera, the adipose tissue beneath the skin is the subcutaneous adipose tissue (SAT). The word “viscera” refers to “organs in the cavities of the body” and considering the three main body cavities, VAT involve adipose tissue distributed in these cavities: intrathoracic, intra-abdominal, and intrapelvic cavities. Consequently, VAT is the adipose tissue within the chest, abdomen, and pelvis [230]. On the other hand, subcutaneous adipose tissue (SAT) consists of three types of white adipose tissue (WAT): deposit WAT (dWAT), structural WAT (sWAT), and fibrous WAT (fWAT). The dWAT is located essentially in large depots in the abdominal area (peri-umbilical), formed by non-lobulated adipose tissue defined as metabolic fat by its large lipidic mass and very poor collagenic component, cells are large and few blood vessels are present. The sWAT is located in some areas in the limbs and in the hips including trochanters, sovrapubic area, armpits, inner faces of the knees, thighs, arms, pectoral, and mammary areas. The stroma is fairly well represented, with a good vascularity and adequate staminality. Cells are wrapped by a basket of collagen fibers. The fWAT is a noteworthy fibrous component and can be found in areas where a severe mechanic stress occurs. Adipocytes are therefore smaller, with a thick fibrous shell wrapping them one-by-one [231]. Abdominal adipose tissue is the accumulation of adipocytes in the abdominal area; however, abdominal fat is composed of abdominal subcutaneous fat and intra-abdominal fat [232]. Abdominal subcutaneous fat is in continuity with the dermal tissue and it is usually considered a unique, homogeneous fat deport. In this abdominal region, SAT is composed by two anatomically different layers, located, respectively, above and below a connective plane named fascia superficialis of the fascia of Scarpa, and is usually indicated as superficial and deep adipose tissues [227]. Intra-abdominal fat is composed of visceral or intraperitoneal fat, mainly consisting of omental and mesenteric fat and retroperitoneal fat masses [232,233].
Excess of both VAT and SAT are associated with different abnormalities, including insulin resistance, impaired glucose tolerance, type 2 diabetes, hypertension, heart failure, coronary heart disease, myocardial infarctions, valve diseases, arrhythmias, sleep apnea, chronic obstructive pulmonary disease, stroke, reduced brain size, reduced grey matter, reduced cognitive function, dementia, reduced bone density, polycystic ovary syndrome, and cancer [234,235,236]. In the context of tissue crosstalk, it is worth noting that these adipose tissues secrete several factors that affect the physiology of different tissues, organs, and body systems. It is known that in obese subjects, VAT/SAT adipose tissue expresses mRNA and secretes soluble factors, including cytokines (e.g., TNF-alpha, IL-1beta, IL6, IL7, IL10, IL8, IL12, IL13, IL15, IL18, IL32, IL33, TNF-like weak inducer of apoptosis or TWEAK, RANTES) [237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256], chemokines (e.g., CXCL2, CXCL9, CXCL10, CCL1, MCP1/CCL2, Fractalkine, MIP1, MIP3) [247,249,257,258,259,260,261], osteopontin [262,263], osteoprotegerin [255], interferon-gamma [264], and adipokines (e.g., omentin, adiponectin, visfatin, leptin, adrenomedullin) [249,252,265,266,267,268,269]. Moreover, VAT/SAT adipose tissue also releases micro-RNAs (e.g., miR-181a-5p, miR-23a-3p, miR-199a-3p, miR-193, and miR-126) [270,271,272].
Osteopontin and IL18 are related to insulin resistance in obese subjects [273]. Under chronic heart stresses (e.g., pressure overload, hypertrophy, cardiomyocyte injury), the quiescent cardiac fibro-adipocyte progenitors (FAPs; quiescent stem cells) become activated and are differentiated in two non-cardiac lineages, including intracardiac adipocytes and intracardiac fibroblast. This activation is mediated by osteopontin [274]. Osteopontin also acts on the cardiovascular system, causing arteriosclerosis, angiogenesis, myocardial remodeling [275], valvular stenosis, hypertrophy, myocardial infarction, heart failure [276], aortic disorders, and coronary artery disease [277]. This protein is also related to several renal diseases, including stone formation, tubulointerstitial nephritis, glomerulonephritis, acute ischemic renal injury, interstitial inflammation and fibrosis, hydronephrosis, chronic cyclosporine-induced nephropathy, and lupus nephritis [278]. Moreover, it plays an important role in mineralization and bone resorption [279]. In the gastrointestinal system, osteopontin is associated with several diseases, such as cancer, inflammatory bowel disease, primary sclerosing cholangitis, cirrhosis, nonalcoholic fatty liver diseases, and helicobacter pylori infection [280]. As for the respiratory system, osteopontin is related to fibrosis development, allergic airways diseases, and pulmonary vascular disease [281]. Finally, osteopontin mediates inflammation in ectopic fat depots present at both the cardiac and renal level [279]. It is true that most body organs secrete osteopontin, and osteopontin levels increase in obese subjects [262], but it cannot be ruled out that the osteopontin secreted by visceral and subcutaneous adipose tissue contributes to the generation of these pathologies of different body organs and systems in obese subjects.
As for osteoprotegerin, it is associated with atherosclerotic disease development in obese subjects [282], together with insulin resistance increasing in obese adolescents [283,284]. In contrast, in obese children, an osteoprotegerin level decrease was reported, suggesting that bone formation relative to resorption was reduced in this population, and consequently increased bone fractures [285]. Osteoprotegerin is also associated with cardiovascular pathologies, including vascular calcification [286], atherosclerosis, endothelial dysfunction, and hypertension [287]. This protein increases in subjects with several lung diseases, including asthma [288], lung cancers [289], and pulmonary arterial hypertension [290,291]. Similarly, osteoprotegerin increases after inflammatory bowel disease [292,293] and gastrointestinal carcinoma [292]. Finally, research shows that osteoprotegerin protects against muscular dystrophy [294,295]. These results suggest that several body tissues and organs are targets of osteoprotegerin and, therefore, respond to the release of this protein by different body cells, including subcutaneous and visceral adipose tissue.
As for interferon-gamma (INF-γ) levels, it was shown to be increased in obese subjects [296,297], in obese subjects with asthma [298], and in obese subjects with prediabetes [299]. Directly or indirectly—INF-γ participates in several stages of atherogenesis, including oxidative stress, promote foam cell accumulation, stimulate smooth muscle cell proliferation and migration into the arterial intima, enhance platelet-derived growth factor expression, and destabilize plaque [300]. Evidence suggests that INF-γ levels are increased in subjects with asthma [301,302,303,304]. Non-eosinophilic asthma, where neutrophils are present in the airway lumen, is mediated by type 1 lymphocytes, which secretes interferon-gamma and TNF-alpha. Both factors promote neutrophil IL-6 secretion. This cytokine contributes to the activation of macrophages of adipose tissue in the lungs, which release pro-inflammatory cytokines that contribute to asthma pathogenesis [305]. Altogether, these findings suggest that, directly or indirectly, INF-γ contributes to both specific cardiovascular and lung diseases development.
It is well known that the repertoire of cytokines, chemokines, and adipokines secreted by visceral and/or subcutaneous adipose tissue are involved in asthma pathogenesis, and the reduction of weight by diet, exercise, or bariatric surgery reduces inflammatory activity, and improve asthma and lung function [305]. Obese adipose tissue releases free fatty acids (FFA), adipokines, and many pro-inflammatory chemokines. These factors are known to play a key role in regulating malignant transformation and colon cancer progression [306,307], and inflammatory bowel disease is associated by secretion of adipokines by adipose tissue [308]. The factors secreted by VAT/SAT adipose tissue (e.g., cytokines, chemokines, adipokines, FFA) also contribute to insulin resistance [309,310] and atherosclerosis in obese subjects [311]. Moreover, pro-inflammatory molecules (e.g., IL6, TNF-alpha) secreted by VAT adipose tissue predispose to cardiac dysfunctions (e.g., end-diastolic septum thickness, end-diastolic posterior wall thickness, absolute and indexed left ventricular mass, deceleration time, myocardial performance index, isovolumetric relaxation time) in obese subjects [312]. Finally, it is known that the production of pro-inflammatory cytokines in VAT/SAT adipose tissue is increased in obese patients with chronic kidney disease [313]. These findings suggest that the factors synthesized and secreted by the visceral and subcutaneous adipose tissue generate a state of generalized body inflammation that contributes to the appearance of dysfunctions of the cardiovascular, respiratory, gastrointestinal, and renal systems. Therefore, there is wide crosstalk between the adipose tissue and various organs and body systems.
In parallel, it is known that VAT/SAT adipocytes secrete exosomes containing several micro-RNAs. For instance, the secretion of miR-181a-5p, miR-23a-3p, and miR-199a-3p are related to insulin resistance in obese subjects [271,272]. Moreover, other micro-RNAs, such as miR-193, miR-126, secreted by VAT adipose tissue promote CCL2 secretion by body adipocytes, a chemokine that facilitates monocyte/macrophage infiltration in adipose tissue, cells that in turn release pro-inflammatory cytokines [270]. On the other hand, a reduction of miR-181a-5p release is associated with atherosclerotic plaque formation [314] and downregulation of miR-181a-5p also prevents cerebral ischemic injury [315] and alleviates oxidative stress and inflammation in coronary microembolization-induced myocardial damage [316]. In this context, it is also known that microRNA-23a-3p attenuates oxidative stress injury in a mouse model of focal cerebral ischemia-reperfusion [317] and microRNA-199a-3p protect cardiomyocytes from simulated ischemia-reperfusion injury [318], participating in the regulation of the NOS (NO Synthase)/NO pathway in the endothelium [319]. This micro-RNA inhibits angiogenesis in an in vitro experimental model of diabetic retinopathy [320].
Finally, in obese subjects, the adipocytes from VAT and SAT also express several molecules, such as lipocalin-2 [321,322], tenascin C, toll-like receptor 4 [323], and protein pentraxin 3 (PTX3) [324], which are related to adipose tissue inflammation and secretion of pro-inflammatory cytokines [321,322,323,325,326,327,328,329,330], insulin resistance [325,331], hyperglycemia and glucose intolerance [325,332,333,334], endothelial dysfunction with hypertension [335], cardiovascular dysfunctions [336,337], and atherosclerosis [338,339].
In summary, there is wide crosstalk between visceral and subcutaneous adipose tissue through a set of diffusible factors secreted by this tissue that exert functional and pathophysiological changes in various tissues and systems, including cardiovascular, respiratory, gastrointestinal, renal, bone, and muscle systems. A schematic illustration of such crosstalk is shown in Figure 1.
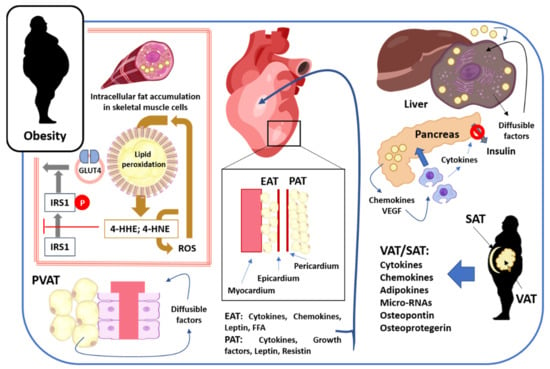
Figure 1.
Overview effects of adipose tissue in obese subjects. In skeletal muscle, fat accumulates inside muscle fibers, causing a dysregulation of cellular metabolism and favoring lipid peroxidation. The peroxidation of intracellular lipid droplets triggers the appearance of 4-HNE and 4-HNE, two mediators that interfere with IRS1 phosphorylation, thereby interrupting the transport of vesicles loaded with the GLUT4 transporter to the muscle membrane. Physiologically, insulin, by binding to its receptor, favors IRS1 phosphorylation and GLUT4 expression in skeletal muscle fibers. Therefore, lipid peroxidation ultimately causes insulin resistance. The adipose tissue that accumulates around the blood vessels (PVAT) secretes a variety of diffusible factors with vasomotor, vascular remodeling, pro- and anti-inflammatory, as well as pro- and anti-atherogenic effects. Adipose tissue also accumulates in the heart, both at the epicardial and pericardial levels (EAT and PAT, respectively). This cardiac adipose tissue secretes cytokines, chemokines, adipokines, growth factors, and FFAs, which indicate cardiomyocyte physiology, causing changes in heart function. In obese subjects, adipose tissue also accumulates in the liver and pancreas. In hepatocytes, the accumulation is intracellular, causing insulin resistance, in-creased hepatic lipogenesis, and the secretion of diffusible factors with effects on the hepatocytes themselves, promoting the functional changes described. In the pancreas, adipose tissue accumulates outside the pancreatic cells, and secretes chemokines and VEGF that promote macrophage infiltration into the pancreatic tissue. These macrophages secrete cytokines that interfere with insulin secretion. Finally, another characteristic of obese subjects is the accumulation of subcutaneous and visceral adipose tissue (SAT and VAT, respectively), preferably in the abdominal region. This adipose tissue secretes a multitude of diffusible factors, which exert effects on various body tissues and organs. Thus, in obese subjects, there is a crosstalk between adipose tissue and other body tissues and organs mediated by diffusible factors with local or generalized effects. For details, see text. Source for figure illustrations: https://scidraw.io/ (accessed on 8 September 2021).
2.5. Obesity: Fat Deposition, Dysfunctional Adipose Tissue, and Metabolic Complications
In the previous subsections, we described the accumulation of fat in various body tissues and organs in obesity conditions. The accumulation of fat changes between the different tissues and organs; thus, in skeletal muscles, the liver, and pancreas, there is an accumulation of fat in the form of lipid droplets or adipocytes in the parenchyma of these tissues or organs, while in the heart and skin, there is an excessive accumulation of fat outside the cells of these tissues. It is well known that some amount of adipose tissue surrounds several organs, and it has been shown to serve a physiological role [340]. Ectopic fat is defined by excess adipose tissue in locations not classically associated with adipose tissue storage, such as the omentum, liver, muscle, pancreas, heart, blood vessels, kidney, or neck, and is associated with the development of cardiovascular diseases [341,342]. Likewise, this excess fat in these regions, especially in the abdominal region, constitutes a sign of obesity [343], and the assessment of certain anthropometric parameters of visceral/abdominal obesity have been proposed as predictive parameters of cardiovascular risk [344]. In this context, it is not unreasonable to associate ectopic fat deposition with obesity, and the development of cardiovascular and metabolic complications.
It should also be noted that adipose tissue is deposited discreetly in the human body. Thus, the subcutaneous adipose tissue (SAT) stores more than 80% of the total fat in the human body, while the accumulation of intra- and retroperitoneal fat represents 10–20% of total body fat in men and 5–10% in women. SAT deports preferably subcutaneously in the abdominal, gluteal, and femoral regions. Intraperitoneal or visceral adipose tissues (VAT) are associated with digestive organs and include the omental (hangs off the stomach), the mesenteric (associated with the intestine), and epiploic (along the colon) [345]. As described above, SAT and VAT are composed by white adipose tissue (WAT), and their primary function is to maintain an energy homeostasis. WAT uptakes excess lipid and stores it in the form of triglycerides; it acts as an energy source and releases lipids in the form of non-esterified fatty acids when there is a demanded for energy. The maintenance of energy homeostasis depends not only on the balance between lipid uptake and release, but also on the sensitivity to stimulatory and inhibitory signals mediated by the sympathetic nervous system and hormonal system [346]. According to previous subsections, in obesity, WAT depots experience abnormal and excess expansion, either by increase in adipocyte number or adipocyte size. They become inflamed and release an increased amount of pro-inflammatory cytokines and other mediators, and a reduced amount of anti-inflammatory adiponectin and other adipokines into the circulation. With the development of obesity, adipose tissue becomes increasingly dysfunctional [346]. In addition, visceral obesity, including the accumulation of subcutaneous fat in the abdominal area, confers an increased risk for metabolic complications of obesity [347,348].
Metabolic syndrome (MetS), metabolically healthy obesity (MHO), and metabolically unhealthy obesity (MUO) are three phenotypes of metabolic complications of obesity. The term metabolic syndrome was first put forward by Reaven (1988) [349] to describe a cluster of interrelated cardiovascular and metabolic risk factors that predispose individuals to the development of type 2 diabetes mellitus and cardiovascular diseases. These well-known risk factors include abdominal obesity, hyperglycemia, hypertension, and atherogenic dyslipidemia. From a physiopathological point of view, the cardiometabolic risk factors that characterize MetS are usually accompanied by the presence of insulin resistance (IR), and by a systemic subclinical inflammation state secondary to the hyperactivity of innate immunity [350,351]. In the etiology of MetS, aging, genetics, inflammation, obesity, and sedentary lifestyle are recognized as predisposing factors. Although abdominal obesity is the major risk factor of the MetS that predispose to the metabolic and cardiovascular diseases [352], understanding by abdominal obesity, the accumulation of ectopic fat in the abdominal region, both viscerally and subcutaneously [343]. Visceral fat is a highly active tissue from the metabolic point of view. It is apparently more susceptible to lipolysis than subcutaneous adipose tissue and is associated with higher production of TNF-α, plasminogen activator inhibitor-1 (PAI-1), IL-6, IL-1β, monocyte chemoattractant protein-1 (MCP-1), macrophage migration inhibitory factor (MIF), C-reactive protein (CRP), chemokines from the CC and CXC families, IL-18, and IL-33, most of which are involved in insulin resistance. The size more than the number of visceral adipocytes is a key factor in abdominal obesity. Big visceral adipocytes are more prone to rupture, and cell rupture will obviously constitute a focus of inflammation, since macrophages infiltrate visceral adipose tissue to engulf cellular debris from dead adipocytes and contribute to the secretion of pro-inflammatory factors [353,354,355,356]. Visceral adipocytes are frequently subject to sudden pressure variations associated with cough, physical exercises, and sleep apnea. Intra-abdominal pressure is higher in obese patients, what may also impact adipocyte stability [357,358,359]. Despite this information, little is understood about the triggers of obesity-associated inflammation, but several mechanisms have been investigated in rodent models of dietary and genetic obesity. In this context, it has been observed that obesity gives rise to increased intestinal permeability, which results in higher circulating levels of LPS from intestinal Gram-positive bacterial species, and gut-derived LPS may initiate an inflammatory cascade via activation of pattern recognition TLR4 receptors in fat cells [360,361,362]. Mice subjected to intravascular infusion of free fatty acids causes an activation of NF-kappa-B in adipose tissue and the secretion of IL6 and MCP-1 by adipocytes and resident and/or infiltrated macrophages in adipose tissue during the course of infusion, and TLR4 is implicated in this response in macrophages and adipocytes to induce inflammatory signaling. Finally, in female C57BL/6J mice, TLR4 is in part required for the ability of high-fat diet to induce inflammatory mediators in peripheral tissues [360]. On the other hand, the proportion of M1 macrophages increases in liver tissue and adipose tissue of mice subjected to a high-fat diet that induces metabolic syndrome [363]. It is well known that M1-macrophages have a pro-inflammatory profile with the production of inflammatory cytokines and reactive oxygen species, but they are also ineffective macrophages in the elimination of dead adipocytes, or in the elimination of lipids [363].
Metabolically healthy obese (MHO) subjects are individuals with high fat mass, low ectopic fat, low triglycerides and inflammation, high HDL-cholesterol and adiponectin, and high insulin sensitivity. MHO are obese subjects with a favorable metabolic profile with the absence of metabolic complications including inflammation, dyslipidemia, and hypertension, and preserved insulin sensitivity despite excessive body fatness. The MHO phenotype is characterized by lower visceral adipose tissue content [364]. Higher insulin sensitivity in MHO subjects is accompanied with lower fasting glucose and insulin levels. MHO individuals present a favorable blood lipid profile as evidenced by lower triglycerides (TGs) and higher high-density lipoprotein (HDL) cholesterol levels, and favorable hepatic enzyme profile as evidence by lower aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT) levels [364]. Recently, a set of criteria to identify people with MHO has been proposed, based on (i) the absence of cardiometabolic disease, including absence of prediabetes, type 2 diabetes, dyslipidemia, nonalcoholic fatty liver disease, chronic kidney disease, cardiovascular disease, and absence of treatment with blood pressure, lipid or diabetic medications; (ii) a healthy cardiometabolic profile, such as fasting triglycerides <95 mg/dL, HDL-C ≥ 40 mg/dL in men and ≥50 mg/dL in women, fasting glucose <100 mg/dL and 2- hour OGTT glucose <140 mg/dL; (iii) normal blood pressure (<130/85 mmHg); (iv) normal intrahepatic triglycerides content (<5% of liver volume by imaging or <5% of hepatocytes with intracellular triglycerides by histology); and (v) normal insulin sensitivity (glucose infusion rate <8 mg/kg fate-free mass during an hyperinsulinemic-euglycemic clamp procedure at insulin infusion rate = 40 mU/m2/min [365].
Various underlying mechanisms have been proposed to explain the existence of MHO. There is growing evidence to suggest that subclinical inflammation may be the underlying mechanism that determines whether an individual is MHO. The subclinical inflammation profile includes low C-reactive protein (CRP) circulating levels, low levels of complement component 3 (C3), low levels of alpha necrosis tumor factor (TNF-a) and interleukin 6 (IL-6), and a low number of blood cells [366,367], but higher levels of pro-inflammatory adipokines, including leptin, resistin, IL-18, retinol-binding protein 4, lipocalin 2, angiopoietin-like protein 2, CC-chemokine ligand 2, and CXC-chemokine ligand 5 [367,368]. A second underlying mechanism is the expansion capacity of adipose tissue, based on when fat tissue needs to increase its storage capacity, can increase the size of adipocytes, or increase their number. These changes in adipose tissue are frequently accompanied by increased vascularization. In subjects in whom adipose tissue has difficulty expanding in the healthiest way, which is increasing cellularity, metabolic disease does appear. This happens in MHO subjects who maintain adequate storage capacity. MHO subjects have higher lipogenic and angiogenic capacities [367,369], and with adipocytes that have a lower average size and a favorable pattern of adipokine secretion [370]. On the other hand, MHO subjects are characterized by lower liver (intrahepatic) and visceral fat deposition, but higher leg fat content [371]. Accumulation of adipose tissue in the upper body (abdominal region) is associated with the development of obesity-related comorbidities (e.g., type 2 diabetes, cardiovascular and metabolic diseases, insulin resistance), whereas the accumulation of fat in the lower body (gluteofemoral region of leg) is associated with a protective lipid and glucose profile as well as a decrease of cardiovascular and metabolic diseases [372].
Metabolically unhealthy obesity (MUO) phenotype is characterized by high visceral fat volume with high liver fat content and low amount of leg fat accumulation, adipose tissue dysfunction, insulin resistance, and higher markers of inflammation [371,372,373]. MUO subjects have a similar risk of cardiovascular disease as MHO subjects, but a much higher risk of type 2 diabetes than MHO subjects [374]. MUO individuals display a pro-inflammatory adipose M1 macrophage profile characterized by the secretion of inflammatory cytokines including interleukin (IL)-6, IL-1b, and monocyte chemoattractant protein-1 (MCP-1). In addition, higher infiltration and recruitment of M1 macrophages has reported in adipose tissue of MUO subjects. Increased consumption of saturated fat, cholesterols, trans-fat, and fructose incites pro-inflammatory macrophage recruitment in MUO adipose tissue. Consumption of these dietary components in conjunction with dysfunctional adipogenesis results in adipocyte hypertrophy. This combined with decreased angiogenic signals, disrupted ECM turnover, and downstream pro-inflammatory cytokine secretion stimulates pro-inflammatory M1 macrophage recruitment. Systemic dysfunction in the form of a decreased vasoreactivity and oxidative stress concurrently fosters insulin resistance while promoting pro-inflammatory M1 macrophage recruitment into MUO adipose tissue. Once M1 macrophages enter the tissue, they secrete additional pro-inflammatory cytokines that recruit more M1 macrophages. Hypertrophic adipocytes in MUO adipose tissue communicate with the recruitment of M1 macrophages and secrete inflammatory leukotrienes (e.g., LTB4), which promotes insulin resistance in peripheral tissues and thus further recruitment of M1-proinflammatory macrophages. As a whole, it constitutes a vicious cycle of initiation and perpetuation of inflammation in the adipose tissue of MUO subjects [375]. In addition, MUO patients have a 44% decrease in capillary density and 58% lower VEGF signaling in the subcutaneous adipose, highlighting the occurrence of vascular rarefaction with hyperglycemia [376].
In summary, the previous evidences in both humans and animal models indicate that the quality of adipose tissue varies among these three metabolic disorders of obesity. Thus, in MetS and MUO subjects, abdominal obesity is observed, with secretion of inflammatory factors by adipose tissue, recruitment of M1 macrophages in adipose tissue, insulin resistance, and a high risk of type 2 diabetes and cardiovascular diseases. While the MHO subjects do not show abdominal obesity, but rather gluteofemoral fat accumulation, with a low to zero risk of type 2 diabetes and cardiovascular diseases, higher insulin sensitivity, and low or very low secretion of inflammatory factors by adipose tissue.
3. Obesity and Pain: Crosstalk between Adipose Tissue and Nociceptive Somatosensory Nervous System
Low back pain [46,47,51,377,378,379,380], foot pain [72,381,382], knee pain [69,383,384], hip pain [71,385], shoulder pain [386], musculoskeletal pain [60,387,388,389], headache/migraine [74,76,390,391,392,393,394], neuropathic pain caused by nerve injuries and diabetic neuropathy [395,396,397,398], and fibromyalgia [399,400], were reported in overweight/obese subjects. These findings suggest that overweight/obesity, which, as mentioned before, is characterized by excess accumulation of adipose tissue, may trigger pathological pain development, both neuropathic and nociplastic.
Distal symmetric polyneuropathy (DSP), a type of peripheral neuropathy, has been described in subjects with metabolic syndrome [401,402]. It is well known that DSP is associated with pain, falls, and reduced quality of life [403,404,405,406,407,408,409,410,411,412]. On the other hand, metabolic syndrome also is associated with an elevated risk for cryptogenic sensory peripheral neuropathy (CSPN), which preferentially affects small unmyelinated axons early in its course, and it may affect autonomic and large fibers [413]. CSPN is usually diagnosed on the basis of pain, numbness, and/or tingling in the distal extremities without symptoms of weakness [414,415]. On the other hand, it was reported that patients with neuropathic pain, the pain intensity in the overweight group was significantly higher than that in the normal-weight group [397]. However, using noxious heat and cold stimuli across multiple body locations and durations of stimulation, no differences in suprathreshold pain intensity or unpleasantness ratings between obese and healthy groups were seen. These results suggest that increased adiposity does not in-and-of-itself alter nociception [416]. It should be noted that, in the scientific literature, there are several excellent reviews on the relationship between obesity and pain in humans. In summary, the scientific evidence reviewed in these publications indicates that obesity is significantly associated with the presence and extent of pain complaints, and the prevalence of pathological pain (e.g., low back pain, osteoarthritis, fibromyalgia) is higher in obese or morbidly obese subjects compared to subjects with normal weight. Longitudinal studies suggest that obesity may be a risk factor for developing chronic pain. Obesity also increases the risk factor for developing headache. Several studies showed that obese people exhibited decreased pain threshold to electrical and mechanical, whereas other studies also show inconsistent results across body parts, suggesting that the reduced pain threshold may not be present for all body areas in obesity. The increased loading due to heavy weight on joints and the spine is one of the most discussed links between obesity and pain. Another link between obesity and pain is adipose tissue. In this sense, obesity may be characterized by a low-grade chronic inflammatory state as reflected by elevated levels in many inflammatory markers in the serum, some of which may act as algogenic substances by stimulating visceral nociceptors or sensitizing nociceptive neurons in the central nervous system. In addition, overweight/obese individuals have an increased risk of various metabolic disorders, thus they have increased vulnerability to the neuropathic disorders associated with conditions, such as diabetes, and some of these peripheral neuropathies present with pain [382,417,418,419,420,421,422,423,424,425,426,427,428,429,430,431]. On the other hand, several studies were reported linking metabolic diseases of obesity with pain. In this context, MetS was associated with neck pain. This association was stronger in males, but the prevalence of neck pain was higher in females [432]. MetS was also significantly associated with low back pain among women only [433,434]. About 40% patients with chronic low backache have metabolic syndrome, and they have more severe pain and disability [435]. Chronic pain due to osteoarthritis in both knees were reported in patients with metabolically healthy obesity [436]. In addition, it was also reported that over the course of two decades, decline in physical functioning and worsening of bodily pain among initially healthy obese adults was two- and six-times greater than among initially healthy normal-weight adults, respectively. These changes occurred at similar rates for both healthy and unhealthy obese adults [437].
According to the International Association for the Study of Pain (IASP), the definition for neuropathic pain is “the pain caused by a lesion or disease of the somatosensory nervous system”, whereas nociplastic pain is “the pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain” [438].
Neuropathic pain includes: (i) trigeminal neuralgia; (ii) peripheral nerve injury by physical trauma; (iii) painful polyneuropathy associated with polyneuropathies caused by metabolic, autoimmune, familial or infectious diseases, exposure to environmental or occupational toxins, or treatment with a neurotoxic drug; (iv) postherpetic neuralgia defined as pain persisting for ≥ 3 months following the onset or healing of herpes zoster; (v) painful radiculopathy defined as pain caused by a lesion or disease involving the cervical, thoracic, lumbar or sacral nerve roots; (vi) neuropathic pain associated with spinal cord injury defined as pain caused by a lesion or disease of the somatosensory pathways in the spinal cord; (vii) neuropathic pain associated with brain injury defined as pain caused by a lesion or disease of the somatosensory cortex, connected brain regions, or associated pathways in the brain; (viii) post-stroke pain defined as pain caused by a cerebrovascular lesion, infarct or hemorrhage, of the brain or brainstem; and (ix) neuropathic pain caused by multiple sclerosis in the somatosensory brain regions or their connecting pathways [439]. On the other hand, under the definition of nociplastic pain are included nonspecific back pain, nonspecific peripheral joint pain, fibromyalgia, complex regional pain syndrome (CRPS) type 1, and visceral pain disorders (e.g., irritable bowel syndrome, bladder pain syndrome) [440,441,442,443].
Together, with the scientific evidence, it can be affirmed that there is a solid rationale in the implication of obesity and its metabolic complications in pathological pain in humans. Given what is stated in the previous paragraphs, both neuropathic pain and nociplastic pain are found in either obese or overweight subjects. In this context, a large number of diffusible factors synthesized and released by the different adipose tissues in obese subjects (Table 1) have been described, and some were shown to be able to interact with nociceptive nerve fibers, favoring the appearance of pain. This interaction may be explained by the fact that nociceptive nerve fibers are located around body tissues and organs. In the heart, calcitonin gene-related peptide (CGRP)-positive nerve fibers, identified as nociceptive afferent fibers, are shown as free endings in the epicardium of mice [444]. Epicardial afferent nociceptive nerve fibers also were reported in the dog’s heart [445], and in the epicardium of the heart of the cat [446]. Human epicardium also shows nerve terminals immunoreactive to either CGRP or substance P (SP), identified as afferent nociceptive fibers [447]. Moreover, peptidergic fibers (e.g., positive for CGRP and/or SP) have been visualized in the pericardium of chicken and human hearts [448,449]. As for the digestive system, CGRP- and SP-positive afferent nociceptive fibers are observed in the head of the mouse pancreas [450] and rat liver shows afferent nociceptive neurons sensitive to capsaicin administration [451]. Moreover, in resected human bowel tissues, nociceptive afferent nerve fibers more sensitive to algogenic mediators were identified [452]. In mice, mechanical distension and stretch-not heat, cutting, or pinching-reliably evoke pain from distal colon and rectum, suggesting that the colon wall showed nociceptive afferent fibers that respond to these stimuli [453]. Regarding respiratory system, in guinea pig, it has reported that nociceptive C-fiber population innervating the lungs [454], TRPV1- and TRPV2-immunoreactive nociceptive afferent nerve ending were reported in rat trachea [455], and TRPA1 nociceptive afferent fibers have been reported in mouse lungs [456]. Moreover, nociceptive afferent nerve fibers are observed in the parietal peritoneum of the rat [457] and it was reported that several drugs produce an antinociceptive effect in the mouse peritoneum by interacting with sensory nerve endings, suggesting that peritoneum may be innervating by nociceptive fibers [458]. Moreover, in the mice, most group IV muscle afferents are chemosensitive rather than mechanically or thermally sensitive, and the majority of these C fibers are muscle polymodal nociceptors [459]. Regarding skeletal muscle systems, in rats, muscle fascia shows a rich fiber network of thin-fiber receptors (Aδ- and C-fibers) [460]. In human skeletal muscle has been observed positive CGRP and SP afferent nociceptive fibers associates with myocytes [461]. The afferent nociceptive nerve fibers located in human muscle respond to mechanical and chemical stimuli [462]. Finally, focusing on skin, in human biopsies, researchers reported nociceptive intraepidermal nerve fibers [463,464,465] and rat and mice skin biopsies revealed intraepidermal free nerve fiber endings identified as afferent nociceptive fibers [466,467,468,469,470]. These evidences indicates that nociceptors or nociceptive afferent fibers are located in most body tissues and organs and, therefore, the substances released by adipose tissue that accumulate in these tissues and organs in obese subjects can interact with the nociceptors, causing their depolarization or their sensitization; thus, developing pain signals.

Table 1.
Diffusible factors synthesized and released by adipose tissues in obese subjects 1.
The adipose tissue is composed of various types of cells, such as adipocytes (e.g., progenitor and mature cells), endothelial cells of the wide vascular network that supplies adipose tissue, fibroblast, and immune cells (e.g., natural killer, T-cells, lymphocytes, neutrophils, macrophages, dendritic cells) [345,471,472]. The different cellular elements that make up adipose tissue are involved in the synthesis and release of various factors secreted by the adipose tissue accumulated in body organs and tissues, including adipokines, cytokines, chemokines, growth factors, and other diffusible factors. All of them may participate in pathological pain development by interacting with muscle, and skin and visceral nociceptors.
3.1. Adipokines
Adipokines are synthesized and released mainly by adipocytes, the main cells of adipose tissue that accumulate in tissues and organs. In an experimental model of inflammatory pain by intraplantar carrageenan injection, the intrathecal adiponectin administration attenuates both thermal and mechanical hyperalgesia whereas intraplantar administration alleviates only thermal hyperalgesia [473]. These findings suggest that adiponectin secreted by SAT may interact with their receptors located in skin afferents nociceptive fibers alleviating thermal hyperalgesia. Another possibility is that adiponectin interacts with AdipoR1/R2 receptors located in macrophages and mast cells that have been activated by carrageenan, inducing in them a lower release of pro-inflammatory factors, which interact with cutaneous nociceptors. Actually, it is well known that macrophages express AdipoR1/R2 and adiponectin reduces the production of pro-inflammatory cytokines by the activated macrophages that showed a M1 phenotype [474]. Mast cells also express AdipoR1/R2 receptors, and adiponectin causes a significant reduction of pro-inflammatory factors and histamine release [475]. These findings indicate that analgesic effect of adiponectin is mediated by regulation of inflammatory factors, secreted by macrophages and mast cells, which interact with the afferent nociceptive fibers in the skin.
On the other hand, intraplantar injection of capsaicin in Sprague–Dawley rats causes an overexpression of adrenomedullin (ADM) in CGRP- and IB4-positive dorsal root ganglion neurons identified as nociceptors. Capsaicin induces a release of ADM by these nociceptors, which promotes thermal hyperalgesia. Moreover, intrathecal administration of ADM causes thermal hyperalgesia and histological results have been revealed that CGRP- and IB4-positive afferent nociceptive fibers express receptors for ADM (e.g., CLR, RAMP2, and RAMP3) [476]. These results suggest that inflammation of skin causes release of ADM by nociceptors that in turn stimulates the nociceptors, playing a role similar to CGRP. Consequently, ADM is a pro-nociceptive molecule secreted by afferent nociceptive fibers, but also the ADM secreted by SAT may interact with their receptors located in skin nociceptors causing pain.
Regarding angiotensin-II (Ang-II), its injection into mouse hind paws induces mechanical but not thermal hyperalgesia and its intrathecal injection did not induce mechanical allodynia. It is known that Ang II-induced mechanical pain sensitivity operates exclusively via AT2R at a peripheral level, not spinally. In addition, Ang-II induces peripheral macrophages infiltration in mouse hind paw skin, which also respond to Ang-II by secreting ROS, since these cells also express the receptor for angiotensin-II (AT2R) [477]. According to these results, Ang-II may be considered as a pro-nociceptive molecule secreted by subcutaneous and perivascular adipose tissues, causing pain via binding with AT2R located in afferents nociceptive fibers.
As for apelin, peripheral injection of a single dose causes thermal hyperalgesia [478], but its intrathecal administration alleviates CFA-induced thermal and mechanical hypersensitivity in mice [479]. In models of formalin-induced pathological pain, intravenous injection of apelin reduces pain response during the second phase of the mouse formalin test, whereas intramuscular injection of apelin reduces licking/biting time during the second phase only at higher dose. [480]. In contrast, after chronic constriction injury (CCI) of the sciatic nerve, intrathecal administration of apelin does not alleviate thermal hyperalgesia and mechanical allodynia [481]. These results suggest that the peripheral administration of apelin is pronociceptive and its central administration exerts analgesic effects in inflammatory pain but not in neuropathic pain. Hence, the local release of apelin by the accumulation of subcutaneous adipose tissue may trigger pain.
With regard to chemerin, its intrathecal administration alleviates both thermal and mechanical hypersensitivity in mice after CCI [482]. In Complete Freund’s adjuvant (CFA, 0.5 mg/mL saline) experimental model of inflammatory pain, chemerin may also exert anti-nociceptive effects. Spinal cord slices from animals subjected to CFA-injection were treated with chemerin while whole-cell patch-clamp recordings are made in spinal lamina I neurons. Under these conditions, when capsaicin was applied, an increase in the firing frequency of excitatory potentials was observed in spinal neurons, which were attenuated by chemerin. At the central level, chemerin reduces the hyperexcitability of spinal neurons, exerting an anti-nociceptive effect [483]. In addition, intrathecal administration of chemerin alleviates hyperalgesia in CFA-induced hind-paw inflammation in rats [484]. All these findings suggest that chemerin may exert anti-nociceptive effects on both inflammatory and neuropathic pain, exerting its effects at spinal cord level.
Another molecule playing roles in adipose tissue and nociceptive somatosensory nervous system crosstalking is the leptin. In obese mice, which show higher levels of circulating leptin, formalin-induced nociceptive responses (flinching behavior) were significantly reduced. These results suggest that leptin may attenuate pain associated with inflammation [485]. In contrast, it was reported that leptin derived from adipocytes in the epineurium of the sciatic nerve, contributes to the development of tactile allodynia through the production of the molecular substrates for neuropathic pain (e.g., overexpression of mRNA for MMP-9, COX-2, and iNOS) by stimulation of the JAK-STAT pathway in macrophages. MMP-9 and iNOS mRNA levels increased in a macrophage cell line treated with leptin. Hence, these findings suggest that leptin in the PNS is a novel mediator of tactile allodynia induced by peripheral nerve injury [486]. On the other hand, intrathecal administration of leptin alleviates both thermal hyperalgesia and mechanical allodynia and reduces the expression of IL-6 and TNFα in Sprague–Dawley rats after CCI, an experimental model of neuropathic pain [487], these results suggest that circulating leptin reduces the expression of pro-inflammatory cytokines in neuropathic and inflammatory animal models of pain, alleviating hyperalgesia in these animal models of pain. However, local release of leptin in injured nerve promotes overexpression of inflammatory mediators that improve hyperalgesia. Consequently, the exact role of leptin on the development of hyperalgesia is still controversial.
There are no experimental studies on the effect of omentin administration on either neuropathic or nociplastic pain. However, it was shown that circulating levels of omentin decrease in patients with temporomandibular pain [488]. On the other hand, it was reported that osteopontin levels are elevated in patients with osteoarthritis, a degenerative joint disease characterized by loss of articular cartilage, inflammation, and pain [489]. In addition, osteopontin levels in synovial fluid is associated with the severity of joint pain and cartilage degradation after anterior cruciate ligament rupture [490]. In mice subjected to spared nerve injury (SNI), mechanical and thermal withdrawal thresholds tested in intact adult wild type and osteopontin knockout animals showed that the mechanical allodynia was attenuated in osteopontin-KO mice respect to wild type mice. In contrast, no differences in thermal latencies (thermal hyperalgesia) were observed between the two genotypes [491]. These results suggest that osteopontin expression after SNI, a model of neuropathic pain, may facilitate mechanical but not thermal hyperalgesia. Clinical studies also suggest that increased expression of osteopontin increases pain sensation. In relation to osteoprotegerin (OPG), pre-clinical studies indicate that it reduces pain behavior development and joint pathology in a model of osteoarthritis [492], and alleviates pain in rats subjected to bone cancer [493,494]. On the other hand, serum resistin increases in subjects with bilateral knee osteoarthritis [495], and in patients with hip and knee osteoarthritis [496]. However, no pre-clinical studies have been performed on the effect of the application of resistin in neuropathic and nociplastic pain. As for vaspin, it was reported a significant association between vaspin circulating levels and low back pain [497], but not pre-clinical studies, have been performed with the aim of studying the effect of vaspin on neuropathic or nociplastic pain. Finally, there are no pre-clinical studies on the role of visfatin in pathological pain or its development. It is known that visfatin levels increases in patients with osteoarthritis, but this increase could not be related to the pain indicated by the patients [498].
3.2. Cytokines and Chemokines
Cytokines and chemokines are mainly synthesized and released by immune cells that are part of the adipose tissue accumulated in body tissues and organs. Growing evidences suggest that both cytokines and chemokines are involved in sensitization of nociceptors, that is, in peripheral sensitization, and in the depolarization of nociceptive primary afferent fibers. For instance, peripheral administration of TNF-alpha [499,500], IL6 [501] and IL1 [502,503,504] cause excitability and/or sensitization of C- and A-delta-nerve fibers. It should be noted that cytokines do not always trigger excitability of nociceptive afferent fibers, thus IL6 causes hypoactivity of nociceptive fibers [505]. On the other hand, in IL17-KO mice it has reported less behavioral hypersensitivity after partial sciatic nerve ligation and a decrease in inflammatory cell infiltration and pro-inflammatory cytokine levels in injured nerves [506]. Intraperitoneal administration of RANTES also attenuates hypersensitivity after partial sciatic nerve ligation. In addition, macrophage infiltration and pro-inflammatory cytokine levels also decreased in injured nerve [507]. These findings suggest that IL17 and RANTES participate in recruitment of inflammatory cells to injury sites releasing inflammatory mediators that causes peripheral sensitization. Consequently, indirectly both IL17 and RANTES participate in the peripheral sensitization of injured nociceptive afferents nerve fibers. Likewise, it was reported that the plantar administration of IL17 causes acute hyperalgesia indirectly by inducing TNF from resident cells [508]. Respect to IL-33, this cytokine contributes to peripheral and spinal cord nociceptor neuron sensitization in innate and adaptive inflammatory immune responses as well as in neuropathic and cancer pain [509].
As for the chemokines, it is known that, for example, the administration of MCP1/CCL2 causes hyperexcitability of C-fibers [510]. Actually, it was reported an upregulation of TRPV1 and Na (v)1.8 channels in DRG nociceptive neurons treated with CCL2, suggesting that this chemokine facilitate pain transmission [511]. In the same context, mechanical hyperalgesia has been evidenced in animals subjected to lysophosphatidylcholine injection that causes sciatic nerve demyelination. This pain response is associated with an upregulation of CCR2, CXCR4, CXCR3, and CCR5 receptors in dorsal root ganglia neurons, and MCP1/CCL2 expression in IB4-positive neurons [512]. These finding suggest that upregulation of chemokines and their receptors contributes to sensitization of peripheral nociceptors. In the same line, CCL5-deficient mice showed less behavioral hypersensitivity and a decrease of both macrophage infiltration and pro-inflammatory cytokines in injured areas after partial sciatic nerve ligation, suggesting that CCL5 participates in the peripheral sensitization [513]. Finally, CXCL1 is a chemokine that promote both nociceptor and central sensitization via its main receptor CXCR2 [514] and it is known that chronic morphine administration enhances incision-induced nociceptive sensitization in mice mediated by the upregulation of peripheral CXCL1/CXCR2 expression [515]. It is worth mentioning that the administration of fractalkine into the DRG (L5) produces mechanical hyperalgesia, causing an activation of satellite glial cells leading to the production of TNFα, IL-1β, and prostanoids, which are likely responsible for the maintenance of inflammatory pain [516].
3.3. Growth Factors and Other Diffusible Factors
Fibroblasts and endothelial cells in adipose tissue can synthesize and secrete growth factors and other diffusible factors that, upon reaching skin, muscle, and visceral nociceptors may trigger pain behavior. It has been evidenced that basic fibroblast growth factor (bFGF) increases the NaV1.8 current density in cultured adult rat DRG neurons. In addition, bFGF sensitizes DRG neurons at the cellular level by induction of Erk1/2 phosphorylation and by an Erk1/2-dependent increase of the NaV1.8 current density. Finally, it is known that intradermal injection of bFGF in rats causes mechanical hyperalgesia [517]. As for transforming growth factor-β1 (TGF-β1), it potentiates ASIC-mediated electrophysiological activity and nociceptive behaviors in rats [518]. Moreover, rats with intratibial inoculation of carcinoma showed thermal hyperalgesia and high levels of TGF-β1. TGF-β1-mediates bone cancer pain by sensitization of TRPV1 in primary sensory neurons [519]. On the other hand, in an experimental model of pancreatitis, it was reported that intrathecal infusion of TGF-beta causes hyperexcitability of nociceptive pancreas nerve fibers, and contributes to pancreatic pain hypersensitivity [520]. After partial sciatic nerve ligation (PSL), mechanical and thermal hyperalgesia are observed in injured rats, and mRNA VEGF levels increase in injured sciatic nerve that is expressed in infiltrated neutrophils and macrophages. In addition, VEGF receptors are expressed in endothelial cells and macrophages, and angiogenesis is observed in the injured sciatic nerve. Taken together, VEGF is upregulated in injured peripheral nerves and participates in angiogenesis and prolonged pain behaviors through its receptors [521]. After partial saphenous nerve injury, the exogenous administration of two isoforms of VEGF has opposing effects: VEGF-A165a exacerbates, whereas VEGF-A165b alleviates thermal and mechanical hyperalgesia [522].
Regarding other diffusible factors, accumulating evidence suggests that reactive oxygen species (ROS) contributes to the sensitization during persistent pain [523]. Spared nerve injury (SNI) causes an increase of ROS in mice. NOX enzymes are important ROS sources in somatic cells, and Nox4 expression was seen in IB4-positive dorsal root ganglia cells. These findings suggest that Nox4 is implicated in the production of ROS after SNI causing hyperalgesia [524]. Nitric oxide directly evokes pain in humans when it is injected into the skin [525]. Intraplantar and intrathecal administration of sodium hydrosulfide (NaHS), a donor of H2S, also causes hyperalgesia in rats [526,527].
In this section, evidence has been presented that neuropathic and nociplastic pain are symptoms and signs associated with obese subjects, and that adipose tissue, skin, heart, pancreas, liver, and other body organs of different mammalian species, including humans, present nociceptors and, therefore, can be excited and/or sensitized by the chemical substances released by the adipose tissue that is deposited on them in obese subjects. A schematic illustration of such processes involving adipose tissue chemical substance release and nociceptor activation is shown in Figure 2.
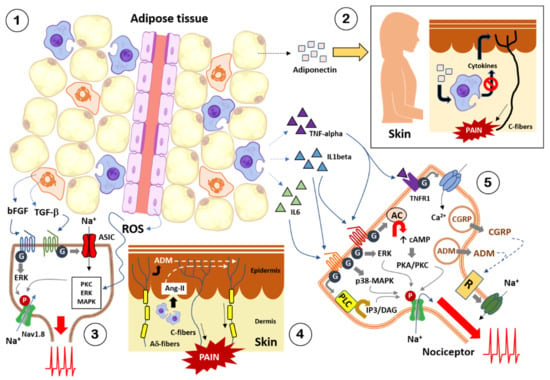
Figure 2.
Crosstalk between adipose tissue, skin, and visceral nociceptors. There is a multitude of diffusible factors secreted by adipose tissue that interact with nociceptors, triggering pain. (1) Adipose tissue is made up of an accumulation of adipocytes, macrophages, lymphocytes, neutrophils, T-cells, natural killer cells, and fibroblasts, as well as an extensive network of blood vessels. (2) Adipocytes secrete adipokines, such as adiponectin, which can reach the skin through the bloodstream. Under an inflammatory condition of the skin, the cutaneous macrophages secrete cytokines, which excite and sensitize the cutaneous nociceptors, triggering pain. Adiponectin acts on these macrophages, inhibiting the release of pain-inducing cytokines. Therefore, this adipokine has analgesic effects. (3) Fibroblasts from adipose tissue accumulated in body tissues and organs secrete growth factors that interact with G protein-coupled receptors on the membrane of nociceptors. Some of these growth factors activate intracellular cascades (e.g., ERK) that allow peripheral sensitization through ion channel phosphorylation (e.g., Nav1.8), thus favoring cation fluxes that trigger depolarization of nociceptors and pain. Other growth factors modulate ion channel opening (e.g., ASIC) and cation entry and depolarization of the nociceptor. Together, growth factors secreted by fibroblasts in adipose tissue cause hyperexcitability of nociceptors and pain. On the other hand, the endothelial cells of the blood vessels that supply adipose tissue secrete oxygen free radicals (ROS) that diffuse to the nociceptors, modulating various intracellular signaling pathways, which cause ion channel phosphorylation, peripheral sensitization and hyperarousal of nociceptors. (4) Inflammation of body tissues, such as the skin, triggers the nociceptors themselves to release adrenomedullin (ADM) or macrophages to release angiotensin-II (Ang-II), two adipokines also secreted by adipose tissue. Both diffusible factors act on receptors in the membrane of cutaneous (or visceral) nociceptors, modulating the opening of ion channels, cation fluxes, and depolarization, causing pain. (5) The macrophages (and other immune cells) of the adipose tissue accumulated in the organs of obese subjects, secrete various cytokines, which by diffusing can interact with specific receptors present in the membrane of visceral nociceptors. The interaction of cytokines is carried out on receptors coupled to G proteins. Some of these interactions (e.g., TNF-alpha) modulate the opening/closing of ion channels, causing variations in the influx of calcium ions. These ions favor the fusion of vesicles loaded with peptides (e.g., pep-tide related to the calcitonin gene or CGRP, adrenomedullin or ADM) present at the terminals of nociceptors. Thus, adipose tissue cytokines cause the release of CGRP and ADM by visceral nociceptors, peptides that interact with their respective receptors (R) that end up regulating the opening of ion channels that favor the entry of sodium ions and depolarization of the nociceptor. At other times, cytokines released by adipose tissue regulate various intracellular cascades (e.g., cAMP/PKA-PKC, ERK, p38-MAPK, IP3/DAG) responsible for the phosphorylation of ion channels and other metabotropic receptors on the nociceptor membrane, triggering peripheral sensitization and hyperexcitability of the nociceptive afferent nerve fiber. In summary, in obese subjects, there is a powerful crosstalk between the accumulated adipose tissue in the body organs and the nociceptive afferent nerve fibers present in these organs, so that the diffusible factors secreted by the adipose tissue cause sensitization and hyperexcitability of the nociceptor, triggering pain. For details, see the main text. Source for figure illustrations: https://scidraw.io/ (accessed on 8 September 2021).
4. Effect of Physical Exercise on Adipose Tissue and Other Body Tissues, Organs, and Systems in Obese Subjects and Its Impact on Pathological Pain
The reduction of adipose tissue is one of the ways to reduce weight in obese subjects, and this can be achieved through changes in diet and modifying energy expenditure through the prescription of physical exercise. In this sense, increasing energy expenditure can help reduce excess adipose tissue in an obese subject [528]. Guidelines by the American College of Sports Medicine (ACSM) include either aerobic or anaerobic exercise. Aerobic exercise (e.g., running, cycling, rowing, etc.) is an exercise that exhausts the oxygen in the muscles, but oxygen consumption is sufficient to supply the energy demands placed on the muscles and does not need to derive energy from another source, whereas anaerobic exercise (or resistance exercise, e.g., weight lifting) is oxygen consumption that is not sufficient to supply the energy demands placed on the muscles, and muscles must break down other energy supplies, such as sugars, to produce energy and lactic acid [529].
International guidelines recommend people living with obesity should be prescribed a minimum of 300 min of moderately intense activity per week for weight loss [529,530]. However, the most efficacious exercise prescription to improve anthropometry, cardiorespiratory fitness (CRF) and metabolic health in this population remains unknown. Despite this, in a recent meta-analysis study, it was reported that while any type of exercise intervention is more effective than control, weight loss induced is modest. Interventions that combine high-intensity aerobic and high load resistance training exert beneficial effects that are superior to any other exercise modality at decreasing abdominal adiposity, improving lean body mass and increasing cardiorespiratory fitness [531]. Another systematical review and meta-analysis study revealed that low-volume high-intensity interval training (HIIT) is inefficient for the modulation of total body fat mass or total body fat percentage in comparison with a non-exercise control and moderate-intensity continuous training (MICT), in overweight and obese adults. In addition, HIIT does not produce a significant change in lean body mass compared to a non-exercising control. However, low-volume HIIT induces greater improvements in cardiorespiratory fitness than a non-exercising control and MICT in normal weight, overweight, and obese adults. Low-volume HIIT, therefore, appears to be a time-efficient treatment for increasing fitness, but not for the improvement of body composition [532]. High-intensity interval training (HIIT) is characterized by short bouts of high-intensity submaximal exercise interspersed with rest periods. Low-volume HIIT, typically involving less than 15 min of high-intensity exercise per session [533].
All of these studies suggest that physical exercise together with the establishment of a diet low in calories may allow body weight to decrease by reducing adipose tissue deposits and increasing muscle mass in obese subjects. This section will review the effect of physical exercise on adipose tissue and other body tissues and organs, mainly in obese but in non-obese subjects, and the impact of such exercise on pain behavior. Here, both studies carried out in humans and in animal models have been included.
Growing evidence suggest that physical exercise interventions are generally effective at reducing body mass. Aerobic training and combination of aerobic and resistant training, reduce both total body mass and fat mass, more than only resistance training. However, resistance training and combination of aerobic and resistance training increase lean body mass, more than aerobic training. These findings suggest that aerobic training may be the optimal mode of exercise for reducing fat mass and body mass, but a program including resistance training is needed for increasing lean mass in overweight/obese individuals [38]. In addition, combination of aerobic and resistant training improves cardio-respiratory parameters (e.g., maximal oxygen consumption or VO2max) and reduces fat percentage and abdominal fat percentage in overweight and obese individuals compared to the lack of exercise [534]. In obese subjects, an exercise training program consisting of aerobic exercise and resistance exercise, with adjustment of exercise intensity every 4 weeks, it was reported to increase maximal oxygen consumption and maximal power output, and to improve peripheral insulin sensitivity but not hepatic and adipose tissue insulin sensitivity. Moreover, total fat mass and body fat percentage slightly decrease but body weight, body mass index, fat free mass, and plasma glucose, non-esterified fatty acid and triacylglycerol concentrations remained unaltered after this training intervention [535]. On the other hand, resistance or aerobic exercise training in sedentary and overweight subjects cause a reduction of C-reactive protein (CRP) but not IL6 [536]. Recently, it was reported that aerobic training, resistance training, and combined training lead to SAT reduction, but aerobic exercise was shown to produce greater efficacy in decreasing SAT [40].
In rats, resistance training induces a significant reduction of body weight and subcutaneous and epididymal white adipose tissue, but the brown adipose tissue is not affected by training. After training, animals showed smaller adipocytes [537].
Aerobic training increase angiogenesis in skeletal muscle but not in subcutaneous adipose tissue (SAT) in insulin-resistant subjects [538], but in obese subjects this training pattern, elevates VGEF expression in SAT and promotes angiogenesis in this adipose tissue [539]. In overweight/obese subjects, aerobic training: (i) reduces the percentage of fat mass without changing the body weight, and modifies adipose tissue lipolysis through an enhancement of beta-adrenergic response [540]; (ii) increases circulating and adipose tissue adiponectin levels [541]; (iii) facilitates a reduction of hepatic fat content without significant changes in weight and visceral fat [542], causing a greater reduction in intra-abdominal adipose tissue than diet [44]. Moreover, after strength and endurance training intervention, insulin sensitivity, VO2max, strength, whole-body, muscle fat content, and abdominal adipose depots were improved in overweight subjects, and hepatic fat, waist circumference, and subcutaneous adipose tissue were reduced in overweight training subjects. Visceral fat was preferentially lost compared with other adipose depots [543]. In addition, endurance-strength training reduces hypertension and decreases fat mass and visceral adipose tissue and increases free fat mass, appendicular lean mass index, and lean mass index [544].
In obese mice, strength and endurance training causes a decrease of whole-body insulin resistance, improve glucose tolerance, and higher activation of the insulin pathway in skeletal muscle. In addition, training increase citrate synthase (CS) activity in skeletal muscle, but not in adipose tissue. These results suggest that the improvement in insulin resistance induced by training is related to mitochondrial adaptation in skeletal muscle, but not in adipose tissue [545].
Combination of resistance and endurance training in overweight subjects cause a reduction of weight, liver fat content and plasma fetuin-A levels. Exercise intervention improve slightly free fatty acids (FFAs) concentration but significantly glucose infusion rate (GIR). These finding suggest that long-term exercise may increase insulin sensitivity by lowering plasma concentration levels of the hepatokine fetuin-A, probably by interfering with adipose tissue [546]. This training pattern also causes a reduction of VAT and IL6 and TNF-alpha in blood plasma, whereas improve insulin sensitivity in humans [547].
Endurance training causes that fat cell weight and lipoprotein lipase activity were lower in abdominal adipose tissue of trained than sedentary subjects. Epinephrine and isoproterenol stimulated lipolytic responses and beta-adrenergic sensitivity of abdominal adipocytes were higher in trained than sedentary subjects, whereas alpha-adrenergic sensitivity was lower in endurance-trained than sedentary subjects. These findings indicate that trained subjects are characterized by a preferential lipid mobilization from the abdominal fat deport, and a reduction of lipoprotein lipase activity with changes in the lipolytic cascades [548]. This training pattern also causes: (i) a reduction of weight body and fat cell size [549]; (ii) a reduction of abdominal fat mass associated with improve of hepatic insulin sensitivity and peripheral insulin sensitivity related with endurance training only [550]; (iii) a decrease of fat weight cell and increased adipocyte epinephrine maximal stimulated lipolysis [551]; (iv) reduction of insulin resistance with increased plasma levels of adiponectin [552]; (v) an increase of blood high-density lipid cholesterol content, maximal exercise capacity, ventilatory threshold, and muscle performance in trained subjects [553]; (vi) a reduction of total cholesterol, triglycerides, and low-density lipoprotein cholesterol (LDL-C), and an increase of high-density lipoprotein cholesterol proteins [554,555]; (vii) reduction of tissue plasminogen activator antigen levels (a cardiovascular risk marker) in overweight subjects [556]; (viii) an improve of maximal oxygen uptake [557].
Strength training improves insulin sensitivity in subcutaneous adipose tissue of obese subjects [558], without altering plasma levels of adipokines (e.g., adiponectin, leptin) [559]. Resistance training in combination with proper diet promotes a reduction of body fat in overweight older adults [560], and this intervention (exercise and diet) improves the cardiovascular conditions of the older subjects [561]. As for pubertal children, resistance training is effective for subjects with low adiposity, but it is ineffective for obese pubertal subjects [562]. Finally, abdominal resistance training, besides diet, did not reduce abdominal subcutaneous fat thickness compared to diet alone in overweight/obese women [563].
Hence, previous experimental evidence in animals and humans shows that different patterns of physical training exert variable effects on obese subjects’ adipose tissue, but most of these studies show changes that lead to improvements in adiposity. It should be noted that part of the heterogeneity of the results of these studies may be due to a diversity of ways of executing physical exercise (e.g., treadmill, swimming, wheel running, etc.), as well as the protocols used on intensity and duration of the physical exercise.
Physical exercise also has an influence on other tissues other than adipose in both obese and non-obese subjects, including skeletal muscle tissue, bone tissue, and the immune system, as well as other systems such as cardiovascular, respiratory, gastrointestinal, endocrine, and nervous system. In the following sections, the physiological effects of physical exercises on body systems are discussed. In addition, Figure 3 and Figure 4 schematically illustrate such effects.
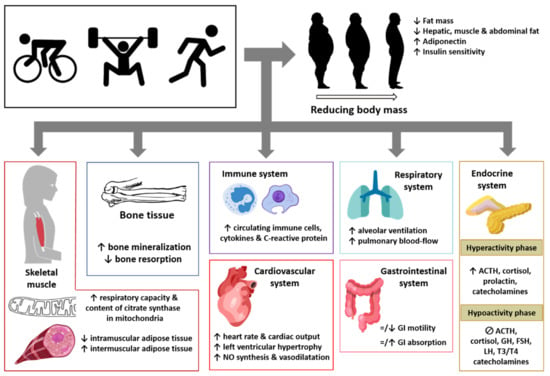
Figure 3.
Overall vision of the effect of physical exercise on body tissues and systems. Physical exercise causes a reduction in body mass with a direct impact on the reduction of accumulated fat, which facilitates an increase in the secretion of adipokines that favor tissue insulin sensitivity in obese subjects. Likewise, physical exercise also has a direct impact on various organs and body systems. On skeletal muscle, it causes metabolic changes especially in mitochondrial physiology, increasing respiratory capacity and the content of Krebs cycle enzymes (e.g., citrate synthase). It also causes a decrease in intracellular lipids, favoring adipose tissue to accumulate mainly at the extracellular level, that is, between skeletal muscle fibers (intermuscular adipose tissue). In bone tissue, physical exercise favors mineralization and therefore reduces bone resorption. At the level of the immune system, physical exercise favors an increase in immune cells in the bloodstream, but also in cytokines and proteins of the acute inflammatory phase (e.g.,). In the cardiovascular system, physical exercise improves various cardiac parameters, and favors the release of diffusible factors that induce vasodilation. On the respiratory–pulmonary system, physical exercise increases alveolar ventilation and pulmonary blood flow. The impact of physical exercise on the gastrointestinal system is dependent on the intensity of this physical exercise. At low or moderate intensities, there are no changes (=) neither in motility nor in gastrointestinal absorption, while with intense physical activity there is a decrease in motility and an increase in gastrointestinal absorption. The endocrine system is also influenced by physical exercise. During the hyperactivity phase, an increase in the levels of various hormones is observed, which suggests that various hormonal axes are highly activated in this hyperactivity phase. On the contrary, in the phase of hypoactivity, the secretion of many hormones is interrupted. For details, see the main text. Source for figure illustrations: https://scidraw.io/ (accessed on 8 September 2021).
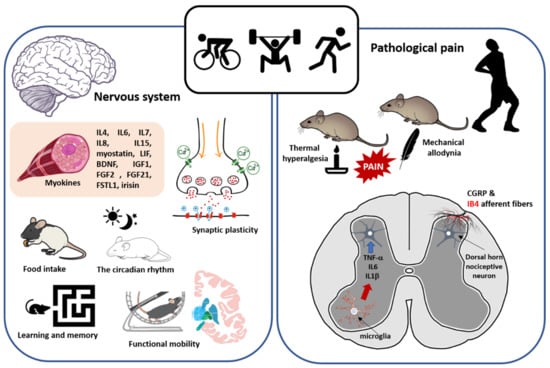
Figure 4.
Effects of physical exercise on the nervous system and pathological pain. Various functions of the nervous system are also affected by physical exercise. Thus, physical exercise enhances synaptic plasticity, regulates food intake, modulates circadian rhythm, enhances learning and memory processes, and modifies neural circuits (e.g., basal ganglia) that improve the functional mobility of subjects. It should be noted that with physical exercise, the continued contraction of skeletal muscle fibers produces a significant release of myokines by this tissue. These myokines, together with other diffusible factors secreted by glial and vascular elements of the nervous system, are responsible for the changes described with physical exercise. On the other hand, physical exercise also has an impact on pathological pain. In animal models, physical exercise has been shown to reduce thermal hyperalgesia and mechanical allodynia. This analgesic effect of physical exercise is mediated by less activation of glial cells (e.g., microglia), which secrete fewer inflammatory cytokines, and with this there is less sensitization and excitation of the nociceptive neurons of the dorsal horn of the spinal cord, but also from nociceptive neurons from other supraspinal pain processing centers. At the spinal level, physical exercise also produces less branching of nociceptive peptidergic (CGRP-positive) and non-peptidergic (IB4-positive) afferent fibers in the dorsal horn of the spinal cord; therefore, there is less input of pain. For details, see the main text. Source for figure illustrations: https://scidraw.io/ (accessed on 8 September 2021).
4.1. Skeletal Muscle
After physical exercise, an increase in the respiratory capacity of the mitochondria of skeletal muscle fibers is observed, as well as the mitochondrial content of citrate synthase [564,565]. Physical exercise (e.g., endurance training) also produces a reorganization of muscle adipose tissue since intramuscular adipose tissue is reduced and intermuscular tissue increased [566], regulates the processes of mitochondrial fusion and fission, increases type IIa fiber type with increased mitochondria and type IIa myosin heavy chain, and improves capacity of skeletal muscle blood flow by increasing VEGF [567]. Moreover, exercise (e.g., treadmill walking, jogging, cycling) increases lipoprotein lipase (LPL) activity in skeletal muscles of sedentary adults, an enzyme implicated in the metabolism of lipids, transforming triglycerides in free fatty acids [568].
Obesity is associated with numerous changes in skeletal muscle including greater muscle mass and muscle fiber cross sectional area, yet fasted muscle protein synthesis is lower. IGF-1 is a growth factor that regulates both anabolic and catabolic pathways in skeletal muscles, including pathways that regulates muscle mass maintenance, muscle hypertrophy, and muscle protein regulation [569]. However, it was reported that subjects with obesity have lower skeletal muscle IGF-1 mRNA and protein, at rest and following resistance exercise training [570]. After cycling activity during 45 min, it was reported that citrate synthase activity and maximal ATP production rate increase in non-obese, but not in obese, subject muscle samples [571]. However, the fatty acid oxidation in muscles increases after exercise training in extremely obese subjects with impaired fatty acid oxidation, at similar levels observed in lean subjects [572]. It was also reported that endurance exercise training upregulates fat oxidative capacity and lipolytic protein expression in skeletal muscle of obese subjects [573].
It should be noted that, in the obese subjects, several endocrinological disturbances have been identified during acute endurance and resistance exercise training including a blunted blood growth hormone, atrial natriuretic peptide and epinephrine release, and greater cortisol and insulin release. All of these endocrinological changes have an impact on the physiology of the skeletal muscle. Specifically, it has been observed that during acute endurance and resistance exercise, blood epinephrine release in obese subjects is smaller in comparison to healthy subjects. This hormone is implicated in lipolytic muscle response. Blood growth hormone secretion is significantly reduced in obese during acute endurance/resistance training, as opposed to healthy subjects. Growth hormone is implicated in the stimulation of skeletal muscle protein synthesis, by facilitating amino acid transport and availability via the release IGF-1 and other local factors. In contrast to healthy subjects, during acute endurance exercise, a greater blood cortisol levels seems to be present in the obese subjects. In skeletal muscle, cortisol causes muscle mass loss by a combination of a suppression of protein synthesis and increased protein degradation. Blood insulin levels also decrease in obese subjects after acute endurance training. This hormone stimulates skeletal muscle protein synthesis. Blood atrial natriuretic peptide levels do not increase with sufficient magnitude in subjects with obesity after acute endurance exercise, contributing to a suppression of lipolysis during exercise [574].
On the other hand, hyperinsulinemia in overweight/obese subjects is associated with increased extremity muscle mass, which is partially reversible with reduction in fasting insulin concentration, consistent with the stimulatory effects of insulin on skeletal muscle after treadmill exercise [575]. It is well known that insulin stimulates glycogen synthase (GS) through dephosphorylation of serine residues, and this effect is impaired in skeletal muscle from insulin-resistant obese subjects. It has been observed that 40 min of moderate-intensity cycle exercise increases GS fractional activity, decreases K(m) for UDP-glucose, and decreases GS phosphorylation. These findings suggest that the molecular regulatory process by which exercise promotes glycogen synthesis in muscle is preserved in insulin-resistant obese subjects [576].
4.2. Bone Tissue
The effect of physical exercise on the bone has been well studied in women. A recent meta-analysis study indicates that the exercise intervention resulted in significant, but rather small positive effect on bone mineral density (BMD) [577]. On the other hand, exercise downregulates sclerostin expression by the osteocyte favoring osteoblastogenesis. These changes are enhanced by dynamic cyclical load with rest periods and may be promoted by low-amplitude high-frequency stimuli [578]. The expression of osteoprotegerin increases after endurance exercise, but this increase is independent of the exercise intensity; however, the accumulation of collagen in the bone depends on the intensity of the exercise. Nevertheless, increased bone-alkaline phosphatase concentrations suggest a beneficial effect of this type of exercise on bone mineralization [579]. Actually, in humans, it was reported that resting femoral bone blood flow increases by physical exercise, but appears to level off with increasing exercise intensities [580]. In addition, it has also been observed that resistance exercise training increased markers of bone formation, while it transiently suppressed a marker of bone resorption, and adaptive changes of bone metabolism to resistance exercise training occurred during the early period of the training, before changes in bone density were observable through densitometry [581].
In rats, strenuous exercise causes a reduction of longitudinal and circumferential growth of the tibia, but the average number of osteons and osteocytes per unit area of the tibial middiaphysis was significantly greater in the exercised group. The number of osteons and osteocytes per unit area in the second metatarsus, however, was significantly less in the exercised group [582]. Other beneficial effects on bone tissue were observed in zebrafish subjected to increased physical exercise for four weeks in a swim tunnel experiment since showed: (i) a higher bone volume in the caudal spine; (ii) increased bone formation in posterior caudal vertebrae; (iii) the bone formation rate (BFR, amount of new bone formed in unit time per unit of bone surface) was 46% higher in the exercise group compared to the control; (iv) osteoblastic bone formation was significantly higher in the exercise group; and (v) higher mineralized bone in the exercise group. These finding suggest that controlled swimming exercise induces bone formation and mineralization in adult zebrafish [583].
4.3. Immune System
Several studies revealed that a single acute bout of prolonged, strenuous exercise has a temporary depressive effect on immune function. Actually, an acute bout of physical activity is accompanied by several responses of immune system, including an increase of circulating lymphocytes and neutrophils, pro-inflammatory cytokines (e.g., TNF-alpha, MCP-1, IL-1beta) and acute phase proteins (e.g., C-reactive protein) and under these circumstances, an increase of hormones (e.g., adrenaline, cortisol, growth hormone, prolactin) with immunomodulatory effects also were shown in plasma. Acute exercise temporarily increases the number of circulating natural killer (NK) cells, but following exercise, normal resting values are usually restored within 24 h. Antigen-presenting cell function is also affected by exercise. Exercise-induced reductions in macrophage major histocompatibility complex class II expression and antigen-presenting capacity. T-memory and T-naïve cells increases temporarily during exercise whereas the production of immunoglobulins by B-lymphocytes is inhibited following prolonged, strenuous exercise [583,584,585,586]. On the other hand, chronic exercise causes a slight increase of NK cell activity. Neutrophil function is suppressed or not significantly influenced by chronic exercise, whereas lymphocyte proliferative responses has been described as decreased, elevated or unchanged after chronic exercise [586].
In obese subjects, aerobic exercise and resistance exercise causes an increase of NK cell activity. Plasma levels of IgA remain stable, whereas IgG levels tend to decrease after training [587]. Several studies showed that leukocyte and lymphocyte subsets are elevated in obese individuals [588], but other studies indicate a reduction of lymphocytes in obese patients [589]. Obese animals also showed T-lymphopenia [590], and NK cell activity is suppressed [591].
4.4. Cardiovascular System
Regarding to cardiovascular system, dynamic physical exercise improves several hemodynamic and cardiac parameters (e.g., heart rate, stroke volume, cardiac output, total vascular conductance) in obese subjects [592]. At a vascular level, exercise increases the activity of endothelial eNOS an enzyme that synthetizes nitric oxide, a chemical mediator that induces vasodilatation [593] and consequently, exercise training consistently induces improvements in both conduit and resistance artery NO-mediated function. In addition, exercise also causes an increase in arterial cross-sectional area, also referred to as “arterial remodeling” [594]. Several cardiovascular adaptations were reported during exercise such as increase of cardiac output, heart rate, blood pressure, and reduction of pulmonary and systemic vascular resistance to blood flow [595,596]. An increase of cardiac output by exercise may be attributable to morphological changes of the heart, such as left ventricular dilatation and hypertrophy, leading to several physiological changes, including increase early diastolic filling, preload and myocardial relaxation, and also increase contractile strength [596,597]. The reduction of vascular resistance was also seen in several organs, such as skeletal muscle during exercise [598,599]. The hypertrophy of left ventricular is primarily the results of an increase in the size of cardiac myocytes [600]. The effect of exercise on cardiomyocyte contractile function may be related to alterations in the rise and decay rates of intracellular Ca2+ transients, possibly due to enhanced coupling efficiency between L-type Ca2+ channel-mediated Ca2+ entry and activation of subsarcolemmal ryanodine receptors (RyR; e.g., calcium-induced calcium release), and increased expression and activity of the sarcoendoplasmic reticulum Ca2+ ATPase (SERCA2a) and sodium-calcium exchanger (NCX) [601,602,603]. Reduction of vascular resistance to blood flow may be related to the release of vasodilatory signals (e.g., adenosine, lactate, potassium ions, CO2) [604,605,606].
4.5. Respiratory System
During exercise, pulmonary ventilation must rise in proportion to increase in metabolic rate so that arterial blood gas homeostasis is maintained, which it involves substantial increases in the work of breathing and thus the energy cost of breathing. However, the work of breathing is minimized, due to decreased resistance of the intra- and extra-thoracic airways during exercise. With exercise, pulmonary blood-flow and pulmonary arterial pressure rise whereas pulmonary vascular resistance falls. The dominant mechanism regulating pulmonary hemodynamic responses during exercise is left ventricular filling pressure, which climbs during exercise, and both recruits and distends the pulmonary capillaries and veins, thus contributing to the fall in pulmonary vascular resistance. Furthermore, vascular distention and recruitment expand the capillary surface area and volume, thus minimizing any fall in red blood cell transit time within the pulmonary capillaries in the face of an increasing cardiac output. Interstitial and alveolar edema is minimized because both capillary absorptive pressure and lymph flow increase during exercise. During exercise, the efficiency of gas exchange worsens in an intensity-dependent manner, but a disproportionate rise in alveolar ventilation relative to metabolic rate during high-intensity exercise prevents arterial hypoxemia [607].
Obese patients show a decline in exercise capacity and diverse degrees of dyspnea in association with mechanical abnormalities, increased ventilatory requirements secondary to the increased metabolic load, and a greater work of breathing. Consequently, obese patients may be particularly predisposed to the development of respiratory muscle fatigue during exercise. In this context, it was reported that in obese subjects the tidal volume, the breathing frequency, the power output, and the peak oxygen uptake were significantly higher after training. At comparable workload, training induces lower minute ventilation, mouth occlusion pressure, ratio of occlusion pressure to maximal inspiratory pressure and rate of perceived breathlessness. These findings suggest that aerobic exercise at ventilatory threshold may induce significant improvement in respiratory muscle strength, maximal exercise capacity, and inspiratory muscle performance and decreased dyspnea perception in obese subjects [608]. Despite these results, it should be noted that mechanical ventilatory constraints increase progressively with degrees of obesity, contributing to exercise limitation in obese subjects [609,610].
On the other hand, one of the mechanisms related to respiratory mechanics alteration caused by obesity is the accumulation of fat in chest, diaphragm, and abdomen [611] since this accumulation may compress the chest wall, diaphragm, and lungs, reducing lung volumes and flow [612]. Consequently, this accumulation of fat may affect several spirometry and respiratory parameters. It was reported that, in obese subjects, the basal values of blood pressure and heart rate were higher whereas oxygen saturation was lower in comparison to eutrophic group. During exercises, oxygen saturation also decreased during exercise, whereas blood pressure and heart rate were higher. With respect to spirometry variables, FVC and FEV1 were lower in obese subjects before and after the exercise. These findings suggest that obesity affects pulmonary function [613] and reduction of functional residual capacity and impairment of diffusion capacity were reported in obese subjects [611]. Finally, in non-asthmatic subjects, the airway responsiveness was significantly correlated with BMI, and this increase was significantly related to lung volume restriction [614].
4.6. Gastrointestinal System
Gastroesophageal reflux occurs more frequently with exercise than at rest and may produce symptoms of chest pain suggestive of ischemic disease. Acid exposure may be reduced by pretreatment with histamine H2-receptor antagonists. Gastric emptying during exercise is subject to a number of factors including calorie count, meal osmolality, and meal, temperature, and exercise conditions. It seems that moderate exercise would have little effects on gastrointestinal tract motility, but when exercise becomes more severe, there may be some inhibiting effects, especially at the level of gastric emptying. Gastric acid secretion probably changes little with exercise although it has been postulated that ulcer patients may increase secretion with exercise. Some exercise-associated digestive symptoms, such as diarrhea and abdominal pain, have been attributed to changes in intestine function. Small bowel transit is delayed by exercise when measured by breath hydrogen oral cecal transit times and motility may be reduced as well. Intestinal absorption during exercise has not been well evaluated, but it likely changes little in ordinary circumstances. Passive absorption of water, electrolytes, and xylose are not affected by submaximal effort, but increased intestinal permeability was reported after a marathon. After higher intensities of exercise, gastrointestinal changes include a reduction in mesenteric blood flow, decreases in esophageal peristaltic activity, decreases in lower esophageal sphincter tone, and increased transient lower sphincter time and a reduction of gastric emptying. Due to reduced gastrointestinal tract perfusion, absorption might be affected and gut barrier function might be compromised [615,616].
As for obese subjects—several studies suggest that physical exercise causes changes in the gastrointestinal system including liver. It was reported that exercise decreased late-phase postprandial gallbladder volume and increased late-phase postprandial gallbladder motility in obese subjects [617]. On the other hand, endurance exercise training reduces plasma cholesterol despite increasing cholesterol absorption in subjects with obesity and metabolic syndrome risk factors [618]. Endurance training did not decrease hepatic fat in obese subjects, but training lowered plasma liver enzymes (e.g., GGT), but not ALT and bilirubin [619]. However, other studies indicate that in obese subjects, blood ALT and AST tend to decrease after endurance-strength training, whereas both enzymes increase after endurance training. On the other hand, a significant reduction in serum of GGT was seen in both trainings and neither endurance nor endurance-strength exercise led to changes in serum ALP activity and total or direct bilirubin level. These findings suggest that the mode of exercise does matter: endurance-strength exercise led to a greater improvement, compared to endurance exercise, in the liver functioning in subjects with abdominal obesity [620]. Finally, no studies have assessed the impact of physical exercise on motility, digestion, and absorption processes of the gastrointestinal system in obese subjects.
4.7. Endocrine System
Hormonal changes related to exercise may be explained by several physiological reasons: (i) to induce cardiovascular adjustments; (ii) to activate energy production pathways and mobilize energy substrates; (iii) to facilitate maintenance of adequate hydration; and (iv) to some extent as part of stress reactivity. During exercise, the endocrine system hormonal responses seem to occur in two phases: an initial hyperactivity phase, followed by a latter hypoactivity phase. In the hyperactivity phase, elevations in the circulating levels of hormones, such as adrenocorticotrophic hormone, cortisol, prolactin, and catecholamines were reported at rest and/or in response to an acute exercise session. In the hypoactivity phase certain hormones including adrenocorticotrophic hormone, catecholamines, cortisol, growth hormone, follicle-stimulating hormone, luteinizing hormone, testosterone, and thyroid hormones are found suppressed. In this hypoactivity phase, several inflammatory mediators (e.g., IL6, TNF-alpha, and UL-1beta) act upon multiple levels of the hypothalamic-pituitary-adrenal axis, most notably the hypothalamic paraventricular nucleus where CRF production occurs. CRF influences this endocrine axis, but also affect mood, sexual, and immune functions [621]. Growing evidences suggest that exercise and training affect the hypothalamic-pituitary-adrenal (HPA) axis. In males, testosterone increases with acute bouts of exercise, but lower testosterone levels were seen in endurance athletes. In females, an increase of total testosterone levels was seen after an acute bout of resistance training and multiple studies have found an acute increase in estradiol immediately after exercise [622]. On the other hand, exercise stimulating HPA axis also induces elevation of cortisol, corticosterone, and catecholamines in plasma. Glucocorticoids play an important role in the mobilization of energy reserves during physical activity by stimulating gluconeogenesis, promoting lipolysis and increasing protein catabolism, whereas catecholamines secreted from the adrenal medulla stimulates the release of IL6 by immune cells. IL-6 is known to stimulate two other hormones: growth hormone (GH) and prolactin (PRL) [623]. Finally, several studies revealed that in diabetic subjects, exercise decrease pancreatic fat content, and increase the release of insulin by endocrine pancreas [624,625,626,627], a key hormone in the regulation of sugars, lipids, and amino acids after food intake.
In older obese subjects, it was reported that exercise training increases beta-cell function. The increase in pancreatic function is clinically important, since it was directly related to improved glucose tolerance [628].
4.8. Nervous System
Physical exercise induces different changes in several neuronal nuclei of the central nervous system. In locus coeruleus (LC) involved in the regulation of attention, arousal, vigilance responses to stress, and spinal cord modulation of pain, exercise causes level variation of galanin, a neurotransmitter attenuates neuronal hyper-excitability. Exercise may therefore be involved in the LC noradrenergic neurons adaptation to stress. Moreover, exercise increases serotonin in the suprachiasmatic nucleus (SCN), the hypothalamic nucleus involved in the control of the circadian rhythm or circadian clock. The circadian clock is a timing mechanism that endogenously coordinates biochemical, physiological, and behavioral processes with the 24 h cycle of light and dark. Moreover, physical exercise also influences hypothalamic neurons involved in regulating food intake. That is, the acute exercise reduces the food intake by activation of the corticotropin-releasing factor (CRF/CRH) pathway in the dorsomedial hypothalamus, and increases leptin levels, whereas chronic exercise inhibits food intake by a pathway leptin-independent. In the basal ganglia, clinical studies demonstrated that exercise might be involved in improving functional mobility of patients with Parkinson’s disease since it increases brain derived neurotrophic factor (BDNF), a growth factor with an important role for the survival of dopaminergic neurons in the striatum [629]. In addition, it is well known that regular exercise has a beneficial impact on depression, quality of sleep, and cognitive function. Chronic physical activity increases the expression of brain growth factors, such as BDNF and VEGF, factors that are involved in enhancing the learning process by regulating synaptic plasticity, or in improving cerebral vascularization [630,631]. Training skeletal muscle contractions stimulates the production and release of myokines in the blood, including IL4, IL6, IL7, IL8, IL15, myostatin, leukemia inhibitory factor (LIF), brain-derived neurotrophic factor (BDNF), insulin-like growth factor (IGF1), fibroblast growth factor 2 (FGF2), fibroblast growth factor 21 (FGF21), follistatin-related protein 1 (FSTL1), irisin, erythropoietin (EPO), β-aminoisobutyric acid (BAIBA), CTRP15 (myonectin), and decorin, which can influence physiology of the nervous system [632]. IL4 modulates the expression of pro-inflammatory cytokines, nitric oxide, and growth factors in astrocytes and microglia cells of the central nervous system, decreasing the secretion of inflammatory mediators and promoting the release of growth factors. Likewise, IL4 participates in the survival of hippocampal and striatum neurons, and reduces pain caused by subsequent intraplantar injection of carrageenan, bradykinin, or TNF-α [633]. IL8 acts in the brain by reducing food intake [634]. IL15 provides neuroprotection to various types of neurons, but at the same time, causes reactivation of microglia cells and astrocytes. In the hypothalamus it regulates temperature, energy metabolism, and circadian rhythms, whereas in hippocampus promotes consolidation of learning and memory [635]. As for IGF-1, it promotes plasticity at synapse levels, such as increasing the length and number of multiple spin bouton complexes as well as the length of the post-synaptic density in hippocampal neurons. Moreover, it increases expression of synapsin-1, a protein important for maintaining the stability of synapses. IGF-1 is also implicated in the modulation of glutamatergic receptor subunits, alterations in calcium channel conductance as well as potentiation of glutamatergic transmission, in the face of a reduction in GABAergic transmission [636]. On the other hand, different brain regions are sensitive to the action of FGF21 including the suprachiasmatic nucleus, area postrema, nucleus of the solitary tract and paraventricular nucleus of the hypothalamus, and smaller amounts in the ventral tegmental area and nucleus accumbens. FGF21 reduces sugar intake in both mice and humans, and regulates alcohol preference [637]. Regarding irisin, it improves learning and memory by increasing BDNF expression in hippocampus [638]. Finally, EPO plays different roles in the nervous system including survival and neuroprotection, modulation of the inflammatory and immune response, hemodynamic, and vasoactive effects, promotes angiogenesis for stimulating mitosis and motility of endothelial cells [639,640].
Finally, it has been shown that regular training increases autonomic nervous system activity in obese subjects [641,642]. Exercise intervention is associated with attenuation in the response to visual food cues in brain regions known to be important in food intake regulation, such as the insula [643]. Aerobic exercise causes a significant increase in plasma BDNF levels in obese subjects [644,645]. A similar pattern was reported after acute high-intensity interval exercise in obese subjects [646].
4.9. Pathological Pain: Relationship with Sedentary Behavior and Modulation by Physical Exercise
Sedentary behavior (SB), defined as activities that do not increase energy expenditure substantially above the resting level (e.g., 1.0–1.5 metabolic equivalents), is characterized by waking time spent sitting, reclining, or lying down [647,648]. Globally, adults spend a considerable percentage of their daily time in SB, such as workplace sitting, television watching, or computer and game-console use [649]. Time spent watching TV, in particular, is positively correlated with increasing body mass index (BMI) [650,651,652]. There is evidence that increased obesity risk is attributed to time spent in sedentary transportation (e.g., car, train, bus) [653,654]. In contrast, it was also reported that greater leisure time moderate-to-vigorous leisure time physical activity (MVPA) (e.g., sports and exercise), active transportation (e.g., walking and bicycling), and reading were independently related to lower BMI, whereas greater TV/movie watching, games, computer use, and sedentary transportation were independently related to higher BMI. However, there were statistical interactions between leisure time MVPA and TV/movie watching, leisure time MVPA and playing games, active transportation and sedentary transportation, and active transportation and reading in relation to BMI. These findings suggest that different combinations or patterns of physical activity and sedentary behaviors may need to be considered in terms of their implications for BMI risk [655].
Cross-sectional and longitudinal research has found that, among healthy adults or adults with chronic pain, individuals with a higher level of SB report a higher level of pain than those who are less sedentary [647,656,657]. It is well known that chronic pain is a highly prevalent and extremely debilitating condition that places a burden on daily living and decreases life expectancy. Lifestyle factors such as physical inactivity, stress, poor sleep, unhealthy diet, and smoking are associated with chronic pain severity and sustainment. This applies to all age categories, that is, chronic pain across the lifespan [658]. Knee osteoarthritis (OA) are the primary cause of chronic pain in older adults. Using a sample of older knee osteoarthritis (OA) patients, it was reported that patients spent less time in moderate-to-vigorous physical activity (MVPA), but more time in sedentary behavior (SB) on days when they endorsed greater levels of catastrophic thinking about pain in the morning. The predictive effects of a patient’s pain catastrophizing also extended to sedentary behavior the next day. In addition, patients reported greater pain catastrophizing in the morning if they spent more time than usual in sedentary behavior the previous day, suggesting that pain catastrophizing could be worsened by avoiding physical activities. These results provide strong evidence to support the hypothesized role of pain catastrophizing in affecting pain patients’ physical activity in everyday life, being able to exist a vicious circle between catastrophic pain and sedentary behavior, in other words, one enhances the other and vice versa [659]. Longer sedentary behavior was correlated with chronic knee pain, and subjects with high levels of physical activity were less likely to suffer from chronic knee pain [660]. On the other hand, in severely obese adult subjects (with BMI ≥ 35 kg/m2), a significant association between pain and MVPA (p = 0.0017) was reported, indicating that individuals with shorter MVPA have more pain. However, no association was seen between pain and light physical activity (LPA) or SB. These findings indicate that, in severely obese individuals, shorter MVPA time is associated with a higher prevalence of pain [661]. Positive associations with daily SB were found for pain intensity (r = 0.31, p < 0.01) and the number of painful joints (r = 0.24, p < 0.05), but not non-articular pain in individuals with rheumatoid arthritis [662]. High levels of sedentary time and lower levels of light physical activity are associated with a worse symptomatology in fibromyalgia [663]. All of these studies suggest the existence of a relationship between sedentary behavior and pain, in pathologies that, according to the International Association for the Study of Pain (IASP), trigger pathological pain.
There is scientific evidence that suggests that physical exercise improves pain in subjects with sedentary behavior and pathological pain diseases. Two-month intensive aquatic therapy program of high frequency (five times/week) decreases levels of back pain and disability, increases quality of life, and improves body composition and health-related fitness in sedentary adults with chronic low back pain [664].
In addition, several studies suggest beneficial effects of physical exercise on pain responses in subjects suffering from pathologies that trigger pathological pain. It was reported that compared to the control group, three exercise modalities (e.g., Tai Chi, Baduanjin, and stationary cycling) significantly increased knee injury and osteoarthritis outcome score pain subscores and regulated serum PD-1 and INF-γ levels in patients with knee osteoarthritis [665]. Isometric exercise in patients with type 2 diabetes mellitus with and without painful diabetic neuropathy promotes exercise-induced hypoalgesia (EIH) [666]. Aerobic exercise [667] muscle strengthening, pool exercises [668], and spa therapy [669] have been found to improve the pathology in subjects with fibromyalgia, including pain. Fibromyalgia patients can attain symptom relief, particularly decreased pain and fatigue as well as improved sleep and mood, with low-to-moderate intensity exercise of any type [670].
Experimental and clinical evidence suggest that physical exercise influences pathological pain, both neuropathic and nociplastic. Studies evaluating the effect of exercise after spinal cord injury indicate that the intervention significantly improves mechanical allodynia and thermal hyperalgesia in injured subjects [671]. In rats subjected to spinal cord contusion, exercise training in treadmill causes an upregulation of BDNF expression, and this is associated with greater thermal hyperalgesia and mechanical allodynia [672]. In addition, after spinal cord contusion, all rats showed mechanical and thermal hypersensitivity, and exercise causes a reduction of animals with mechanical allodynia. Exercise also reduces the sprouting of IB4-positive afferent fibers in the dorsal horn of injured rats [673] and it alleviates hyperalgesia in rats subjected to spinal cord compression [674]. Furthermore, after spinal cord contusion in mice, exercise alleviates hyperalgesia and reduces CGRP-immunoreactivity in dorsal horn [675]. In the same line, chronic exercise training also alleviates mechanical and thermal hyperalgesia in mice subjected to spinal cord compression [676].
On the other hand, after chronic constriction injury (CCI) of the sciatic nerve, exercise alleviates thermal and mechanical hyperalgesia by reducing the expression of pro-inflammatory cytokines in rats (e.g., TNF-alpha, IL6) [677]. Transcranial direct current stimulation combined with exercise also alleviates both mechanical and thermal hyperalgesia and modulates the expression of pro-inflammatory cytokines in several central nervous system areas in rats [678]. The combination of ultrasound and exercise also alleviates hyperalgesia and reduces the expression of pro-inflammatory cytokines, and improve the expression of anti-inflammatory cytokines in rats [679]. It is worth mentioning that exercise reduces irisin expression in rats subjected to CCI [680].
After spared nerve injury (SNI), exercise alleviates both mechanical and thermal hyperalgesia [681], and this pattern was seen in mice subjected to partial ligation of sciatic nerve, where exercise also reduces gliosis [682]. In this experimental model of partial ligation of sciatic nerve, it was also reported that exercise alleviates hyperalgesia and causes a significant reduction of inhibitory GABAergic neurons in the dorsal horn [683].
As for nociplastic pain, it has been shown that resistance exercise improves muscle strength, health status and pain intensity in women with fibromyalgia [684] and spinal stabilization exercise (SSE) plus kinesio taping (KT) improve pain responses in patients with this health concern [685]. Moreover, exercise in warm water decreases pain and improve cognitive function in subjects with fibromyalgia [686]. Actually, it was reported that physical exercise tends to reduce pain in patients with fibromyalgia and this effect is associated with a decrease in IGF-1 levels in the cerebrospinal fluid of these patients [687]. Hence, low to moderate intensity global exercises performed for a long period of treatment should be performed in patients with nociplastic pain predominance. Additionally, focused and intense exercises for a short period of treatment can be prescribed for patients with nociceptive pain predominance [688].
It should be noted that in the context of pain, inflammatory cytokines (e.g., TNF-alpha, IL6, and IL1beta) as well as BDNF, and IGF-1 causes hyperalgesia for: (i) stimulating nociceptive neurons and causing hyperexcitability, and (ii) inducing central sensitization [689,690,691]. However, irisin has anti-inflammatory properties by attenuating the expression of pro-inflammatory cytokines [692] and their intrathecal administration alleviates hyperalgesia after CCI [693]. However, it is well known that irisin promotes the expression of BDNF [694], a growth factors that may cause pain behaviors.
Finally, few studies have focused on studying the effects that exercise exert on pain in obese subjects. It has been shown in mice that high-fat diet induces prediabetes, causing hyperalgesia and exercise attenuates this pain response in obese mice. Actually, obese prediabetic mice showed higher levels of GDNF and NGF in nerves and spinal cord [695]. In contrast, swimming does not alter nociception threshold in obese rats submitted to median nerve compression [696]. Regarding human studies, it has been suggested that increased physical exercise has no mitigating effects on low back pain in obese subjects [49], but further studies on the beneficial effects of physical exercise are needed to obtain robust conclusions on pain modulation on obese subjects.
5. Conclusions
A sedentary lifestyle leads to the development of overweight/obesity, which involve excessive fat body accumulation. This fat accumulation may trigger several pathophysiological comorbidities, which would result from adipose tissue and body systems crosstalking. Our review offered an overview of structural and functional changes in tissues, organs, and body systems due to the crosstalk between fat and body tissues, and how physical exercise may modulate fat accumulation-related comorbidities, including pathological pain development. To conclude, firstly, the literature review provided evidence that in overweight/obese subjects, the following is present: adipose tissue and other body tissue crosstalk mediated by diffusible factors with local or generalized effects, occurring mainly on skeletal muscle, cardiovascular system, liver, pancreas, and visceral, abdominal, and subcutaneous adipose tissues. Consistent with the literature, we showed that, besides these general body system pathological changes, neuropathic and nociplastic pain development might be associated with overweight/obesity. Concretely, it has been evidenced that in obese subjects, there is a powerful crosstalk between body organ adipose tissue and the nociceptive afferent nerve fibers present in these organs; diffusible factors secreted by the adipose tissue would lead to nociceptor sensitization and hyperexcitability, resulting in pain development. On the other hand, several beneficial effects of physical exercise has been evidenced, not only on adipose tissue, but also on organs and body systems, including skeletal muscle, bone tissue, immune system, cardiovascular system, respiratory-pulmonary system, gastrointestinal system, endocrine system, and the nervous system. That is, physical exercise not only favors a reduction in body mass with a direct impact on decreasing overweight/obese-induced comorbidities associated with adipose tissue factors released, but it also directly affects other organs and tissues. Finally, focusing on pathological fat accumulation-related pathological pain, the literature allowed describing experimental and clinical evidence, suggesting that physical exercise may modulate both neuropathic and nociplastic pain symptoms. However, it has been evidenced that further studies on the beneficial effects of physical exercise are needed to obtain robust conclusions about pain modulation, specifically in obese subjects.
Author Contributions
E.V. and P.B.-V. conceived the review. E.V. conducted the screening, searched for articles, performed data extraction, created the table and figures, and wrote the first draft of the manuscript. P.B.-V. refined the search strategy, assisted in screening, edited the table, and helped write the main text and conclusion. J.H. and P.B.-V. edited the second draft of the manuscript and E.V. supervised the review process. J.H. reviewed and formatted the bibliography. P.B.-V. prepared the final draft for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank Scidraw.io and all collaborators for the free repository of high quality drawings of cells, organs, and others, used to illustrate the figures of the present manuscript: Adipocyte https://doi.org/10.5281/zenodo.3926133; Macrophage, https://doi.org/10.5281/zenodo.3926101; Heart, https://doi.org/10.5281/zenodo.3926161; Liver, https://doi.org/10.5281/zenodo.3926109; Lungs, https://doi.org/10.5281/zenodo.3926367; Pancreas, https://doi.org/10.5281/zenodo.3926363; Large intestine, https://doi.org/10.5281/zenodo.3926408; Brain outline, https://doi.org/10.5281/zenodo.3925989; Hepatocyte, https://doi.org/10.5281/zenodo.3926287; Lipid droplet no receptors, https://doi.org/10.5281/zenodo.3926370; Myocyte, https://doi.org/10.5281/zenodo.3926129; Epithelial layer, https://doi.org/10.5281/zenodo.3926185); Silhouette Muscle, https://doi.org/10.5281/zenodo.3926201; Silhouette, https://doi.org/10.5281/zenodo.3926189; mouse profile, https://doi.org/10.5281/zenodo.3925921; Mouse on a running wheel, https://10.0.20.161/zenodo.4742583; mouse sleeping, https://doi.org/10.5281/zenodo.3925977; Long-Evans Rat Eating, https://doi.org/10.5281/zenodo.3926281.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hu, F.B.; Li, T.Y.; Colditz, G.A.; Willett, W.C.; Manson, J.E. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 2003, 289, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.J.; Fiatarone Singh, M.A. The influence of physical activity on abdominal fat: A systematic review of the literature. Obes. Rev. 2006, 7, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, S. Physical activity and abdominal obesity in youth. Appl. Physiol. Nutr. Metab. 2009, 34, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.; Edwardson, C.L.; Morgan, B.; Horsfield, M.A.; Bodicoat, D.H.; Biddle, S.J.; Gorely, T.; Nimmo, M.A.; McCann, G.P.; Khunti, K.; et al. Associations of Sedentary Time with Fat Distribution in a High-Risk Population. Med. Sci. Sports Exerc. 2015, 47, 1727–1734. [Google Scholar] [CrossRef]
- Galmes-Panades, A.M.; Konieczna, J.; Abete, I.; Colom, A.; Rosique-Esteban, N.; Zulet, M.A.; Vázquez, Z.; Estruch, R.; Vidal, J.; Toledo, E.; et al. PREDIMED-Plus investigators. Lifestyle factors and visceral adipose tissue: Results from the PREDIMED-PLUS study. PLoS ONE 2019, 14, e0210726. [Google Scholar] [CrossRef]
- Moschonis, G.; Kalliora, A.C.; Costarelli, V.; Papandreou, C.; Koutoukidis, D.; Lionis, C.; Chrousos, G.P.; Manios, Y.; Healthy Growth Study Group. Identification of lifestyle patterns associated with obesity and fat mass in children: The Healthy Growth Study. Public Health Nutr. 2014, 17, 614–624. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018; National Center for Health Statistics: Hyattsville, MD, USA, 2021. [Google Scholar]
- Eurostat. Overweight and Obesity—BMI Statistics; European Statistical System: Luxembourg, 2021. [Google Scholar]
- Spieker, E.A.; Pyzocha, N. Economic Impact of Obesity. Prim. Care 2016, 43, 83–95. [Google Scholar] [CrossRef]
- Hartman, M.; Martin, A.B.; Espinosa, N.; Catlin, A.; The National Health Expenditure Accounts Team. National Health Care Spending. In 2016: Spending and Enrollment Slow After Initial Growth Coverage Expansions. Health Aff. 2018, 37, 150–160. [Google Scholar] [CrossRef]
- OECD. The Heavy Burden of Obesity: The Economics of Prevention, OECD Health Policy Studies; OECD Publishing: Paris, France, 2019. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Pi-Sunyer, F.X. The obesity epidemic: Pathophysiology and consequences of obesity. Obes Res. 2002, 10, 97S–104S. [Google Scholar] [CrossRef]
- Standl, E. Dysglycemia and abdominal obesity. Curr. Vasc. Pharmacol. 2012, 10, 678–679. [Google Scholar] [CrossRef]
- Feakins, R.M. Obesity and metabolic syndrome: Pathological effects on the gastrointestinal tract. Histopathology 2016, 68, 630–640. [Google Scholar] [CrossRef]
- Chang, J.W.; Chen, H.L.; Su, H.J.; Lee, C.C. Abdominal Obesity and Insulin Resistance in People Exposed to Moderate-to-High Levels of Dioxin. PLoS ONE 2016, 11, e0145818. [Google Scholar] [CrossRef]
- Landin, K.; Stigendal, L.; Eriksson, E.; Krotkiewski, M.; Risberg, B.; Tengborn, L.; Smith, U. Abdominal obesity is associated with an impaired fibrinolytic activity and elevated plasminogen activator inhibitor-1. Metabolism 1990, 39, 1044–1048. [Google Scholar] [CrossRef]
- Katzel, L.I.; Busby-Whitehead, M.J.; Goldberg, A.P. Adverse effects of abdominal obesity on lipoprotein lipids in healthy older men. Exp. Gerontol. 1993, 28, 411–420. [Google Scholar] [CrossRef]
- Strasser, B.; Arvandi, M.; Pasha, E.P.; Haley, A.P.; Stanforth, P.; Tanaka, H. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 495–502. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, P.; Sun, H.; Liu, Y.; Liu, D.; Zhou, Q.; Guo, C.; Li, Q.; Tian, G.; Wu, X.; et al. Metabolically healthy general and abdominal obesity are associated with increased risk of hypertension. Br. J. Nutr. 2021, 123, 583–591. [Google Scholar] [CrossRef]
- Krzesiński, P.; Stańczyk, A.; Piotrowicz, K.; Gielerak, G.; Uziębło-Zyczkowska, B.; Skrobowski, A. Abdominal obesity and hypertension: A double burden to the heart. Hypertens. Res. 2016, 39, 349–355. [Google Scholar] [CrossRef]
- Thumann, B.F.; Michels, N.; Felső, R.; Hunsberger, M.; Kaprio, J.; Moreno, L.A.; Siani, A.; Tornaritis, M.; Veidebaum, T.; De Henauw, S.; et al. Associations between sleep duration and insulin resistance in European children and adolescents considering the mediating role of abdominal obesity. PLoS ONE 2021, 15, e0235049. [Google Scholar] [CrossRef]
- Ross, R.; Després, J.P. Abdominal obesity, insulin resistance, and the metabolic syndrome: Contribution of physical activity/exercise. Obesity 2009, 17, S1–S2. [Google Scholar] [CrossRef]
- Velásquez-Rodríguez, C.M.; Velásquez-Villa, M.; Gómez-Ocampo, L.; Bermúdez-Cardona, J. Abdominal obesity and low physical activity are associated with insulin resistance in overweight adolescents: A cross-sectional study. BMC Pediatr. 2014, 14, 258. [Google Scholar] [CrossRef]
- Marchand, N.E.; Sparks, J.A.; Tedeschi, S.K.; Malspeis, S.; Costenbader, K.H.; Karlson, E.W.; Lu, B. Abdominal Obesity in Comparison with General Obesity and Risk of Developing Rheumatoid Arthritis in Women. J. Rheumatol. 2021, 48, 165–173. [Google Scholar] [CrossRef]
- Chan, J.M.; Rimm, E.B.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994, 17, 961–969. [Google Scholar] [CrossRef]
- Souza, F.A.; Dias, R.; Fernandes, C.E.; Pimentel, F.; Dias, D. Menstrual irregularity: A possible clinical marker of metabolic dysfunction in women with class III obesity. Gynecol. Endocrinol. 2010, 10, 768–772. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Vatten, L.J. Body mass index, abdominal fatness and the risk of gallbladder disease. Eur. J. Epidemiol. 2015, 9, 1009–1019. [Google Scholar] [CrossRef]
- Shojaee-Moradie, F.; Baynes, K.C.; Pentecost, C.; Bell, J.D.; Thomas, E.L.; Jackson, N.C.; Stolinski, M.; Whyte, M.; Lovell, D.; Bowes, S.B.; et al. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia 2007, 50, 404–413. [Google Scholar] [CrossRef]
- Keating, S.E.; Coombes, J.S.; Stowasser, M.; Bailey, T.G. The Role of Exercise in Patients with Obesity and Hypertension. Curr. Hypertens. Rep. 2021, 22, 77. [Google Scholar] [CrossRef]
- Collier, S.R.; Sandberg, K.; Moody, A.M.; Frechette, V.; Curry, C.D.; Ji, H.; Gowdar, R.; Chaudhuri, D.; Meucci, M. Reduction of plasma aldosterone and arterial stiffness in obese pre- and stage1 hypertensive subjects after aerobic exercise. J. Hum. Hypertens. 2015, 29, 53–57. [Google Scholar] [CrossRef]
- Aziz, C.B.; Omar, N.; Abdullah, W.Z.; Jalil, R.A.; Nik, W.S.; Zakaria, R. Reduced fibrinogen, fibrinolytic biomarkers, and physical parameters after a weight-loss program in obese subjects. N. Am. J. Med. Sci. 2014, 6, 377–382. [Google Scholar] [CrossRef]
- Lemmey, A.B.; Williams, S.L.; Marcora, S.M.; Jones, J.; Maddison, P.J. Are the benefits of a high-intensity progressive resistance training program sustained in rheumatoid arthritis patients? A 3-year follow up study. Arthritis Care Res. 2012, 64, 71–75. [Google Scholar] [CrossRef]
- Huang, M.H.; Chen, C.H.; Chen, T.W.; Weng, M.C.; Wang, W.T.; Wang, Y.L. The effects of weight reduction on the rehabilitation of patients with knee osteoarthritis and obesity. Arthritis Care Res. 2000, 13, 398–405. [Google Scholar] [CrossRef]
- Mena, G.P.; Mielke, G.I.; Brown, W.J. Prospective associations between physical activity and BMI with irregular periods and heavy menstrual bleeding in a large cohort of Australian women. Hum. Reprod. 2021, 36, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Martín-Moraleda, E.; Delisle, C.; Collado Mateo, D.; Aznar-Lain, S. Weight loss and body composition changes through ketogenic diet and physical activity: A methodological and systematic review. Nutr. Hosp. 2019, 36, 1196–1204. [Google Scholar] [PubMed]
- Farias Edos, S.; Gonçalves, E.M.; Morcillo, A.M.; Guerra-Júnior, G.; Amancio, O.M. Effects of programmed physical activity on body composition in post-pubertal schoolchildren. J. Pediatr. 2015, 91, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.H.; Slentz, C.A.; Bateman, L.A.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, W.E. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J. Appl. Physiol. 2012, 113, 1831–1837. [Google Scholar] [CrossRef]
- Westerterp, K.R. Exercise, energy balance and body composition. Eur. J. Clin. Nutr. 2018, 72, 1246–1250. [Google Scholar] [CrossRef]
- Yarizadeh, H.; Eftekhar, R.; Anjom-Shoae, J.; Speakman, J.R.; Djafarian, K. The Effect of Aerobic and Resistance Training and Combined Exercise Modalities on Subcutaneous Abdominal Fat: A Systematic Review and Meta-analysis of Randomized Clinical Trials. Adv. Nutr. 2021, 12, 179–196. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Dias, S.; Strasser, B.; Hoffmann, G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: A systematic review and network meta-analysis. PLoS ONE 2013, 8, e82853. [Google Scholar] [CrossRef]
- Coker, R.H.; William, R.H.; Kortebein, P.M.; Sullivan, D.H.; Evans, W.J. Influence of exercise intensity on abdominal fat and adiponectin in elderly adults. Metab. Syndr. Relat. Disord. 2009, 7, 363–368. [Google Scholar] [CrossRef]
- Ohkawara, K.; Tanaka, S.; Miyachi, M.; Ishikawa-Takata, K.; Tabata, I. A dose-response relation between aerobic exercise and visceral fat reduction: Systematic review of clinical trials. Int. J. Obes. 2007, 31, 1786–1797. [Google Scholar] [CrossRef]
- Okura, T.; Nakata, Y.; Lee, D.J.; Ohkawara, K.; Tanaka, K. Effects of aerobic exercise and obesity phenotype on abdominal fat reduction in response to weight loss. Int. J. Obes. 2005, 29, 1259–1266. [Google Scholar] [CrossRef]
- Vissers, D.; Hens, W.; Taeymans, J.; Baeyens, J.P.; Poortmans, J.; Van Gaal, L. The effect of exercise on visceral adipose tissue in overweight adults: A systematic review and meta-analysis. PLoS ONE 2013, 8, e56415. [Google Scholar] [CrossRef]
- Ibrahimi-Kaçuri, D.; Murtezani, A.; Rrecaj, S.; Martinaj, M.; Haxhiu, B. Low back pain and obesity. Med. Arch. 2015, 69, 114–116. [Google Scholar] [CrossRef]
- Melissas, J.; Volakakis, E.; Hadjipavlou, A. Low-back pain in morbidly obese patients and the effect of weight loss following surgery. Obes. Surg. 2003, 13, 389–393. [Google Scholar] [CrossRef]
- Vismara, L.; Menegoni, F.; Zaina, F.; Galli, M.; Negrini, S.; Capodaglio, P. Effect of obesity and low back pain on spinal mobility: A cross sectional study in women. J. Neuroeng. Rehabil. 2010, 18, 73. [Google Scholar] [CrossRef]
- Shiri, R.; Solovieva, S.; Husgafvel-Pursiainen, K.; Telama, R.; Yang, X.; Viikari, J.; Raitakari, O.T.; Viikari-Juntura, E. The role of obesity and physical activity in non-specific and radiating low back pain: The Young Finns study. Semin. Arthritis Rheum. 2013, 42, 640–650. [Google Scholar] [CrossRef]
- Leboeuf-Yde, C.; Kyvik, K.O.; Bruun, N.H. Low back pain and lifestyle. Part II—Obesity. Information from a population-based sample of 29,424 twin subjects. Spine 1999, 24, 779–783. [Google Scholar] [CrossRef]
- Cimolin, V.; Vismara, L.; Galli, M.; Zaina, F.; Negrini, S.; Capodaglio, P. Effects of obesity and chronic low back pain on gait. J. Neuroeng. Rehabil. 2011, 8, 55. [Google Scholar] [CrossRef]
- Bener, A.; Alwash, R.; Gaber, T.; Lovasz, G. Obesity and low back pain. Coll. Antropol. 2003, 27, 95–104. [Google Scholar]
- Mangwani, J.; Giles, C.; Mullins, M.; Salih, T.; Natali, C. Obesity and recovery from low back pain: A prospective study to investigate the effect of body mass index on recovery from low back pain. Ann. R Coll. Surg. Engl. 2010, 92, 23–26. [Google Scholar] [CrossRef]
- Deyo, R.A.; Bass, J.E. Lifestyle and low-back pain. The influence of smoking and obesity. Spine 1989, 14, 501–506. [Google Scholar] [CrossRef]
- Han, T.S.; Schouten, J.S.; Lean, M.E.; Seidell, J.C. The prevalence of low back pain and associations with body fatness, fat distribution and height. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 600–607. [Google Scholar] [CrossRef]
- Häuser, W.; Schmutzer, G.; Brähler, E.; Schiltenwolf, M.; Hilbert, A. The impact of body weight and depression on low back pain in a representative population sample. Pain Med. 2014, 15, 1316–1327. [Google Scholar] [CrossRef]
- Jannini, S.N.; Dória-Filh, U.; Damiani, D.; Silva, C.A. Musculoskeletal pain in obese adolescents. J. Pediatr 2011, 87, 329–335. [Google Scholar] [CrossRef][Green Version]
- Caberlon, C.F.; Padoin, A.V.; Mottin, C.C. Importance of musculoskeletal pain in work activities in obese individuals. Obes. Surg. 2013, 23, 2092–2095. [Google Scholar] [CrossRef]
- Stovitz, S.D.; Pardee, P.E.; Vazquez, G.; Duval, S.; Schwimmer, J.B. Musculoskeletal pain in obese children and adolescents. Acta Paediatr. 2008, 97, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, M.; Lindroos, A.K.; Torgerson, J.S. Musculoskeletal pain in the obese: A comparison with a general population and long-term changes after conventional and surgical obesity treatment. Pain 2003, 104, 549–557. [Google Scholar] [CrossRef]
- Tamin, T.Z.; Murdana, N.; Pitoyo, Y.; Safitri, E.D. Exercise Intervention for Chronic Pain Management, Muscle Strengthening, and Functional Score in Obese Patients with Chronic Musculoskeletal Pain: A Systematic Review and Meta-analysis. Acta Med. Indones. 2018, 50, 299–308. [Google Scholar] [PubMed]
- Sperotto, F.; Balzarin, M.; Parolin, M.; Monteforte, N.; Vittadello, F.; Zulian, F. Joint hypermobility, growing pain and obesity are mutually exclusive as causes of musculoskeletal pain in schoolchildren. Clin. Exp. Rheumatol. 2014, 32, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Tsuritani, I.; Honda, R.; Noborisaka, Y.; Ishida, M.; Ishizaki, M.; Yamada, Y. Impact of obesity on musculoskeletal pain and difficulty of daily movements in Japanese middle-aged women. Maturitas 2002, 42, 23–30. [Google Scholar] [CrossRef]
- Widhalm, H.K.; Seemann, R.; Hamboeck, M.; Mittlboeck, M.; Neuhold, A.; Friedrich, K.; Hajdu, S.; Widhalm, K. Osteoarthritis in morbidly obese children and adolescents, an age-matched controlled study. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Hartz, A.J.; Fischer, M.E.; Bril, G.; Kelber, S.; Rupley, D., Jr.; Oken, B.; Rimm, A.A. The association of obesity with joint pain and osteoarthritis in the HANES data. J. Chronic. Dis. 1986, 39, 311–319. [Google Scholar] [CrossRef]
- Alfieri, F.M.; Silva, N.C.O.V.E.; Battistella, L.R. Study of the relation between body weight and functional limitations and pain in patients with knee osteoarthritis. Einstein 2017, 15, 307–312. [Google Scholar] [CrossRef]
- Goulston, L.M.; Kiran, A.; Javaid, M.K.; Soni, A.; White, K.M.; Hart, D.J.; Spector, T.D.; Arden, N.K. Does obesity predict knee pain over fourteen years in women, independently of radiographic changes? Arthritis Care Res. 2011, 63, 1398–1406. [Google Scholar] [CrossRef]
- Brandt, K.D.; Heilman, D.K.; Slemenda, C.; Katz, B.P.; Mazzuca, S.; Braunstein, E.M.; Byrd, D. A comparison of lower extremity muscle strength, obesity, and depression scores in elderly subjects with knee pain with and without radiographic evidence of knee osteoarthritis. J. Rheumatol. 2000, 27, 1937–1946. [Google Scholar]
- Miller, G.D.; Nicklas, B.J.; Loeser, R.F. Inflammatory biomarkers and physical function in older, obese adults with knee pain and self-reported osteoarthritis after intensive weight-loss therapy. J. Am. Geriatr. Soc. 2008, 56, 644–651. [Google Scholar] [CrossRef]
- Tanamas, S.K.; Wluka, A.E.; Davies-Tuck, M.; Wang, Y.; Strauss, B.J.; Proietto, J.; Dixon, J.B.; Jones, G.; Forbes, A.; Cicuttini, F.M. Association of weight gain with incident knee pain, stiffness, and functional difficulties: A longitudinal study. Arthritis Care Res. 2013, 65, 34–43. [Google Scholar] [CrossRef]
- Schwarze, M.; Häuser, W.; Schmutzer, G.; Brähler, E.; Beckmann, N.A.; Schiltenwolf, M. Obesity, depression and hip pain. Musculoskelet. Care 2019, 17, 126–132. [Google Scholar] [CrossRef]
- Tanamas, S.K.; Wluka, A.E.; Berry, P.; Menz, H.B.; Strauss, B.J.; Davies-Tuck, M.; Proietto, J.; Dixon, J.B.; Jones, G.; Cicuttini, F.M. Relationship between obesity and foot pain and its association with fat mass, fat distribution, and muscle mass. Arthritis Care Res. 2012, 64, 262–268. [Google Scholar] [CrossRef]
- Mekhail, N.; Mehanny, D.; Armanyous, S.; Saweris, Y.; Costandi, S. The impact of obesity on the effectiveness of spinal cord stimulation in chronic spine-related pain patients. Spine J. 2019, 19, 476–486. [Google Scholar] [CrossRef]
- Bigal, M.E.; Liberman, J.N.; Lipton, R.B. Obesity and migraine: A population study. Neurology 2006, 66, 545–550. [Google Scholar] [CrossRef]
- Bigal, M.E.; Gironda, M.; Tepper, S.J.; Feleppa, M.; Rapoport, A.M.; Sheftell, F.D.; Lipton, R.B. Headache prevention outcome and body mass index. Cephalalgia 2006, 26, 445–450. [Google Scholar] [CrossRef]
- Kristoffersen, E.S.; Børte, S.; Hagen, K.; Zwart, J.A.; Winsvold, B.S. Migraine, obesity and body fat distribution—A population-based study. J. Headache Pain 2021, 21, 97. [Google Scholar] [CrossRef]
- Trovato, G.; Brischetto, D.; Pace, P.; Fabio Martines, G. Perceived body weight status of youngsters interferes with headache in obese and non-obese subjects. Headache 2014, 54, 1062–1063. [Google Scholar] [CrossRef]
- Cha, N.C.; Scher, A.I.; Moghekar, A.; Bond, D.S.; Peterlin, B.L. Perceived body weight status of youngsters interferes with headache in obese and non-obese subjects: A response. Headache 2014, 54, 1063–1065. [Google Scholar] [CrossRef]
- Farris, S.G.; Thomas, J.G.; Abrantes, A.M.; Lipton, R.B.; Pavlovic, J.; Smitherman, T.A.; Irby, M.B.; Penzien, D.B.; Roth, J.; O’Leary, K.C.; et al. Pain worsening with physical activity during migraine attacks in women with overweight/obesity: A prospective evaluation of frequency, consistency, and correlates. Cephalalgia 2018, 38, 1707–1715. [Google Scholar] [CrossRef]
- Afshinmajd, S.; Davati, A.; Akbari, F. The effects of body mass index on the treatment of the patients with migraine headaches. Iran. J. Neurol. 2011, 10, 35–38. [Google Scholar]
- Saloom, H.F.; Papageorgiou, S.N.; Carpenter, G.H.; Cobourne, M.T. The effect of obesity on orofacial pain during early orthodontic treatment with fixed appliances: A prospective cohort study. Eur. J. Orthod. 2018, 40, 343–349. [Google Scholar] [CrossRef]
- Bonato, R.C.S.; Mapengo, M.A.A.; de Azevedo-Silva, L.J.; Janson, G.; de Carvalho Sales-Peres, S.H. Tooth movement, orofacial pain, and leptin, interleukin-1beta, and tumor necrosis factor-alpha levels in obese adolescents. Angle Orthod. 2021, 1–6. [Google Scholar] [CrossRef]
- Balderas-Peña, L.M.; Macías-López, G.G.; Zepeda-González, A.; González-Hernández, I.; Herrera-Rodríguez, R.; Fafutis-Morris, M. Association between obesity, gender and preoperative inflammatory markers with postsurgical pain in live kidney donors. Cir. Cir. 2011, 79, 526–533. [Google Scholar] [PubMed]
- Paley, C.A.; Johnson, M.I. Physical Activity to Reduce Systemic Inflammation Associated with Chronic Pain and Obesity: A Narrative Review. Clin. J. Pain. 2016, 32, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Zdziarski, L.A.; Wasser, J.G.; Vincent, H.K. Chronic pain management in the obese patient: A focused review of key challenges and potential exercise solutions. J. Pain Res. 2015, 8, 63–77. [Google Scholar]
- Wasser, J.G.; Vasilopoulos, T.; Zdziarski, L.A.; Vincent, H.K. Exercise Benefits for Chronic Low Back Pain in Overweight and Obese Individuals. PM&R 2017, 9, 181–192. [Google Scholar]
- Perez-Huerta, B.D.; Díaz-Pulido, B.; Pecos-Martin, D.; Beckwee, D.; Lluch-Girbes, E.; Fernandez-Matias, R.; Rubio, M.J.B.; Gallego-Izquierdo, T. Effectiveness of a Program Combining Strengthening, Stretching, and Aerobic Training Exercises in a Standing versus a Sitting Position in Overweight Subjects with Knee Osteoarthritis: A Randomized Controlled Trial. J. Clin. Med. 2021, 9, 4113. [Google Scholar] [CrossRef]
- White, D.K.; Neogi, T.; Rejeski, W.J.; Walkup, M.P.; Lewis, C.E.; Nevitt, M.C.; Foy, C.G.; Felson, D.T.; Look Ahead Research Group. Can an intensive diet and exercise program prevent knee pain among overweight adults at high risk? Arthritis Care Res. 2015, 67, 965–971. [Google Scholar] [CrossRef]
- Irandoust, K.; Taheri, M. The effects of aquatic exercise on body composition and nonspecific low back pain in elderly males. J. Phys. Ther. Sci. 2015, 27, 433–435. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Theriault, R.; Watkins, S.C.; Kelley, D.E. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 2000, 49, 467–472. [Google Scholar] [CrossRef]
- Malenfant, P.; Joanisse, D.R.; The’riault, R.; Goodpaster, B.H.; Kelley, D.E.; Simoneau, J.A. Fat content in individual muscle fibers of lean and obese subjects. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1316–1321. [Google Scholar] [CrossRef]
- Velan, S.S.; Said, N.; Durst, C.; Frisbee, S.; Frisbee, J.; Raylman, R.R.; Thomas, M.A.; Rajendran, V.M.; Spencer, R.G.; Alway, S.E. Distinct patterns of fat metabolism in skeletal muscle of normal-weight, overweight, and obese humans. Am. J. Physiol Regul. Integr. Comp. Physiol. 2008, 295, R1060–R1065. [Google Scholar] [CrossRef][Green Version]
- Morris, R.T.; Laye, M.J.; Lees, S.J.; Rector, R.S.; Thyfault, J.P.; Booth, F.W. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J. Appl. Physiol. 2008, 104, 708–715. [Google Scholar] [CrossRef]
- Ingram, K.H.; Hill, H.; Moellering, D.R.; Hill, B.G.; Lara-Castro, C.; Newcomer, B.; Brandon, L.J.; Ingalls, C.P.; Penumetcha, M.; Rupp, J.C.; et al. Skeletal muscle lipid peroxidation and insulin resistance in humans. J. Clin. Endocrinol. Metab. 2012, 97, E1182–E1186. [Google Scholar] [CrossRef]
- Di Meo, S.; Iossa, S.; Venditti, P. Skeletal muscle insulin resistance: Role of mitochondria and other ROS sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef] [PubMed]
- Thiebaud, D.; Jacot, E.; DeFronzo, R.A.; Maeder, E.; Jequier, E.; Felber, J.P. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes 1982, 31, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Soulage, C.O.; Sardón Puig, L.; Soulère, L.; Zarrouki, B.; Guichardant, M.; Lagarde, M.; Pillon, N.J. Skeletal muscle insulin resistance is induced by 4-hydroxy-2-hexenal, a by-product of n-3 fatty acid peroxidation. Diabetologia 2018, 61, 688–699. [Google Scholar] [CrossRef]
- Pillon, N.J.; Croze, M.L.; Vella, R.E.; Soulère, L.; Lagarde, M.; Soulage, C.O. The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology 2012, 153, 2099–2111. [Google Scholar] [CrossRef]
- Funai, K.; Song, H.; Yin, L.; Lodhi, I.J.; Wei, X.; Yoshino, J.; Coleman, T.; Semenkovich, C.F. Muscle lipogenesis balances insulin sensitivity and strength through calcium signaling. J. Clin. Invest. 2013, 123, 1229–1240. [Google Scholar] [CrossRef]
- Funai, K.; Lodhi, I.J.; Spears, L.D.; Yin, L.; Song, H.; Klein, S.; Semenkovich, C.F. Skeletal Muscle Phospholipid Metabolism Regulates Insulin Sensitivity and Contractile Function. Diabetes 2016, 65, 358–370. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef]
- Seebacher, F.; Tallis, J.; McShea, K.; James, R.S. Obesity-induced decreases in muscle performance are not reversed by weight loss. Int J. Obes. 2017, 41, 1271–1278. [Google Scholar] [CrossRef]
- Hilton, T.N.; Tuttle, L.J.; Bohnert, K.L.; Mueller, M.J.; Sinacore, D.R. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: Association with performance and function. Phys. Ther. 2008, 88, 1336–1344. [Google Scholar] [CrossRef]
- Bittel, D.C.; Bittel, A.J.; Tuttle, L.J.; Hastings, M.K.; Commean, P.K.; Mueller, M.J.; Cade, W.T.; Sinacore, D.R. Adipose tissue content, muscle performance and physical function in obese adults with type 2 diabetes mellitus and peripheral neuropathy. J. Diabetes Complicat. 2015, 29, 250–257. [Google Scholar] [CrossRef][Green Version]
- Sachs, S.; Zarini, S.; Kahn, D.E.; Harrison, K.A.; Perreault, L.; Phang, T.; Newsom, S.A.; Strauss, A.; Kerege, A.; Schoen, J.A.; et al. Intermuscular adipose tissue directly modulates skeletal muscle insulin sensitivity in humans. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E866–E879. [Google Scholar] [CrossRef]
- Griffin, M.E.; Marcucci, M.J.; Cline, G.W.; Bell, K.; Barucci, N.; Lee, D.; Goodyear, L.J.; Kraegen, E.W.; White, M.F.; Shulman, G.I. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 1999, 48, 1270–1274. [Google Scholar] [CrossRef]
- Yu, C.; Chen, Y.; Cline, G.W.; Zhang, D.; Zong, H.; Wang, Y.; Bergeron, R.; Kim, J.K.; Cushman, S.W.; Cooney, G.J.; et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002, 277, 50230–50236. [Google Scholar] [CrossRef]
- Itani, S.I.; Ruderman, N.B.; Schmieder, F.; Boden, G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002, 51, 2005–2011. [Google Scholar] [CrossRef]
- Szendroedi, J.; Yoshimura, T.; Phielix, E.; Koliaki, C.; Marcucci, M.; Zhang, D.; Jelenik, T.; Müller, J.; Herder, C.; Nowotny, P.; et al. Role of diacylglycerol activation of PKCteta in lipid-induced muscle insulin resistance in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 9597–9602. [Google Scholar] [CrossRef]
- Timmers, S.; Schrauwen, P.; de Vogel, J. Muscular diacylglycerol metabolism and insulin resistance. Physiol. Behav. 2008, 94, 242–251. [Google Scholar] [CrossRef]
- Haam, J.H.; Kim, Y.S.; Koo, H.S.; Haam, J.; Seo, N.K.; Kim, H.Y.; Park, K.C.; Park, K.S.; Kim, M.J. Intermuscular adipose tissue is associated with monocyte chemoattractant protein-1, independent of visceral adipose tissue. Clin. Biochem. 2016, 49, 439–443. [Google Scholar] [CrossRef]
- Khan, I.M.; Perrard, X.Y.; Brunner, G.; Lui, H.; Sparks, L.M.; Smith, S.R.; Wang, X.; Shi, Z.Z.; Lewis, D.E.; Wu, H.; et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int J. Obes. 2015, 39, 1607–1618. [Google Scholar] [CrossRef]
- Ma, J.; Yu, S.; Wang, F.; Bai, L.; Xiao, J.; Jiang, Y.; Chen, L.; Wang, J.; Jiang, A.; Li, M.; et al. MicroRNA transcriptomes relate intermuscular adipose tissue to metabolic risk. Int J. Mol. Sci. 2013, 14, 8611–8624. [Google Scholar] [CrossRef]
- Gao, Y.J.; Lu, C.; Su, L.Y.; Sharma, A.M.; Lee, R.M.K.W. Modulation of vascular function by perivascular adipose tissue: The role of endothelium and hydrogen peroxide. Br. J. Pharmacol. 2007, 151, 323–331. [Google Scholar] [CrossRef]
- Brown, N.K.; Zhou, Z.; Zhang, J.; Zeng, R.; Wu, J.; Eitzman, D.T.; Chen, Y.E.; Chang, L. Perivascular adipose tissue in vascular function and disease: A review of current research and animal models. Arter. Thromb. Vasc. Biol. 2014, 34, 1621–1630. [Google Scholar] [CrossRef]
- Gil-Ortega, M.; Somoza, B.; Huang, Y.; Gollasch, M.; Fernández-Alfonso, M.S. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol. Metab. 2015, 26, 367–375. [Google Scholar] [CrossRef]
- Xia, N.; Li, H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br. J. Pharmacol. 2017, 174, 3425–3442. [Google Scholar] [CrossRef]
- Grigoras, A.; Amalinei, C.; Balan, R.A.; Giusca, S.E.; Caruntu, I.D. Perivascular adipose tissue in cardiovascular diseases—An update. Anatol. J. Cardiol. 2019, 22, 219–231. [Google Scholar] [CrossRef]
- Saxton, S.N.; Clark, B.J.; Withers, S.B.; Eringa, E.C.; Heagerty, A.M. Mechanistic Links Between Obesity, Diabetes, and Blood Pressure: Role of Perivascular Adipose Tissue. Physiol. Rev. 2019, 99, 1701–1763. [Google Scholar] [CrossRef]
- Ramirez, J.G.; O’Malley, E.J.; Ho, W.S.V. Pro-contractile effects of perivascular fat in health and disease. Br. J. Pharmacol. 2017, 174, 3482–3495. [Google Scholar] [CrossRef]
- Chang, L.; Garcia-Barrio, M.T.; Chen, Y.E. Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2021, 40, 1094–1109. [Google Scholar] [CrossRef]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef]
- Cheng, C.K.; Bakar, H.A.; Gollasch, M.; Huang, Y. Perivascular Adipose Tissue: The Sixth Man of the Cardiovascular System. Cardiovasc. Drugs Ther. 2018, 32, 481–502. [Google Scholar] [CrossRef]
- Kangawa, K.; Kitamura, K.; Minamino, N.; Matsuo, H. Adrenomedullin: A new modulator of vascular tone. J. Card. Fail. 1996, 2, S135–S140. [Google Scholar] [CrossRef]
- Schroeter, M.R.; Eschholz, N.; Herzberg, S.; Jerchel, I.; Leifheit-Nestler, M.; Czepluch, F.S.; Chalikia, G.; Konstantinides, S.; Schafer, K. Leptin-dependent and leptin-independent paracrine effects of perivascular adipose tissue on neointima formation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Reidy, M.A.; Jackson, C.L. Factors controlling growth of arterial cells following injury. Toxicol. Pathol. 1990, 18, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballantyne, L.L.; Yu, Y.; Funk, C.D. Perivascular adipose tissue-derived extracellular vesicle miR-221–3p mediates vascular remodeling. FASEB J. 2019, 33, 12704–12722. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.H.; Egner, I.; Dong, Y.; Porter, E.L.; Heagerty, A.M.; Edwards, G. Stimulated release of a hyperpolarizing factor (ADHF) from mesenteric artery perivascular adipose tissue: Involvement of myocyte BKCa channels and adiponectin. Br. J. Pharmacol. 2013, 169, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Uemura, Y.; Shibata, R.; Ohashi, K.; Enomoto, T.; Kambara, T.; Yamamoto, T.; Ogura, Y.; Yuasa, D.; Joki, Y.; Matsuo, K.; et al. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J. 2013, 27, 25–33. [Google Scholar] [CrossRef]
- Butcher, M.J.; Waseem, T.C.; Galkina, E.V. Smooth muscle cell derived interleukin-17C plays an atherogenic role via the recruitment of proinflammatory interleukin-17A+ T cells to the aorta. Arter. Thromb. Vasc. Biol. 2016, 36, 1496–1506. [Google Scholar] [CrossRef]
- Xia, N.; Horke, S.; Habermeier, A.; Closs, E.I.; Reifenberg, G.; Gericke, A.; Mikhed, Y.; Münzel, T.; Daiber, A.; Förstermann, U.; et al. Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 78–85. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wu, J.; Guo, W.; Zhu, Y.Z. Atherosclerosis and the Hydrogen Sulfide Signaling Pathway—Therapeutic Approaches to Disease Prevention. Cell. Physiol. Biochem. 2017, 42, 859–875. [Google Scholar] [CrossRef]
- Bussey, C.E.; Withers, S.B.; Aldous, R.G.; Edwards, G.; Heagerty, A.M. Obesity-Related Perivascular Adipose Tissue Damage Is Reversed by Sustained Weight Loss in the Rat. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1377–1385. [Google Scholar] [CrossRef]
- Henrichot, E.; Juge-Aubry, C.E.; Pernin, A.; Pache, J.C.; Velebit, V.; Dayer, J.M.; Meda, P.; Chizzolini, C.; Meier, C.A. Production of chemokines by perivascular adipose tissue: A role in the pathogenesis of atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2594–2599. [Google Scholar] [CrossRef]
- Xia, N.; Weisenburger, S.; Koch, E.; Burkart, M.; Reifenberg, G.; Förstermann, U.; Li, H. Restoration of perivascular adipose tissue function in diet-induced obese mice without changing bodyweight. Br. J. Pharmacol. 2017, 174, 3443–3453. [Google Scholar] [CrossRef]
- Mazzotta, C.; Basu, S.; Gower, A.C.; Karki, S.; Farb, M.G.; Sroczynski, E.; Zizza, E.; Sarhan, A.; Pande, A.N.; Walsh, K.; et al. Perivascular Adipose Tissue Inflammation in Ischemic Heart Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1239–1250. [Google Scholar] [CrossRef]
- Munjal, A.; Khandia, R. Atherosclerosis: Orchestrating cells and biomolecules involved in its activation and inhibition. Adv. Protein Chem. Struct. Biol. 2021, 120, 85–122. [Google Scholar]
- Xu, H.; Jiang, J.; Chen, W.; Li, W.; Chen, Z. Vascular Macrophages in Atherosclerosis. J. Immunol. Res. 2019, 2019, 4354786. [Google Scholar] [CrossRef]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef]
- Zhang, K.; Gharaee-Kermani, M.; McGarry, B.; Remick, D.; Phan, S.H. TNF-alpha-mediated lung cytokine networking and eosinophil recruitment in pulmonary fibrosis. J. Immunol. 1997, 158, 954–959. [Google Scholar]
- Lukacs, N.W.; Strieter, R.M.; Chensue, S.W.; Widmer, M.; Kunkel, S.L. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J. Immunol. 1995, 154, 5411–5417. [Google Scholar]
- Lampinen, M.; Carlson, M.; Sangfelt, P.; Taha, Y.; Thörn, M.; Lööf, L.; Raab, Y.; Venge, P. IL-5 and TNF-alpha participate in recruitment of eosinophils to intestinal mucosa in ulcerative colitis. Dig. Dis. Sci. 2001, 46, 2004–2009. [Google Scholar] [CrossRef]
- Marx, C.; Novotny, J.; Salbeck, D.; Zellner, K.R.; Nicolai, L.; Pekayvaz, K.; Kilani, B.; Stockhausen, S.; Bürgener, N.; Kupka, D.; et al. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood 2019, 134, 1859–1872. [Google Scholar] [CrossRef]
- Ouwens, D.M.; Sell, H.; Greulich, S.; Eckel, J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J. Cell. Mol. Med. 2010, 14, 2223–2234. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 460–466. [Google Scholar] [CrossRef]
- Baker, A.R.; da Silva, N.F.; Quinn, D.W.; Harte, A.L.; Pagano, D.; Bonser, R.S.; Kumar, S.; McTernan, P.G. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc. Diabetol. 2006, 5, 1. [Google Scholar] [CrossRef]
- Song, D.K.; Hong, Y.S.; Lee, H.; Oh, J.Y.; Sung, Y.A.; Kim, Y. Increased Epicardial Adipose Tissue Thickness in Type 2 Diabetes Mellitus and Obesity. Diabetes Metab. J. 2015, 39, 405–413. [Google Scholar] [CrossRef][Green Version]
- Aitken-Buck, H.M.; Moharram, M.; Babakr, A.A.; Reijers, R.; Van Hout, I.; Fomison-Nurse, I.C.; Sugunesegran, R.; Bhagwat, K.; Davis, P.J.; Bunton, R.W.; et al. Relationship between epicardial adipose tissue thickness and epicardial adipocyte size with increasing body mass index. Adipocyte 2019, 8, 412–420. [Google Scholar] [CrossRef]
- Eren, E.; Koca, B.; Ture, M.; Guzel, B. Epicardial adiposity in children with obesity and metabolic syndrome. Iran. J. Pediatr. 2014, 24, 411–417. [Google Scholar]
- Boyraz, M.; Pirgon, O.; Akyol, B.; Dundar, B.; Cekmez, F.; Eren, N. Importance of epicardial adipose tissue thickness measurement in obese adolescents, its relationship with carotid intima-media thickness, and echocardiographic findings. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 3309–3317. [Google Scholar]
- Akyol, B.; Boyraz, M.; Aysoy, C. Relationship of epicardial adipose tissue thickness with early indicators of atherosclerosis and cardiac functional changes in obese adolescents with metabolic syndrome. J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 156–163. [Google Scholar]
- Fu, C.P.; Sheu, W.H.; Lee, I.T.; Tsai, I.C.; Lee, W.J.; Liang, K.W.; Lee, W.L.; Lin, S.Y. Effects of weight loss on epicardial adipose tissue thickness and its relationship between serum soluble CD40 ligand levels in obese men. Clin. Chim. Acta 2013, 421, 98–103. [Google Scholar] [CrossRef]
- Chumakova, G.; Gritsenko, O.; Gruzdeva, O.; Dyleva, Y. Analysis of probable lipotoxic damage and myocardial fibrosis in epicardial obesity. Aging 2021, 13, 14806–14815. [Google Scholar] [CrossRef] [PubMed]
- Kankaanpää, M.; Lehto, H.R.; Pärkkä, J.P.; Komu, M.; Viljanen, A.; Ferrannini, E.; Knuuti, J.; Nuutila, P.; Parkkola, R.; Iozzo, P. Myocardial triglyceride content and epicardial fat mass in human obesity: Relationship to left ventricular function and serum free fatty acid levels. J. Clin. Endocrinol. Metab. 2006, 91, 4689–4695. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Montoliu, I.; Qanadli, S.D.; Collino, S.; Rezzi, S.; Kussmann, M.; Giusti, V.; Martin, F.P. Blood plasma lipidomic signature of epicardial fat in healthy obese women. Obesity 2015, 23, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; Heuer, S.; Meder, K.; Boehler, J.; Lange, V.; Quaschning, T.; Ertl, G.; Bonz, A. The proinflammatory cytokines TNF-alpha and IL-1 beta impair economy of contraction in human myocardium. Cytokine 2007, 39, 157–162. [Google Scholar] [CrossRef]
- Abe, I.; Teshima, Y.; Kondo, H.; Kaku, H.; Kira, S.; Ikebe, Y.; Saito, S.; Fukui, A.; Shinohara, T.; Yufu, K.; et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm. 2018, 15, 1717–1727. [Google Scholar] [CrossRef]
- Yu, M.; Wen, S.; Wang, M.; Liang, W.; Li, H.H.; Long, Q.; Guo, H.P.; Liao, Y.H.; Yuan, J. TNF-alpha-secreting B cells contribute to myocardial fibrosis in dilated cardiomyopathy. J. Clin. Immunol. 2013, 33, 1002–1008. [Google Scholar] [CrossRef]
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clément, K.; et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 2015, 36, 795–805a. [Google Scholar] [CrossRef]
- Han, L.; Liu, J.; Zhu, L.; Tan, F.; Qin, Y.; Huang, H.; Yu, Y. Free fatty acid can induce cardiac dysfunction and alter insulin signaling pathways in the heart. Lipids Health Dis. 2018, 17, 185. [Google Scholar] [CrossRef]
- Toemen, L.; Santos, S.; Roest, A.A.W.; Vernooij, M.W.; Helbing, W.A.; Gaillard, R.; Jaddoe, V.W.V. Pericardial adipose tissue, cardiac structures, and cardiovascular risk factors in school-age children. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 307–313. [Google Scholar] [CrossRef]
- Gill, C.M.; Azevedo, D.C.; Oliveira, A.L.; Martinez-Salazar, E.L.; Torriani, M.; Bredella, M.A. Sex differences in pericardial adipose tissue assessed by PET/CT and association with cardiometabolic risk. Acta Radiol. 2018, 59, 1203–1209. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, S.J.; Wu, T.W.; Lin, H.J.; Chen, J.W.; Mersmann, H.J.; Ding, S.T.; Chen, C.Y. The role of pericardial adipose tissue in the heart of obese minipigs. Eur. J. Clin. Invest. 2018, 48, e12942. [Google Scholar] [CrossRef]
- Li, S.J.; Wu, T.W.; Chien, M.J.; Mersmann, H.J.; Chen, C.Y. Involvement of pericardial adipose tissue in cardiac fibrosis of dietary-induced obese minipigs—Role of mitochondrial function. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 2019, 1864, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Pucci, G.; Battista, F.; de Vuono, S.; Boni, M.; Scavizzi, M.; Ricci, M.A.; Lupattelli, G.; Schillaci, G. Pericardial fat, insulin resistance, and left ventricular structure and function in morbid obesity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, S.W.; Lee, J.S.; Lee, S.K.; Abbott, R.; Lee, K.Y.; Lim, H.E.; Sung, K.C.; Cho, G.Y.; Koh, K.K.; et al. Association of pericardial adipose tissue with left ventricular structure and function: A region-specific effect? Cardiovasc. Diabetol. 2021, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Goumans, M.J.; Ten Dijke, P. TGF-beta Signaling in Control of Cardiovascular Function. Cold Spring Harb. Perspect. Biol. 2018, 10, a022210. [Google Scholar] [CrossRef]
- Gallini, R.; Lindblom, P.; Bondjers, C.; Betsholtz, C.; Andrae, J. PDGF-A and PDGF-B induces cardiac fibrosis in transgenic mice. Exp. Cell Res. 2016, 349, 282–290. [Google Scholar] [CrossRef]
- Kang, S.; Chemaly, E.R.; Hajjar, R.J.; Lebeche, D. Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J. Biol. Chem. 2011, 286, 18465–18473. [Google Scholar] [CrossRef]
- Kim, M.; Oh, J.K.; Sakata, S.; Liang, I.; Park, W.; Hajjar, R.J.; Lebeche, D. Role of resistin in cardiac contractility and hypertrophy. J. Mol. Cell Cardiol. 2008, 45, 270–280. [Google Scholar] [CrossRef]
- Karmazyn, M.; Rajapurohitam, V. Leptin as a cardiac pro-hypertrophic factor and its potential role in the development of heart failure. Curr. Pharm. Des. 2014, 20, 646–651. [Google Scholar] [CrossRef]
- Ren, J. Leptin and hyperleptinemia—From friend to foe for cardiovascular function. J. Endocrinol. 2004, 181, 1–10. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Younossi, Z.M. Obesity-associated nonalcoholic fatty liver disease. Clin. Liver Dis. 2014, 18, 19–31. [Google Scholar] [CrossRef]
- Bellentani, S.; Saccoccio, G.; Masutti, F.; Crocè, L.S.; Brandi, G.; Sasso, F.; Cristanini, G.; Tiribelli, C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann. Intern. Med. 2000, 132, 112–117. [Google Scholar] [CrossRef]
- Santoro, N.; Feldstein, A.E.; Enoksson, E.; Pierpont, B.; Kursawe, R.; Kim, G.; Caprio, S. The association between hepatic fat content and liver injury in obese children and adolescents: Effects of ethnicity, insulin resistance, and common gene variants. Diabetes Care 2013, 36, 1353–1360. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Brunt, E.M.; Kleiner, D.E.; Kowdley, K.V.; Chalasani, N.; Lavine, J.E.; Ratziu, V.; Mccullough, A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011, 54, 344–353. [Google Scholar] [CrossRef]
- Lian, C.Y.; Zhai, Z.Z.; Li, Z.F.; Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem. Biol. Interact. 2021, 330, 109199. [Google Scholar] [CrossRef]
- Al-Dayyat, H.M.; Rayyan, Y.M.; Tayyem, R.F. Non-alcoholic fatty liver disease and associated dietary and lifestyle risk factors. Diabetes Metab. Syndr. 2018, 12, 569–575. [Google Scholar] [CrossRef]
- Lu, F.B.; Hu, E.D.; Xu, L.M.; Chen, L.; Wu, J.L.; Li, H.; Chen, D.Z.; Chen, Y.P. The relationship between obesity and the severity of non-alcoholic fatty liver disease: Systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 491–502. [Google Scholar] [CrossRef]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus Panel. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2021, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2021, 73, 202–209. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Mantzoros, C.S. Making progress in nonalcoholic fatty liver disease (NAFLD) as we are transitioning from the era of NAFLD to dys-metabolism associated fatty liver disease (DAFLD). Metabolism 2021, 111, 154318. [Google Scholar] [CrossRef]
- Makri, E.; Goulas, A.; Polyzos, S.A. Epidemiology, Pathogenesis, Diagnosis and Emerging Treatment of Nonalcoholic Fatty Liver Disease. Arch. Med. Res. 2021, 52, 25–37. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Koo, S.H. Nonalcoholic fatty liver disease: Molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 2013, 19, 210–215. [Google Scholar] [CrossRef]
- Wang, G.; Bonkovsky, H.L.; de Lemos, A.; Burczynski, F.J. Recent insights into the biological functions of liver fatty acid binding protein 1. J. Lipid Res. 2015, 56, 2238–2247. [Google Scholar] [CrossRef]
- Sanders, F.W.; Griffin, J.L. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol. Rev. Camb. Philos. Soc. 2016, 91, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Eberle, D.; Hegarty, B.; Bossard, P.; Ferre, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Takenoshita, M.; Sakurai, M.; Bruick, R.K.; Henzel, W.J.; Shillinglaw, W.; Arnot, D.; Uyeda, K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. USA 2001, 98, 9116–9121. [Google Scholar] [CrossRef] [PubMed]
- Kohjima, M.; Enjoji, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; et al. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2007, 20, 351–358. [Google Scholar] [CrossRef]
- Kohjima, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; Enjoji, M.; et al. SREBP-1c. regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2008, 21, 507–511. [Google Scholar] [CrossRef]
- Benhamed, F.; Denechaud, P.D.; Lemoine, M.; Robichon, C.; Moldes, M.; Bertrand-Michel, J.; Ratziu, V.; Serfaty, L.; Housset, C.; Capeau, J.; et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Invest. 2012, 122, 2176–2194. [Google Scholar] [CrossRef]
- Bril, F.; Barb, D.; Portillo-Sanchez, P.; Biernacki, D.; Lomonaco, R.; Suman, A.; Weber, M.H.; Budd, J.T.; Lupi, M.E.; Cusi, K. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology 2017, 65, 1132–1144. [Google Scholar] [CrossRef]
- Korenblat, K.M.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 2008, 134, 1369–1375. [Google Scholar] [CrossRef]
- Chen, Z.; Han, C.K.; Pan, L.L.; Zhang, H.J.; Ma, Z.M.; Huang, Z.F.; Chen, S.; Zhuang, X.J.; Li, Z.B.; Li, X.Y.; et al. Serum alanine aminotransferase independently correlates with intrahepatic triglyceride contents in obese subjects. Dig. Dis. Sci. 2014, 59, 2470–2476. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, X.F.; Ma, Z.M.; Pan, L.L.; Chen, Z.; Han, H.W.; Han, C.K.; Zhuang, X.J.; Lu, Y.; Li, X.J.; et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J. Hepatol. 2013, 59, 557–562. [Google Scholar] [CrossRef]
- Flisiak-Jackiewicz, M.; Bobrus-Chociej, A.; Wasilewska, N.; Tarasow, E.; Wojtkowska, M.; Lebensztejn, D.M. Can hepatokines be regarded as novel non-invasive serum biomarkers of intrahepatic lipid content in obese children? Adv. Med. Sci. 2019, 64, 280–284. [Google Scholar] [CrossRef]
- Tang, H.; Yu, R.; Liu, S.; Huwatibieke, B.; Li, Z.; Zhang, W. Irisin Inhibits Hepatic Cholesterol Synthesis via AMPK-SREBP2 Signaling. EBioMedicine 2016, 6, 139–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, T.; Huang, J.F.; Wang, S.B.; Zhou, L.L.; Yang, Z.Q.; Chen, X.D. The link between fibroblast growth factor 21 and sterol regulatory element binding protein 1c during lipogenesis in hepatocytes. Mol. Cell. Endocrinol. 2011, 342, 41–47. [Google Scholar] [CrossRef]
- Novotny, D.; Vaverkova, H.; Karasek, D.; Lukes, J.; Slavik, L.; Malina, P.; Orsag, J. Evaluation of total adiponectin, adipocyte fatty acid binding protein and fibroblast growth factor 21 levels in individuals with metabolic syndrome. Physiol. Res. 2014, 63, 219–228. [Google Scholar] [CrossRef]
- Maggio, A.B.; Mueller, P.; Wacker, J.; Viallon, M.; Belli, D.C.; Beghetti, M.; Farpour-Lambert, N.J.; McLin, V.A. Increased pancreatic fat fraction is present in obese adolescents with metabolic syndrome. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 720–726. [Google Scholar] [CrossRef]
- Rossi, A.P.; Fantin, F.; Zamboni, G.A.; Mazzali, G.; Rinaldi, C.A.; Del Giglio, M.; Di Francesco, V.; Barillari, M.; Pozzi Mucelli, R.; Zamboni, M. Predictors of ectopic fat accumulation in liver and pancreas in obese men and women. Obesity 2011, 19, 1747–1754. [Google Scholar] [CrossRef]
- Targher, G.; Rossi, A.P.; Zamboni, G.A.; Fantin, F.; Antonioli, A.; Corzato, F.; Bambace, C.; Pozzi Mucelli, R.; Zamboni, M. Pancreatic fat accumulation and its relationship with liver fat content and other fat depots in obese individuals. J. Endocrinol. Investig. 2012, 35, 748–753. [Google Scholar]
- Lee, Y.; Lingvay, I.; Szczepaniak, L.S.; Ravazzola, M.; Orci, L.; Unger, R.H. Pancreatic steatosis: Harbinger of type 2 diabetes in obese rodents. Int. J. Obes. 2010, 34, 396–400. [Google Scholar] [CrossRef]
- Wang, C.Y.; Ou, H.Y.; Chen, M.F.; Chang, T.C.; Chang, C.J. Enigmatic ectopic fat: Prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J. Am. Heart Assoc. 2014, 3, e000297. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.L.; Zhang, D.D.; Lin, H.Y.; Dai, X.H.; Sun, X.L.; Li, J.T.; Song, L.Y.; Peng, H.; Wen, M.M. The correlation between pancreatic steatosis and metabolic syndrome in a Chinese population. Pancreatology 2016, 16, 578–583. [Google Scholar] [CrossRef]
- Smits, M.M.; van Geenen, E.J. The clinical significance of pancreatic steatosis. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 169–177. [Google Scholar] [CrossRef]
- Shah, N.; Rocha, J.P.; Bhutiani, N.; Endashaw, O. Nonalcoholic Fatty Pancreas Disease. Nutr. Clin. Pract. 2019, 34, S49–S56. [Google Scholar] [CrossRef]
- Silva, L.L.S.E.; Fernandes, M.S.S.; Lima, E.A.; Stefano, J.T.; Oliveira, C.P.; Jukemura, J. Fatty Pancreas: Disease or Finding? Clinics 2021, 76, e2439. [Google Scholar] [CrossRef]
- Rebuffat, S.A.; Sidot, E.; Guzman, C.; Azay-Milhau, J.; Jover, B.; Lajoix, A.D.; Peraldi-Roux, S. Adipose tissue derived-factors impaired pancreatic beta-cell function in diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3378–3387. [Google Scholar] [CrossRef]
- Watada, H. Role of VEGF-A in pancreatic beta cells. Endocr. J. 2010, 57, 185–191. [Google Scholar] [CrossRef]
- Lai, Y.; Schneider, D.; Kidszun, A.; Hauck-Schmalenberger, I.; Breier, G.; Brandhorst, D.; Brandhorst, H.; Iken, M.; Brendel, M.D.; Bretzel, R.G.; et al. Vascular endothelial growth factor increases functional beta-cell mass by improvement of angiogenesis of isolated human and murine pancreatic islets. Transplantation 2005, 79, 1530–1536. [Google Scholar] [CrossRef]
- De Leu, N.; Heremans, Y.; Coppens, V.; Van Gassen, N.; Cai, Y.; D’Hoker, J.; Magenheim, J.; Salpeter, S.; Swisa, A.; Khalaileh, A.; et al. Short-term overexpression of VEGF-A in mouse beta cells indirectly stimulates their proliferation and protects against diabetes. Diabetologia 2014, 57, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Agudo, J.; Ayuso, E.; Jimenez, V.; Casellas, A.; Mallol, C.; Salavert, A.; Tafuro, S.; Obach, M.; Ruzo, A.; Moya, M.; et al. Vascular endothelial growth factor-mediated islet hypervascularization and inflammation contribute to progressive reduction of beta-cell mass. Diabetes 2012, 61, 2851–2861. [Google Scholar] [CrossRef] [PubMed]
- Silha, J.V.; Krsek, M.; Sucharda, P.; Murphy, L.J. Angiogenic factors are elevated in overweight and obese individuals. Int. J. Obes. 2005, 29, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Imachi, H.; Lyu, J.; Miyai, Y.; Fukunaga, K.; Dong, T.; Ibata, T.; Kobayashi, T.; Yoshimoto, T.; Kikuchi, F.; et al. Effect of TNF-alpha on the expression of ABCA1 in pancreatic beta-cells. J. Mol. Endocrinol. 2018, 61, 185–193. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef]
- Azizian, M.; Mahdipour, E.; Mirhafez, S.R.; Shoeibi, S.; Nematy, M.; Esmaily, H.; Ferns, G.A.; Ghayour-Mobarhan, M. Cytokine profiles in overweight and obese subjects and normal weight individuals matched for age and gender. Ann. Clin. Biochem. 2016, 53, 663–668. [Google Scholar] [CrossRef]
- Dirice, E.; Kahraman, S.; Jiang, W.; El Ouaamari, A.; De Jesus, D.F.; Teo, A.K.; Hu, J.; Kawamori, D.; Gaglia, J.L.; Mathis, D.; et al. Soluble factors secreted by T cells promote beta-cell proliferation. Diabetes 2014, 63, 188–202. [Google Scholar] [CrossRef]
- Duan, L.F.; Xu, X.F.; Zhu, L.J.; Liu, F.; Zhang, X.Q.; Wu, N.; Fan, J.W.; Xin, J.Q.; Zhang, H. Dachaihu decoction ameliorates pancreatic fibrosis by inhibiting macrophage infiltration in chronic pancreatitis. World J. Gastroenterol. 2017, 23, 7242–7252. [Google Scholar] [CrossRef]
- Westerbacka, J.; Cornér, A.; Kolak, M.; Makkonen, J.; Turpeinen, U.; Hamsten, A.; Fisher, R.M.; Yki-Järvinen, H. Insulin regulation of MCP-1 in human adipose tissue of obese and lean women. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E841–E845. [Google Scholar] [CrossRef]
- Levelt, E.; Pavlides, M.; Banerjee, R.; Mahmod, M.; Kelly, C.; Sellwood, J.; Ariga, R.; Thomas, S.; Francis, J.; Rodgers, C.; et al. Ectopic and Visceral Fat Deposition in Lean and Obese Patients with Type 2 Diabetes. J. Am. Coll. Cardiol. 2016, 68, 53–63. [Google Scholar] [CrossRef]
- Kang, M.; Lee, A.; Yoo, H.J.; Kim, M.; Kim, M.; Shin, D.Y.; Lee, J.H. Association between increased visceral fat area and alterations in plasma fatty acid profile in overweight subjects: A cross-sectional study. Lipids Health Dis. 2017, 16, 248. [Google Scholar] [CrossRef]
- Walker, G.E.; Marzullo, P.; Prodam, F.; Bona, G.; Di Blasio, A.M. Obesity modifies expression profiles of metabolic markers in superficial and deep subcutaneous abdominal adipose tissue depots. Endocrine 2014, 46, 99–106. [Google Scholar] [CrossRef]
- Cancello, R.; Zulian, A.; Gentilini, D.; Maestrini, S.; Della Barba, A.; Invitti, C.; Corà, D.; Caselle, M.; Liuzzi, A.; Di Blasio, A.M. Molecular and morphologic characterization of superficial- and deep-subcutaneous adipose tissue subdivisions in human obesity. Obesity 2013, 21, 2562–2570. [Google Scholar] [CrossRef]
- Thomas, E.L.; Frost, G.; Taylor-Robinson, S.D.; Bell, J.D. Excess body fat in obese and normal-weight subjects. Nutr. Res. Rev. 2012, 25, 150–161. [Google Scholar] [CrossRef]
- Ezure, T.; Amano, S. Increased subcutaneous adipose tissue impairs dermal function in diet-induced obese mice. Exp. Dermatol. 2010, 19, 878–882. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Z.; Punyanita, M.; Lei, J.; Sinav, A.; Kral, J.G.; Imielinska, C.; Ross, R.; Heymsfield, S.B. Adipose tissue quantification by imaging methods: A proposed classification. Obes. Res. 2003, 11, 5–16. [Google Scholar] [CrossRef]
- Sbarbati, A.; Accorsi, D.; Benati, D.; Marchetti, L.; Orsini, G.; Rigotti, G.; Panettiere, P. Subcutaneous adipose tissue classification. Eur. J. Histochem. 2010, 54, e48. [Google Scholar] [CrossRef]
- Märin, P.; Andersson, B.; Ottosson, M.; Olbe, L.; Chowdhury, B.; Kvist, H.; Holm, G.; Sjöström, L.; Björntorp, P. The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism 1992, 41, 1242–1248. [Google Scholar] [CrossRef]
- Abate, N.; Burns, D.; Peshock, R.M.; Garg, A.; Grundy, S.M. Estimation of adipose tissue mass by magnetic resonance imaging: Validation against dissection in human cadavers. J. Lipid Res. 1994, 35, 1490–1496. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. International Atherosclerosis Society; International Chair on Cardiometabolic Risk Working Group on Visceral Obesity. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Walker, G.E.; Marzullo, P.; Ricotti, R.; Bona, G.; Prodam, F. The pathophysiology of abdominal adipose tissue depots in health and disease. Horm. Mol. Biol. Clin. Investig. 2014, 19, 57–74. [Google Scholar] [CrossRef]
- Cao, Y.L.; Hu, C.Z.; Meng, X.; Wang, D.F.; Zhang, J. Expression of TNF-alpha protein in omental and subcutaneous adipose tissue in obesity. Diabetes Res. Clin. Pract. 2008, 79, 214–219. [Google Scholar] [CrossRef]
- Terra, X.; Auguet, T.; Quesada, I.; Aguilar, C.; Luna, A.M.; Hernández, M.; Sabench, F.; Porras, J.A.; Martínez, S.; Lucas, A.; et al. Increased levels and adipose tissue expression of visfatin in morbidly obese women: The relationship with pro-inflammatory cytokines. Clin. Endocrinol. 2012, 77, 691–698. [Google Scholar] [CrossRef]
- Madani, R.; Karastergiou, K.; Ogston, N.C.; Miheisi, N.; Bhome, R.; Haloob, N.; Tan, G.D.; Karpe, F.; Malone-Lee, J.; Hashemi, M.; et al. RANTES release by human adipose tissue in vivo and evidence for depot-specific differences. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1262–E1268. [Google Scholar] [CrossRef]
- Jonas, M.I.; Kurylowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Wierzbicki, Z.; Chmura, A.; Pruszczyk, P.; Puzianowska-Kuznicka, M. Interleukins 6 and 15 Levels Are Higher in Subcutaneous Adipose Tissue, but Obesity Is Associated with Their Increased Content in Visceral Fat Depots. Int. J. Mol. Sci. 2015, 16, 25817–25830. [Google Scholar] [CrossRef]
- Chacón, M.R.; Richart, C.; Gómez, J.M.; Megía, A.; Vilarrasa, N.; Fernández-Real, J.M.; García-España, A.; Miranda, M.; Masdevall, C.; Ricard, W.; et al. Expression of TWEAK and its receptor Fn14 in human subcutaneous adipose tissue. Relationship with other inflammatory cytokines in obesity. Cytokine 2006, 33, 129–137. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Valentí, V.; Moncada, R.; Landecho, M.F.; Silva, C.; Salvador, J.; Frühbeck, G. Increased Interleukin-32 Levels in Obesity Promote Adipose Tissue Inflammation and Extracellular Matrix Remodeling: Effect of Weight Loss. Diabetes 2016, 65, 3636–3648. [Google Scholar] [CrossRef]
- Pierce, J.R.; Maples, J.M.; Hickner, R.C. IL-15 concentrations in skeletal muscle and subcutaneous adipose tissue in lean and obese humans: Local effects of IL-15 on adipose tissue lipolysis. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1131–E1139. [Google Scholar] [CrossRef]
- Samaras, K.; Botelho, N.K.; Chisholm, D.J.; Lord, R.V. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity 2010, 18, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.K.; Bunkin, D.A.; Greenberg, A.S. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 1998, 83, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Good, M.; Newell, F.M.; Haupt, L.M.; Whitehead, J.P.; Hutley, L.J.; Prins, J.B. TNF and TNF receptor expression and insulin sensitivity in human omental and subcutaneous adipose tissue—Influence of BMI and adipose distribution. Diab. Vasc. Dis. Res. 2006, 3, 26–33. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; García-Lorda, P.; Figueredo, R.; Del Castillo, D.; Bonada, A.; Balanzà, R. Subcutaneous adipose tissue cytokine production is not responsible for the restoration of systemic inflammation markers during weight loss. Int. J. Obes. 2006, 30, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Leick, L.; Lindegaard, B.; Stensvold, D.; Plomgaard, P.; Saltin, B.; Pilegaard, H. Adipose tissue interleukin-18 mRNA and plasma interleukin-18: Effect of obesity and exercise. Obesity 2007, 15, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.; Klimcakova, E.; Lolmède, K.; Berlan, M.; Lafontan, M.; Stich, V.; Bouloumié, A.; Galitzky, J.; Arner, P.; Langin, D. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia 2007, 50, 1038–1047. [Google Scholar] [CrossRef]
- Nielsen, N.B.; Højbjerre, L.; Sonne, M.P.; Alibegovic, A.C.; Vaag, A.; Dela, F.; Stallknecht, B. Interstitial concentrations of adipokines in subcutaneous abdominal and femoral adipose tissue. Regul. Pept. 2009, 155, 39–45. [Google Scholar] [CrossRef]
- Vendrell, J.; Maymó-Masip, E.; Tinahones, F.; García-España, A.; Megia, A.; Caubet, E.; García-Fuentes, E.; Chacón, M.R. Tumor necrosis-like weak inducer of apoptosis as a proinflammatory cytokine in human adipocyte cells: Up-regulation in severe obesity is mediated by inflammation but not hypoxia. J. Clin. Endocrinol. Metab. 2010, 95, 2983–2992. [Google Scholar] [CrossRef]
- Murdolo, G.; Herder, C.; Wang, Z.; Rose, B.; Schmelz, M.; Jansson, P.A. In situ profiling of adipokines in subcutaneous microdialysates from lean and obese individuals. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1095–E1105. [Google Scholar] [CrossRef][Green Version]
- Skopková, M.; Penesová, A.; Sell, H.; Rádiková, Z.; Vlcek, M.; Imrich, R.; Koska, J.; Ukropec, J.; Eckel, J.; Klimes, I.; et al. Protein array reveals differentially expressed proteins in subcutaneous adipose tissue in obesity. Obesity 2007, 15, 2396–2406. [Google Scholar] [CrossRef]
- Al-Attar, A.; Presnell, S.R.; Clasey, J.L.; Long, D.E.; Walton, R.G.; Sexton, M.; Starr, M.E.; Kern, P.A.; Peterson, C.A.; Lutz, C.T. Human Body Composition and Immunity: Visceral Adipose Tissue Produces IL-15 and Muscle Strength Inversely Correlates with NK Cell Function in Elderly Humans. Front. Immunol. 2018, 9, 440. [Google Scholar] [CrossRef]
- Jorge, A.S.B.; Andrade, J.M.O.; Paraíso, A.F.; Jorge, G.C.B.; Silveira, C.M.; de Souza, L.R.; Santos, E.P.; Guimaraes, A.L.S.; Santos, S.H.S.; De-Paula, A.M.B. Body mass index and the visceral adipose tissue expression of IL-6 and TNF-alpha are associated with the morphological severity of non-alcoholic fatty liver disease in individuals with class III obesity. Obes. Res. Clin. Pract. 2018, 12, 1–8. [Google Scholar] [CrossRef]
- Zeyda, M.; Wernly, B.; Demyanets, S.; Kaun, C.; Hämmerle, M.; Hantusch, B.; Schranz, M.; Neuhofer, A.; Itariu, B.K.; Keck, M.; et al. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int. J. Obes. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Ramirez, B.; Rotellar, F.; Pastor, C.; Silva, C.; Rodríguez, A.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Proinflammatory cytokines in obesity: Impact of type 2 diabetes mellitus and gastric bypass. Obes. Surg. 2007, 17, 1464–1474. [Google Scholar] [CrossRef]
- Bruun, J.M.; Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): Implication of macrophages resident in the AT. J. Clin. Endocrinol. Metab. 2005, 90, 2282–2289. [Google Scholar] [CrossRef]
- Hueso, L.; Ortega, R.; Selles, F.; Wu-Xiong, N.Y.; Ortega, J.; Civera, M.; Ascaso, J.F.; Sanz, M.J.; Real, J.T.; Piqueras, L. Upregulation of angiostatic chemokines IP-10/CXCL10 and I-TAC/CXCL11 in human obesity and their implication for adipose tissue angiogenesis. Int J. Obes. 2018, 42, 1406–1417. [Google Scholar] [CrossRef]
- Rouault, C.; Pellegrinelli, V.; Schilch, R.; Cotillard, A.; Poitou, C.; Tordjman, J.; Sell, H.; Clément, K.; Lacasa, D. Roles of chemokine ligand-2 (CXCL2) and neutrophils in influencing endothelial cell function and inflammation of human adipose tissue. Endocrinology 2013, 154, 1069–1079. [Google Scholar] [CrossRef]
- Sbierski-Kind, J.; Mai, K.; Kath, J.; Jurisch, A.; Streitz, M.; Kuchenbecker, L.; Babel, N.; Nienen, M.; Jürchott, K.; Spranger, L.; et al. Association between Subcutaneous Adipose Tissue Inflammation, Insulin Resistance, and Calorie Restriction in Obese Females. J. Immunol. 2021, 205, 45–55. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Catalán, V.; Ramírez, B.; Rodríguez, A.; Colina, I.; Silva, C.; Rotellar, F.; Mugueta, C.; Gil, M.J.; Cienfuegos, J.A.; et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J. Clin. Endocrinol. Metab. 2007, 92, 3719–3727. [Google Scholar] [CrossRef]
- Kiefer, F.W.; Zeyda, M.; Todoric, J.; Huber, J.; Geyeregger, R.; Weichhart, T.; Aszmann, O.; Ludvik, B.; Silberhumer, G.R.; Prager, G.; et al. Osteopontin expression in human and murine obesity: Extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology 2008, 149, 1350–1357. [Google Scholar] [CrossRef]
- Mogilenko, D.A.; Caiazzo, R.; L’homme, L.; Pineau, L.; Raverdy, V.; Noulette, J.; Derudas, B.; Pattou, F.; Staels, B.; Dombrowicz, D. IFNgamma-producing NK cells in adipose tissue are associated with hyperglycemia and insulin resistance in obese women. Int. J. Obes. 2021, 45, 1607–1617. [Google Scholar] [CrossRef]
- Berndt, J.; Klöting, N.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes 2005, 54, 2911–2916. [Google Scholar] [CrossRef] [PubMed]
- Paulmyer-Lacroix, O.; Desbriere, R.; Poggi, M.; Achard, V.; Alessi, M.C.; Boudouresque, F.; Ouafik, L.; Vuaroqueaux, V.; Labuhn, M.; Dutourand, A.; et al. Expression of adrenomedullin in adipose tissue of lean and obese women. Eur. J. Endocrinol. 2006, 155, 177–185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Phillips, S.A.; Ciaraldi, T.P.; Oh, D.K.; Savu, M.K.; Henry, R.R. Adiponectin secretion and response to pioglitazone is depot dependent in cultured human adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E842–E850. [Google Scholar] [CrossRef] [PubMed]
- Urbanová, M.; Dostálová, I.; Trachta, P.; Drápalová, J.; Kaválková, P.; Haluzíková, D.; Matoulek, M.; Lacinová, Z.; Mráz, M.; Kasalický, M.; et al. Serum concentrations and subcutaneous adipose tissue mRNA expression of omentin in morbid obesity and type 2 diabetes mellitus: The effect of very-low-calorie diet, physical activity and laparoscopic sleeve gastrectomy. Physiol. Res. 2014, 63, 207–218. [Google Scholar] [CrossRef]
- Reneau, J.; Goldblatt, M.; Gould, J.; Kindel, T.; Kastenmeier, A.; Higgins, R.; Rengel, L.R.; Schoyer, K.; James, R.; Obi, B.; et al. Effect of adiposity on tissue-specific adiponectin secretion. PLoS ONE 2018, 13, e0198889. [Google Scholar]
- Arner, E.; Mejhert, N.; Kulyté, A.; Balwierz, P.J.; Pachkov, M.; Cormont, M.; Lorente-Cebrián, S.; Ehrlund, A.; Laurencikiene, J.; Hedén, P.; et al. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes 2012, 61, 1986–1993. [Google Scholar] [CrossRef]
- Gu, N.; You, L.; Shi, C.; Yang, L.; Pang, L.; Cui, X.; Ji, C.; Zheng, W.; Guo, X. Expression of miR-199a-3p in human adipocytes is regulated by free fatty acids and adipokines. Mol. Med. Rep. 2016, 14, 1180–1186. [Google Scholar] [CrossRef]
- Lozano-Bartolomé, J.; Llauradó, G.; Portero-Otin, M.; Altuna-Coy, A.; Rojo-Martínez, G.; Vendrell, J.; Jorba, R.; Rodríguez-Gallego, E.; Chacón, M.R. Altered Expression of miR-181a-5p and miR-23a-3p Is Associated with Obesity and TNFalpha-Induced Insulin Resistance. J. Clin. Endocrinol. Metab. 2018, 103, 1447–1458. [Google Scholar] [CrossRef]
- Ahmad, R.; Al-Mass, A.; Al-Ghawas, D.; Shareif, N.; Zghoul, N.; Melhem, M.; Hasan, A.; Al-Ghimlas, F.; Dermime, S.; Behbehani, K. Interaction of osteopontin with IL-18 in obese individuals: Implications for insulin resistance. PLoS ONE 2013, 8, e63944. [Google Scholar]
- Vianello, E.; Kalousová, M.; Dozio, E.; Tacchini, L.; Zima, T.; Corsi Romanelli, M.M. Osteopontin: The Molecular Bridge between Fat and Cardiac-Renal Disorders. Int. J. Mol. Sci. 2021, 21, 5568. [Google Scholar] [CrossRef]
- Okamoto, H. Osteopontin and cardiovascular system. Mol. Cell. Biochem. 2007, 300, 1–7. [Google Scholar] [CrossRef]
- Singh, M.; Ananthula, S.; Milhorn, D.M.; Krishnaswamy, G.; Singh, K. Osteopontin: A novel inflammatory mediator of cardiovascular disease. Front. Biosci. 2007, 12, 214–221. [Google Scholar] [CrossRef]
- Yuan, S.M.; Wang, J.; Huang, H.R.; Jing, H. Osteopontin expression and its possible functions in the aortic disorders and coronary artery disease. Rev. Bras. Cir. Cardiovasc. 2011, 26, 173–182. [Google Scholar] [CrossRef]
- Kaleta, B. The role of osteopontin in kidney diseases. Inflamm. Res. 2019, 68, 93–102. [Google Scholar] [CrossRef]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2021, 26, e919159. [Google Scholar] [CrossRef]
- Iida, T.; Wagatsuma, K.; Hirayama, D.; Nakase, H. Is Osteopontin a Friend or Foe of Cell Apoptosis in Inflammatory Gastrointestinal and Liver Diseases? Int. J. Mol. Sci. 2017, 19, 7. [Google Scholar] [CrossRef]
- O’Regan, A. The role of osteopontin in lung disease. Cytokine Growth Factor Rev. 2003, 14, 479–488. [Google Scholar] [CrossRef]
- Del Toro, R.; Cavallari, I.; Tramontana, F.; Park, K.; Strollo, R.; Valente, L.; De Pascalis, M.; Grigioni, F.; Pozzilli, P.; Buzzetti, R.; et al. Association of bone biomarkers with advanced atherosclerotic disease in people with overweight/obesity. Endocrine 2021, 73, 339–346. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdanski, P.; Gajewska, E.; Kalmus, G.; Sobieska, M.; Samborski, W. The association of insulin resistance with serum osteoprotegerin in obese adolescents. J. Physiol. Biochem. 2013, 69, 847–853. [Google Scholar] [CrossRef]
- Kotanidou, E.P.; Kotanidis, C.P.; Giza, S.; Serbis, A.; Tsinopoulou, V.R.; Karalazou, P.; Tzimagiorgis, G.; Galli-Tsinopoulou, A. Osteoprotegerin increases parallel to insulin resistance in obese adolescents. Endocr. Res. 2019, 44, 9–15. [Google Scholar] [CrossRef]
- Dimitri, P.; Wales, J.K.; Bishop, N. Adipokines, bone-derived factors and bone turnover in obese children; evidence for altered fat-bone signalling resulting in reduced bone mass. Bone 2011, 48, 189–196. [Google Scholar] [CrossRef]
- Makarović, S.; Makarović, Z.; Steiner, R.; Mihaljević, I.; Milas-Ahić, J. Osteoprotegerin and Vascular Calcification: Clinical and Prognostic Relevance. Coll. Antropol. 2015, 39, 461–468. [Google Scholar]
- Montagnana, M.; Lippi, G.; Danese, E.; Guidi, G.C. The role of osteoprotegerin in cardiovascular disease. Ann. Med. 2013, 45, 254–264. [Google Scholar] [CrossRef]
- Nacaroglu, H.T.; Büke, Ö.; Gayret, Ö.B.; Erol, M.; Zengi, O. Serum osteoprotegerin levels in school-aged children with asthma. Allergol. Immunopathol. 2021, 48, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, E.; Yasasever, V.; Soydinc, H.O.; Yasasever, C.T.; Ozturk, N.; Duranyildiz, D. Markers of bone metastases in breast and lung cancers. Asian Pac. J. Cancer Prev. 2012, 13, 4331–4334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lawrie, A.; Waterman, E.; Southwood, M.; Evans, D.; Suntharalingam, J.; Francis, S.; Crossman, D.; Croucher, P.; Morrell, N.; Newman, C. Evidence of a role for osteoprotegerin in the pathogenesis of pulmonary arterial hypertension. Am. J. Pathol. 2008, 172, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Jasiewicz, M.; Knapp, M.; Waszkiewicz, E.; Musiał, W.J.; Kamiński, K.A. Potential pathogenic role of soluble receptor activator of nuclear factor-qB ligand and osteoprotegerin in patients with pulmonary arterial hypertension. Pol. Arch. Med. Wewn. 2014, 124, 579–586. [Google Scholar] [CrossRef]
- De Voogd, F.A.; Gearry, R.B.; Mulder, C.J.; Day, A.S. Osteoprotegerin: A novel biomarker for inflammatory bowel disease and gastrointestinal carcinoma. J. Gastroenterol. Hepatol. 2016, 31, 1386–1392. [Google Scholar] [CrossRef]
- Krela-Kaźmierczak, I.; Szymczak-Tomczak, A.; Łykowska-Szuber, L.; Wysocka, E.; Michalak, M.; Stawczyk-Eder, K.; Waszak, K.; Linke, K.; Eder, P. Interleukin 6, osteoprotegerin, sRANKL and bone metabolism in inflammatory bowel diseases. Adv. Clin. Exp. Med. 2018, 27, 449–453. [Google Scholar] [CrossRef]
- Dufresne, S.S.; Dumont, N.A.; Bouchard, P.; Lavergne, É.; Penninger, J.M.; Frenette, J. Osteoprotegerin protects against muscular dystrophy. Am. J. Pathol. 2015, 185, 920–926. [Google Scholar] [CrossRef]
- Dufresne, S.S.; Boulanger-Piette, A.; Frenette, J. Osteoprotegerin and beta (2)-Agonists Mitigate Muscular Dystrophy in Slow- and Fast-Twitch Skeletal Muscles. Am. J. Pathol. 2017, 187, 498–504. [Google Scholar] [CrossRef]
- Pacifico, L.; Di Renzo, L.; Anania, C.; Osborn, J.F.; Ippoliti, F.; Schiavo, E.; Chiesa, C. Increased T-helper interferon-gamma-secreting cells in obese children. Eur. J. Endocrinol. 2006, 154, 691–697. [Google Scholar] [CrossRef]
- Asadikaram, G.; Ram, M.; Izadi, A.; Sheikh Fathollahi, M.; Nematollahi, M.H.; Najafipour, H.; Shahoozehi, B.; Mirhoseini, M.; Masoumi, M.; Shahrokhi, N.; et al. The study of the serum level of IL-4, TGF-beta, IFN-gamma, and IL-6 in overweight patients with and without diabetes mellitus and hypertension. J. Cell. Biochem. 2019, 120, 4147–4157. [Google Scholar] [CrossRef]
- Youssef, D.M.; Elbehidy, R.M.; Shokry, D.M.; Elbehidy, E.M. The influence of leptin on Th1/Th2 balance in obese children with asthma. J. Bras. Pneumol. 2013, 39, 562–568. [Google Scholar] [CrossRef]
- Lucas, R.; Parikh, S.J.; Sridhar, S.; Guo, D.H.; Bhagatwala, J.; Dong, Y.; Caldwell, R.; Mellor, A.; Caldwell, W.; Zhu, H.; et al. Cytokine profiling of young overweight and obese female African American adults with prediabetes. Cytokine 2013, 64, 310–315. [Google Scholar] [CrossRef]
- Elyasi, A.; Voloshyna, I.; Ahmed, S.; Kasselman, L.J.; Behbodikhah, J.; De Leon, J.; Reiss, A.B. The role of interferon-gamma in cardiovascular disease: An update. Inflamm. Res. 2021, 69, 975–988. [Google Scholar] [CrossRef]
- Raundhal, M.; Morse, C.; Khare, A.; Oriss, T.B.; Milosevic, J.; Trudeau, J.; Huff, R.; Pilewski, J.; Holguin, F.; Kolls, J.; et al. High IFN-gamma and low SLPI mark severe asthma in mice and humans. J. Clin. Invest. 2015, 125, 3037–3050. [Google Scholar] [CrossRef]
- Nie, W.; Meng, L.; Wang, X.; Xiu, Q. Interferon-gamma +874A/T polymorphism is associated with asthma risk: A meta-analysis. J. Investig. Allergol. Clin. Immunol. 2014, 24, 324–330. [Google Scholar]
- Ten Hacken, N.H.; Oosterhoff, Y.; Kauffman, H.F.; Guevarra, L.; Satoh, T.; Tollerud, D.J.; Postma, D.S. Elevated serum interferon-gamma in atopic asthma correlates with increased airways responsiveness and circadian peak expiratory flow variation. Eur. Respir. J. 1998, 11, 312–316. [Google Scholar] [CrossRef]
- Cembrzynska-Nowak, M.; Szklarz, E.; Inglot, A.D.; Teodorczyk-Injeyan, J.A. Elevated release of tumor necrosis factor-alpha and interferon-gamma by bronchoalveolar leukocytes from patients with bronchial asthma. Am. Rev. Respir. Dis. 1993, 147, 291–295. [Google Scholar] [CrossRef]
- Bantulà, M.; Roca-Ferrer, J.; Arismendi, E.; Picado, C. Asthma and Obesity: Two Diseases on the Rise and Bridged by Inflammation. J. Clin. Med. 2021, 10, 169. [Google Scholar] [CrossRef]
- Yehuda-Shnaidman, E.; Schwartz, B. Mechanisms linking obesity, inflammation and altered metabolism to colon carcinogenesis. Obes. Rev. 2012, 13, 1083–1095. [Google Scholar] [CrossRef]
- Vazzana, N.; Riondino, S.; Toto, V.; Guadagni, F.; Roselli, M.; Davi, G.; Ferroni, P. Obesity-driven inflammation and colorectal cancer. Curr. Med. Chem. 2012, 19, 5837–5853. [Google Scholar] [CrossRef]
- Olszanecka-Glinianowicz, M.; Handzlik-Orlik, G.; Orlik, B.; Chudek, J. Adipokines in the pathogenesis of idiopathic inflammatory bowel disease. Endokrynol. Pol. 2013, 64, 226–231. [Google Scholar]
- Tewari, N.; Awad, S.; Macdonald, I.A.; Lobo, D.N. Obesity-related insulin resistance: Implications for the surgical patient. Int. J. Obes. 2015, 39, 1575–1588. [Google Scholar] [CrossRef]
- Janochova, K.; Haluzik, M.; Buzga, M. Visceral fat and insulin resistance—What we know? Biomed. Pap. Med Fac. Palacky Univ. Olomouc 2019, 163, 19–27. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Katritsis, D.; Raggi, P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis 2014, 233, 104–112. [Google Scholar] [CrossRef]
- Malavazos, A.E.; Corsi, M.M.; Ermetici, F.; Coman, C.; Sardanelli, F.; Rossi, A.; Morricone, L.; Ambrosi, B. Proinflammatory cytokines and cardiac abnormalities in uncomplicated obesity: Relationship with abdominal fat deposition. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 294–302. [Google Scholar] [CrossRef]
- Teplan, V., Jr.; Vyhnánek, F.; Gürlich, R.; Haluzík, M.; Racek, J.; Vyhnankova, I.; Stollová, M.; Teplan, V. Increased proinflammatory cytokine production in adipose tissue of obese patients with chronic kidney disease. Wien. Klin. Wochenschr. 2010, 122, 466–473. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, J.; Zhang, F.; Lei, Q.; Zhang, T.; Li, K.; Guo, J.; Hong, Y.; Bu, G.; Lv, X.; et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death Dis. 2019, 10, 365. [Google Scholar] [CrossRef]
- Song, X.; Xue, Y.; Cai, H. Down-Regulation of miR-181a-5p Prevents Cerebral Ischemic Injury by Upregulating En2 and Activating Wnt/beta-catenin Pathway. J. Stroke Cerebrovasc. Dis. 2021, 30, 105485. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Long, M.Y.; Chen, Z.Q.; Huang, J.W.; Qin, Z.B.; Li, L. Downregulation of miR-181a-5p alleviates oxidative stress and inflammation in coronary microembolization-induced myocardial damage by directly targeting XIAP. J. Geriatr. Cardiol. 2021, 18, 426–439. [Google Scholar] [PubMed]
- Zhao, H.; Tao, Z.; Wang, R.; Liu, P.; Yan, F.; Li, J.; Zhang, C.; Ji, X.; Luo, Y. MicroRNA-23a-3p attenuates oxidative stress injury in a mouse model of focal cerebral ischemia-reperfusion. Brain Res. 2014, 1592, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Teoh, J.P.; Wang, Y.; Broskova, Z.; Bayoumi, A.S.; Tang, Y.; Su, H.; Weintraub, N.L.; Kim, I.M. Carvedilol-responsive microRNAs, miR-199a-3p and -214 protect cardiomyocytes from simulated ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H371–H383. [Google Scholar] [CrossRef]
- Joris, V.; Gomez, E.L.; Menchi, L.; Lobysheva, I.; Di Mauro, V.; Esfahani, H.; Condorelli, G.; Balligand, J.L.; Catalucci, D.; Dessy, C. MicroRNA-199a-3p and MicroRNA-199a-5p Take Part to a Redundant Network of Regulation of the NOS (NO Synthase)/NO Pathway in the Endothelium. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2345–2357. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.X.; Huang, X.G. MicroRNA-199a-3p inhibits angiogenesis by targeting the VEGF/PI3K/AKT signalling pathway in an in vitro model of diabetic retinopathy. Exp. Mol. Pathol. 2021, 116, 104488. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Silva, C.; Rotellar, F.; Gil, M.J.; Cienfuegos, J.A.; Salvador, J.; Frühbeck, G. Increased adipose tissue expression of lipocalin-2 in obesity is related to inflammation and matrix metalloproteinase-2 and metalloproteinase-9 activities in humans. J. Mol. Med. 2009, 87, 803–813. [Google Scholar] [CrossRef]
- Auguet, T.; Quintero, Y.; Terra, X.; Martínez, S.; Lucas, A.; Pellitero, S.; Aguilar, C.; Hernández, M.; del Castillo, D.; Richart, C. Upregulation of lipocalin 2 in adipose tissues of severely obese women: Positive relationship with proinflammatory cytokines. Obesity 2011, 19, 2295–2300. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Frühbeck, G. Increased tenascin C and Toll-like receptor 4 levels in visceral adipose tissue as a link between inflammation and extracellular matrix remodeling in obesity. J. Clin. Endocrinol. Metab. 2012, 97, E1880–E1889. [Google Scholar] [CrossRef]
- Osorio-Conles, O.; Guitart, M.; Chacón, M.R.; Maymo-Masip, E.; Moreno-Navarrete, J.M.; Montori-Grau, M.; Näf, S.; Fernandez-Real, J.M.; Vendrell, J.; Gómez-Foix, A.M. Plasma PTX3 protein levels inversely correlate with insulin secretion and obesity, whereas visceral adipose tissue PTX3 gene expression is increased in obesity. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1254–E1261. [Google Scholar] [CrossRef]
- Wang, Y.; Lam, K.S.; Kraegen, E.W.; Sweeney, G.; Zhang, J.; Tso, A.W.; Chow, W.S.; Wat, N.M.; Xu, J.Y.; Hoo, R.L.; et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007, 53, 34–41. [Google Scholar] [CrossRef]
- Hoo, R.L.; Yeung, D.C.; Lam, K.S.; Xu, A. Inflammatory biomarkers associated with obesity and insulin resistance: A focus on lipocalin-2 and adipocyte fatty acid-binding protein. Expert Rev. Endocrinol. Metab. 2008, 3, 29–41. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Valentí, V.; Moncada, R.; Silva, C.; Salvador, J.; Frühbeck, G. Peripheral mononuclear blood cells contribute to the obesity-associated inflammatory state independently of glycemic status: Involvement of the novel proinflammatory adipokines chemerin, chitinase-3-like protein 1, lipocalin-2 and osteopontin. Genes Nutr. 2015, 10, 460. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Zhang, Y.; Leroith, D.; Bernlohr, D.A.; Chen, X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol. Endocrinol. 2008, 22, 1416–1426. [Google Scholar] [CrossRef]
- Cao, W.; Huang, H.; Xia, T.; Liu, C.; Muhammad, S.; Sun, C. Homeobox a5 Promotes White Adipose Tissue Browning Through Inhibition of the Tenascin C/Toll-Like Receptor 4/Nuclear Factor Kappa B Inflammatory Signaling in Mice. Front. Immunol. 2018, 9, 647. [Google Scholar] [CrossRef]
- Renovato-Martins, M.; Moreira-Nunes, C.; Atella, G.C.; Barja-Fidalgo, C.; Moraes, J.A. Obese Adipose Tissue Secretion Induces Inflammation in Preadipocytes: Role of Toll-Like Receptor-4. Nutrients 2021, 12, 2828. [Google Scholar] [CrossRef]
- Kamble, P.G.; Pereira, M.J.; Sidibeh, C.O.; Amini, S.; Sundbom, M.; Börjesson, J.L.; Eriksson, J.W. Lipocalin 2 produces insulin resistance and can be upregulated by glucocorticoids in human adipose tissue. Mol. Cell. Endocrinol. 2016, 427, 124–132. [Google Scholar] [CrossRef]
- Tsukumo, D.M.; Carvalho-Filho, M.A.; Carvalheira, J.B.; Prada, P.O.; Hirabara, S.M.; Schenka, A.A.; Araújo, E.P.; Vassallo, J.; Curi, R.; Velloso, L.A.; et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007, 56, 1986–1998. [Google Scholar] [CrossRef]
- Yan, Q.W.; Yang, Q.; Mody, N.; Graham, T.E.; Hsu, C.H.; Xu, Z.; Houstis, N.E.; Kahn, B.B.; Rosen, E.D. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 2007, 56, 2533–2540. [Google Scholar] [CrossRef]
- Jun, L.S.; Siddall, C.P.; Rosen, E.D. A minor role for lipocalin 2 in high-fat diet-induced glucose intolerance. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E825–E835. [Google Scholar] [CrossRef]
- Song, E.; Fan, P.; Huang, B.; Deng, H.B.; Cheung, B.M.; Félétou, M.; Vilaine, J.P.; Villeneuve, N.; Xu, A.; Vanhoutte, P.M.; et al. Deamidated lipocalin-2 induces endothelial dysfunction and hypertension in dietary obese mice. J. Am. Heart Assoc. 2014, 3, e000837. [Google Scholar] [CrossRef] [PubMed]
- Alberti, L.; Gilardini, L.; Zulian, A.; Micheletto, G.; Peri, G.; Doni, A.; Mantovani, A.; Invitti, C. Expression of long pentraxin PTX3 in human adipose tissue and its relation with cardiovascular risk factors. Atherosclerosis 2009, 202, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Manabe, I. Toll-like receptor, lipotoxicity and chronic inflammation: The pathological link between obesity and cardiometabolic disease. J. Atheroscler. Thromb. 2014, 21, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.D.; Surani, S.; Horseman, M.A. Endotoxin, Toll-like Receptor-4, and Atherosclerotic Heart Disease. Curr. Cardiol. Rev. 2017, 13, 86–93. [Google Scholar] [CrossRef]
- Shibata, K.; Sato, K.; Shirai, R.; Seki, T.; Okano, T.; Yamashita, T.; Koide, A.; Mitsuboshi, M.; Mori, Y.; Hirano, T.; et al. Lipocalin-2 exerts pro-atherosclerotic effects as evidenced by in vitro and in vivo experiments. Heart Vessel. 2021, 35, 1012–1024. [Google Scholar] [CrossRef]
- Zwick, R.K.; Guerrero-Juarez, C.F.; Horsley, V.; Plikus, M.V. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018, 27, 68–83. [Google Scholar] [CrossRef]
- Britton, K.A.; Fox, C.S. Ectopic fat depots and cardiovascular disease. Circulation 2011, 124, 837–841. [Google Scholar] [CrossRef]
- Lim, S.; Meigs, J.B. Links between ectopic fat and vascular disease in humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 820–826. [Google Scholar] [CrossRef]
- Smith, U. Abdominal obesity: A marker of ectopic fat accumulation. J. Clin. Invest. 2015, 125, 1790–1792. [Google Scholar] [CrossRef]
- Schneider, H.J.; Glaesmer, H.; Klotsche, J.; Böhler, S.; Lehnert, H.; Zeiher, A.M.; März, W.; Pittrow, D.; Stalla, G.K.; Wittchen, H.U. DETECT Study Group. Accuracy of anthropometric indicators of obesity to predict cardiovascular risk. J. Clin. Endocrinol. Metab. 2007, 92, 589–594. [Google Scholar] [CrossRef]
- Lee, M.J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Aspects Med. 2013, 34, 1–11. [Google Scholar] [CrossRef]
- Kwok, K.H.M.; Lam, K.S.L.; Xu, A. Heterogeneity of white adipose tissue: Molecular basis and clinical implications. Exp. Mol. Med. 2016, 48, 215. [Google Scholar] [CrossRef]
- Von Eyben, F.E.; Mouritsen, E.; Holm, J.; Montvilas, P.; Dimcevski, G.; Suciu, G.; Helleberg, I.; Kristensen, L.; von Eyben, R. Intra-abdominal obesity and metabolic risk factors: A study of young adults. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 941–949. [Google Scholar] [CrossRef]
- Vega, G.L.; Adams-Huet, B.; Peshock, R.; Willett, D.; Shah, B.; Grundy, S.M. Influence of body fat content and distribution on variation in metabolic risk. J. Clin. Endocrinol. Metab. 2006, 91, 4459–4466. [Google Scholar] [CrossRef]
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1600. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C.; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D.; Karnieli, E. The metabolic syndrome-from insulin resistance to obesity and diabetes. Endocrinol. Metab. Clin. N. Am. 2008, 37, 559–579. [Google Scholar] [CrossRef]
- Karelis, A.D.; St-Pierre, D.H.; Conus, F.; Rabasa-Lhoret, R.; Poehlman, E.T. Metabolic and body composition factors in subgroups of obesity: What do we know? J. Clin. Endocrinol. Metab. 2004, 89, 2569–2575. [Google Scholar] [CrossRef]
- Monteiro, R.; de Castro, P.M.S.T.; Calhau, C.; Azevedo, I. Adipocyte size and liability to cell death. Obes. Surg. 2006, 16, 804–806. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Fang, L.; Guo, F.; Zhou, L.; Stahl, R.; Grams, J. The cell size and distribution of adipocytes from subcutaneous and visceral fat is associated with type 2 diabetes mellitus in humans. Adipocyte 2015, 4, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Laforest, S.; Labrecque, J.; Michaud, A.; Cianflone, K.; Tchernof, A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Crit. Rev. Clin. Lab. Sci. 2015, 52, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Cobb, W.S.; Burns, J.M.; Kercher, K.W.; Matthews, B.D.; James Norton, H.; Todd Heniford, B. Normal intraabdominal pressure in healthy adults. J. Surg. Res. 2005, 129, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.M.; Marceau, S.; Forse, R.A. Intra-abdominal pressure in the morbidly obese. Obes. Surg. 2005, 15, 1225–1232. [Google Scholar] [CrossRef]
- Monteiro, R.; Calhau, C.; Azevedo, I. Obstructive sleep apnea and adipocyte death. Eur. J. Heart Fail. 2007, 9, 103–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Song, M.J.; Kim, K.H.; Yoon, J.M.; Kim, J.B. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem. Biophys. Res. Commun. 2006, 346, 739–745. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Yang, J.; Ran, Y.; Yang, Y.; Song, S.; Wu, Y.; Qi, Y.; Gao, Y.; Li, G. 4-Hydroxyisoleucine Alleviates Macrophage-Related Chronic Inflammation and Metabolic Syndrome in Mice Fed a High-Fat Diet. Front. Pharmacol. 2021, 11, 606514. [Google Scholar] [CrossRef]
- Primeau, V.; Coderre, L.; Karelis, A.D.; Brochu, M.; Lavoie, M.E.; Messier, V.; Sladek, R.; Rabasa-Lhoret, R. Characterizing the profile of obese patients who are metabolically healthy. Int. J. Obes. 2011, 35, 971–981. [Google Scholar] [CrossRef]
- Smith, G.I.; Mittendorfer, B.; Klein, S. Metabolically healthy obesity: Facts and fantasies. J. Clin. Invest. 2019, 129, 3978–3989. [Google Scholar] [CrossRef]
- Phillips, C.M.; Perry, I.J. Does inflammation determine metabolic health status in obese and nonobese adults? J. Clin. Endocrinol. Metab. 2013, 98, E1610–E1619. [Google Scholar] [CrossRef]
- Muñoz-Garach, A.; Cornejo-Pareja, I.; Tinahones, F.J. Does Metabolically Healthy Obesity Exist? Nutrients 2016, 8, 320. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Lionetti, L.; Mollica, M.P.; Lombardi, A.; Cavaliere, G.; Gifuni, G.; Barletta, A. From chronic overnutrition to insulin resistance: The role of fat-storing capacity and inflammation. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 146–152. [Google Scholar] [CrossRef]
- Klöting, N.; Fasshauer, M.; Dietrich, A.; Kovacs, P.; Schön, M.R.; Kern, M.; Stumvoll, M.; Blüher, M. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E506–E515. [Google Scholar] [CrossRef]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, 405–420. [Google Scholar] [CrossRef]
- Goossens, G.H. The metabolic phenotype in obesity: Fat mass, body fat distribution, and adipose tissue function. Obes. Facts. 2017, 10, 207–215. [Google Scholar] [CrossRef]
- Stefan, N.; Schick, F.; Häring, H.U. Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. Cell Metab. 2017, 26, 292–300. [Google Scholar] [CrossRef]
- Gaiţă, D.; Moşteoru, S. Metabolically healthy versus unhealthy obesity and risk for diabetes mellitus and cardiovascular diseases. Cardiovasc. Endocrinol. 2017, 6, 23–26. [Google Scholar] [CrossRef]
- Ruggiero, A.D.; Key, C.C.; Kavanagh, K. Adipose Tissue Macrophage Polarization in Healthy and Unhealthy Obesity. Front. Nutr. 2021, 8, 625331. [Google Scholar] [CrossRef]
- Pasarica, M.; Sereda, O.R.; Redman, L.M.; Albarado, D.C.; Hymel, D.T.; Roan, L.E.; Rood, J.C.; Burk, D.H.; Smith, S.R. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef]
- Dario, A.B.; Ferreira, M.L.; Refshauge, K.M.; Lima, T.S.; Ordoñana, J.R.; Ferreira, P.H. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: A systematic review of twin studies. Spine J. 2015, 15, 1106–1117. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Matsudaira, K.; Sawada, S.S.; Gando, Y.; Kawakami, R.; Kinugawa, C.; Okamoto, T.; Tsukamoto, K.; Miyachi, M.; Naito, H. Obesity and low back pain: A retrospective cohort study of Japanese males. J. Phys. Ther. Sci. 2017, 29, 978–983. [Google Scholar] [CrossRef]
- Da Cruz Fernandes, I.M.; Pinto, R.Z.; Ferreira, P.; Lira, F.S. Low back pain, obesity, and inflammatory markers: Exercise as potential treatment. J. Exerc. Rehabil. 2018, 14, 168–174. [Google Scholar] [CrossRef]
- Puri, J.; Sharma, S.; Samuel, A.J.; Chahal, A. Investigate Correlation between Diastasis of Rectus Abdominis Muscle and Low Back Pain in Obese Women. J. Lifestyle Med. 2021, 11, 38–42. [Google Scholar] [CrossRef]
- Dufour, A.B.; Losina, E.; Menz, H.B.; LaValley, M.P.; Hannan, M.T. Obesity, foot pain and foot disorders in older men and women. Obes. Res. Clin. Pract. 2017, 11, 445–453. [Google Scholar] [CrossRef]
- Moon, J.L.; Moon, K.M.; Carlisle, D.M. Obesity-Related Foot Pain: Diagnosis and Surgical Planning. Clin. Podiatr. Med. Surg. 2019, 36, 141–151. [Google Scholar] [CrossRef]
- Li, J.S.; Tsai, T.Y.; Clancy, M.M.; Li, G.; Lewis, C.L.; Felson, D.T. Weight loss changed gait kinematics in individuals with obesity and knee pain. Gait Posture 2019, 68, 461–465. [Google Scholar] [CrossRef]
- Landsmeer, M.L.A.; Runhaar, J.; van Middelkoop, M.; Oei, E.H.G.; Schiphof, D.; Bindels, P.J.E.; Bierma-Zeinstra, S.M.A. Predicting Knee Pain and Knee Osteoarthritis Among Overweight Women. J. Am. Board Fam. Med. 2019, 32, 575–584. [Google Scholar] [CrossRef]
- Haebich, S.J.; Mark, P.; Khan, R.J.K.; Fick, D.P.; Brownlie, C.; Wimhurst, J.A. The Influence of Obesity on Hip Pain, Function, and Satisfaction 10 Years Following Total Hip Arthroplasty. J. Arthroplasty. 2021, 35, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Özkuk, K.; Ateş, Z. The effect of obesity on pain and disability in chronic shoulder pain patients. J. Back Musculoskelet. Rehabil. 2021, 33, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Adams, M.C.; Vincent, K.R.; Hurley, R.W. Musculoskeletal pain, fear avoidance behaviors, and functional decline in obesity: Potential interventions to manage pain and maintain function. Reg. Anesth. Pain Med. 2013, 38, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.; Ells, L.; Ryan, C.; Martin, D. Perceptions of adults with overweight/obesity and chronic musculoskeletal pain: An interpretative phenomenological analysis. J. Clin. Nurs. 2018, 27, e776–e786. [Google Scholar] [CrossRef]
- Hooper, M.M.; Stellato, T.A.; Hallowell, P.T.; Seitz, B.A.; Moskowitz, R.W. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J. Obes. 2007, 31, 114–120. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Pinto, A.; Ienca, R.; Coppola, G.; Sirianni, G.; Di Lorenzo, G.; Parisi, V.; Serrao, M.; Spagnoli, A.; Vestri, A.; et al. A Randomized Double-Blind, Cross-Over Trial of very Low-Calorie Diet in Overweight Migraine Patients: A Possible Role for Ketones? Nutrients 2019, 11, 1742. [Google Scholar] [CrossRef]
- Wu, J.; Davis-Ajami, M.L.; Lu, Z.K. Impact of migraine on health care utilization and expenses in obese adults: A US population-based study. Clinicoecon. Outcomes Res. 2018, 11, 51–59. [Google Scholar] [CrossRef]
- Miri, A.; Nasiri, M.; Zonoori, S.; Yarahmad, F.; Dabbagh-Moghadam, A.; Askari, G.; Sadeghi, O.; Asadi, M. The association between obesity and migraine in a population of Iranian adults: A case-control study. Diabetes Metab. Syndr. 2018, 12, 733–736. [Google Scholar] [CrossRef]
- Verrotti, A.; Di Fonzo, A.; Penta, L.; Agostinelli, S.; Parisi, P. Obesity and headache/migraine: The importance of weight reduction through lifestyle modifications. Biomed. Res. Int. 2014, 2014, 420858. [Google Scholar] [CrossRef]
- Verrotti, A.; Carotenuto, M.; Altieri, L.; Parisi, P.; Tozzi, E.; Belcastro, V.; Esposito, M.; Guastaferro, N.; Ciuti, A.; Mohn, A.; et al. Migraine and obesity: Metabolic parameters and response to a weight loss programme. Pediatr. Obes. 2015, 10, 220–225. [Google Scholar] [CrossRef]
- Hatami, M.; Soveid, N.; Lesani, A.; Djafarian, K.; Shab-Bidar, S. Migraine and Obesity: Is There a Relationship? A Systematic Review and Meta-Analysis of Observational Studies. CNS Neurol. Disord. Drug Targets 2021, 20, 863–870. [Google Scholar] [CrossRef]
- Miscio, G.; Guastamacchia, G.; Brunani, A.; Priano, L.; Baudo, S.; Mauro, A. Obesity and peripheral neuropathy risk: A dangerous liaison. J. Peripher. Nerv. Syst. 2005, 10, 354–358. [Google Scholar] [CrossRef]
- Hozumi, J.; Sumitani, M.; Matsubayashi, Y.; Abe, H.; Oshima, Y.; Chikuda, H.; Takeshita, K.; Yamada, Y. Relationship between Neuropathic Pain and Obesity. Pain Res. Manag. 2016, 2016, 2487924. [Google Scholar] [CrossRef]
- Herman, R.M.; Brower, J.B.; Stoddard, D.G.; Casano, A.R.; Targovnik, J.H.; Herman, J.H.; Tearse, P. Prevalence of somatic small fiber neuropathy in obesity. Int. J. Obes. 2007, 31, 226–235. [Google Scholar] [CrossRef]
- De Araújo, T.A.; Mota, M.C.; Crispim, C.A. Obesity and sleepiness in women with fibromyalgia. Rheumatol. Int. 2015, 35, 281–287. [Google Scholar] [CrossRef]
- Paiva, E.S.; Andretta, A.; Batista, E.D.; Lobo, M.M.M.T.; Miranda, R.C.; Nisihara, R.; Schieferdecker, M.E.M.; Boguszewski, C.L. Serum levels of leptin and adiponectin and clinical parameters in women with fibromyalgia and overweight/obesity. Arch. Endocrinol. Metab. 2017, 61, 249–256. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Xia, R.; Banerjee, M.; de Rekeneire, N.; Harris, T.B.; Newman, A.B.; Satterfield, S.; Schwartz, A.V.; Vinik, A.I.; Feldman, E.L.; et al. Health ABC Study. Metabolic Syndrome Components Are Associated with Symptomatic Polyneuropathy Independent of Glycemic Status. Diabetes Care 2016, 39, 801–807. [Google Scholar] [CrossRef]
- Stino, A.M.; Smith, A.G. Peripheral neuropathy in prediabetes and the metabolic syndrome. J. Diabetes Investig. 2017, 8, 646–655. [Google Scholar] [CrossRef]
- Truini, A.; Biasiotta, A.; Cesa, S.; Stefano, D.G.; Galeotti, F.; Petrucci, M.T.; Inghilleri, M.; Cartoni, C.; Pergolini, M.; Cruccu, G. Mechanisms of pain in distal symmetric polyneuropathy: A combined clinical and neurophysiological study. Pain 2010, 150, 516–521. [Google Scholar] [CrossRef]
- Truini, A.; Biasiotta, A.; Di Stefano, G.; La Cesa, S.; Leone, C.; Cartoni, C.; Leonetti, F.; Casato, M.; Pergolini, M.; Petrucci, M.T.; et al. Peripheral nociceptor sensitization mediates allodynia in patients with distal symmetric polyneuropathy. J. Neurol. 2013, 260, 761–766. [Google Scholar] [CrossRef]
- Mondelli, M.; Aretini, A.; Baldasseroni, A. Distal symmetric polyneuropathy in diabetes. Differences between patients with and without neuropathic pain. Exp. Clin. Endocrinol. Diabetes 2012, 120, 45–50. [Google Scholar] [CrossRef]
- Tesfaye, S.; Boulton, A.J.; Dickenson, A.H. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care 2013, 36, 2456–2465. [Google Scholar] [CrossRef]
- Callaghan, B.; Kerber, K.; Langa, K.M.; Banerjee, B.; Rodgers, A.; McCammon, R.; Burke, J.; Feldman, E. Longitudinal patient-oriented outcomes in neuropathy: Importance of early detection and falls. Neurology 2015, 85, 71–79. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Price, R.S.; Feldman, E.L. Distal Symmetric Polyneuropathy: A Review. JAMA 2015, 314, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Veresiu, A.I.; Bondor, C.I.; Florea, B.; Vinik, E.J.; Vinik, A.I.; Gâvan, N.A. Detection of undisclosed neuropathy and assessment of its impact on quality of life: A survey in 25,000 Romanian patients with diabetes. J. Diabetes Complicat. 2015, 29, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, P.; Hincker, A.M.; Jensen, T.S.; Freeman, R.; Haroutounian, S. Structural, functional, and symptom relations in painful distal symmetric polyneuropathies: A systematic review. Pain 2019, 160, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Shillo, P.; Sloan, G.; Greig, M.; Hunt, L.; Selvarajah, D.; Elliott, J.; Gandhi, R.; Wilkinson, I.D.; Tesfaye, S. Painful and Painless Diabetic Neuropathies: What Is the Difference? Curr. Diab. Rep. 2019, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Galosi, E.; Hu, X.; Michael, N.; Nyengaard, J.R.; Truini, A.; Karlsson, P. Redefining distal symmetrical polyneuropathy features in type 1 diabetes: A systematic review. Acta Diabetol. 2021, 1–19. [Google Scholar] [CrossRef]
- Kazamel, M.; Stino, A.M.; Smith, A.G. Metabolic syndrome and peripheral neuropathy. Muscle Nerve 2021, 63, 285–293. [Google Scholar] [CrossRef]
- Wolfe, G.I.; Baker, N.S.; Amato, A.A.; Jackson, C.E.; Nations, S.P.; Saperstein, D.S.; Cha, C.H.; Katz, J.S.; Bryan, W.W.; Barohn, R.J. Chronic cryptogenic sensory polyneuropathy: Clinical and laboratory characteristics. Arch. Neurol. 1999, 56, 540–547. [Google Scholar] [CrossRef]
- Pasnoor, M.; Dimachkie, M.M.; Barohn, R.J. Cryptogenic sensory polyneuropathy. Neurol. Clin. 2013, 31, 463–476. [Google Scholar] [CrossRef]
- Emerson, N.M.; Nahman-Averbuch, H.; Peugh, J.L.; Coghill, R.C. Pain sensitivity does not differ between obese and healthy weight individuals. Pain Rep. 2021, 6, e942. [Google Scholar] [CrossRef]
- Garzillo, M.J.; Garzillo, T.A. Does obesity cause low back pain? J. Manip. Physiol. Ther. 1994, 17, 601–604. [Google Scholar]
- Janke, E.A.; Collins, A.; Kozak, A.T. Overview of the relationship between pain and obesity: What do we know? Where do we go next? J. Rehabil. Res. Dev. 2007, 44, 245–262. [Google Scholar] [CrossRef]
- Ursini, F.; Naty, S.; Grembiale, R.D. Fibromyalgia and obesity: The hidden link. Rheumatol. Int. 2011, 31, 1403–1408. [Google Scholar] [CrossRef]
- Arranz, L.I.; Rafecas, M.; Alegre, C. Effects of obesity on function and quality of life in chronic pain conditions. Curr. Rheumatol. Rep. 2014, 16, 390. [Google Scholar] [CrossRef]
- Chai, N.C.; Scher, A.I.; Moghekar, A.; Bond, D.S.; Peterlin, B.L. Obesity and headache: Part I—A systematic review of the epidemiology of obesity and headache. Headache 2014, 54, 219–234. [Google Scholar] [CrossRef]
- Smith, S.M.; Sumar, B.; Dixon, K.A. Musculoskeletal pain in overweight and obese children. Int. J. Obes. 2014, 38, 11–15. [Google Scholar] [CrossRef]
- Taylor, R., Jr.; Pergolizzi, J.V.; Raffa, R.B.; Nalamachu, S.; Balestrieri, P.J. Pain and obesity in the older adult. Curr. Pharm. Des. 2014, 20, 6037–6041. [Google Scholar]
- Narouze, S.; Souzdalnitski, D. Obesity and chronic pain: Systematic review of prevalence and implications for pain practice. Reg. Anesth. Pain Med. 2015, 40, 91–111. [Google Scholar] [CrossRef]
- Okifuji, A.; Hare, B.D. The association between chronic pain and obesity. J. Pain Res. 2015, 8, 399–408. [Google Scholar] [CrossRef]
- Laino, D.; Vitaliti, G.; Parisi, P.; Pavone, P.; Verrotti, A.; Lubrano, R.; Matin, N.; Falsaperla, R. Headache, migraine and obesity: An overview on plausible links. J. Biol. Regul. Homeost. Agents 2016, 30, 333–338. [Google Scholar]
- Torensma, B.; Thomassen, I.; van Velzen, M.; In‘t Veld, B.A. Pain Experience and Perception in the Obese Subject Systematic Review (Revised Version). Obes. Surg. 2016, 26, 631–639. [Google Scholar] [CrossRef]
- Pavlovic, J.M.; Vieira, J.R.; Lipton, R.B.; Bond, D.S. Association Between Obesity and Migraine in Women. Curr. Pain Headache Rep. 2017, 21, 41. [Google Scholar] [CrossRef]
- Chin, S.H.; Huang, W.L.; Akter, S.; Binks, M. Obesity and pain: A systematic review. Int. J. Obes. 2020, 44, 969–979. [Google Scholar] [CrossRef]
- D’Onghia, M.; Ciaffi, J.; Lisi, L.; Mancarella, L.; Ricci, S.; Stefanelli, N.; Meliconi, R.; Ursini, F. Fibromyalgia and obesity: A comprehensive systematic review and meta-analysis. Semin. Arthritis Rheum. 2021, 51, 409–424. [Google Scholar] [CrossRef]
- Qian, M.; Shi, Y.; Yu, M. The association between obesity and chronic pain among community-dwelling older adults: A systematic review and meta-analysis. Geriatr. Nurs. 2021, 42, 8–15. [Google Scholar] [CrossRef]
- Mäntyselkä, P.; Kautiainen, H.; Vanhala, M. Prevalence of neck pain in subjects with metabolic syndrome--a cross-sectional population-based study. BMC Musculoskelet. Disord. 2010, 11, 171. [Google Scholar] [CrossRef]
- Ono, R.; Yamazaki, S.; Takegami, M.; Otani, K.; Sekiguchi, M.; Onishi, Y.; Hayashino, Y.; Kikuchi, S.; Konno, S.; Fukuhara, S. Gender difference in association between low back pain and metabolic syndrome: Locomotive syndrome and health outcome in Aizu cohort study (LOHAS). Spine 2012, 37, 1130–1137. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Ochiai, H.; Shirasawa, T.; Nagahama, S.; Uehara, A.; Sai, S.; Kokaze, A. Sex differences in the association of metabolic syndrome with low back pain among middle-aged Japanese adults: A large-scale cross-sectional study. Biol. Sex Differ. 2019, 10, 33. [Google Scholar] [CrossRef]
- Kalita, J.; Sonkar, K.K.; Misra, U.K.; Bhoi, S.K. Does Metabolic Syndrome Determine Severity and Disability of Chronic Low Backache? J. Neurosci. Rural. Pract. 2018, 9, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.A.; Sabia, S.; Singh-Manoux, A.; Hamer, M.; Kivimäki, M. Healthy obesity and risk of accelerated functional decline and disability. Int J. Obes 2017, 41, 866–872. [Google Scholar] [CrossRef] [PubMed]
- IASP—International Association for the Study of Pain. Terminology. Updated August 2021. Available online: https://www.iasp-pain.org/resources/terminology (accessed on 9 August 2021).
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef]
- Trouvin, A.P.; Perrot, S. New concepts of pain. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101415. [Google Scholar] [CrossRef]
- Freynhagen, R.; Parada, H.A.; Calderon-Ospina, C.A.; Chen, J.; Rakhmawati Emril, D.; Fernández-Villacorta, F.J.; Franco, H.; Ho, K.Y.; Lara-Solares, A.; Li, C.C.; et al. Current understanding of the mixed pain concept: A brief narrative review. Curr. Med. Res. Opin. 2019, 35, 1011–1018. [Google Scholar] [CrossRef]
- Nijs, J.; Lahousse, A.; Kapreli, E.; Bilika, P.; Saraçoğlu, İ.; Malfliet, A.; Coppieters, I.; De Baets, L.; Leysen, L.; Roose, E.; et al. Nociplastic Pain Criteria or Recognition of Central Sensitization? Pain Phenotyping in the Past, Present and Future. J. Clin. Med. 2021, 10, 3203. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef]
- Li, L.; Hatcher, J.T.; Hoover, D.B.; Gu, H.; Wurster, R.D.; Cheng, Z.J. Distribution and morphology of calcitonin gene-related peptide and substance P immunoreactive axons in the whole-mount atria of mice. Auton. Neurosci. 2014, 181, 37–48. [Google Scholar] [CrossRef]
- Staszewska-Woolley, J.; Luk, D.E.; Nolan, P.N. Cardiovascular reflexes mediated by capsaicin sensitive cardiac afferent neurones in the dog. Cardiovasc. Res. 1986, 20, 897–906. [Google Scholar] [CrossRef]
- Baker, D.G.; Coleridge, H.M.; Coleridge, J.C.; Nerdrum, T. Search for a cardiac nociceptor: Stimulation by bradykinin of sympathetic afferent nerve endings in the heart of the cat. J. Physiol. 1980, 306, 519–536. [Google Scholar] [CrossRef]
- Marron, K.; Wharton, J.; Sheppard, M.N.; Fagan, D.; Royston, D.; Kuhn, D.M.; de Leval, M.R.; Whitehead, B.F.; Anderson, R.H.; Polak, J.M. Distribution, morphology, and neurochemistry of endocardial and epicardial nerve terminal arborizations in the human heart. Circulation 1995, 92, 2343–2351. [Google Scholar] [CrossRef]
- Corvetti, G.; Andreotti, L.; Sisto Daneo, L. Chick heart peptidergic innervation: Localization and development. Basic Appl. Histochem. 1988, 32, 485–493. [Google Scholar]
- Gordon, L.; Polak, J.M.; Moscoso, G.J.; Smith, A.; Kuhn, D.M.; Wharton, J. Development of the peptidergic innervation of human heart. J. Anat. 1993, 183, 131–140. [Google Scholar]
- Saricaoglu, Ö.C.; Teller, S.; Wang, X.; Wang, S.; Stupakov, P.; Heinrich, T.; Istvanffy, R.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Localisation analysis of nerves in the mouse pancreas reveals the sites of highest nerve density and nociceptive innervation. Neurogastroenterol. Motil. 2021, 32, e13880. [Google Scholar] [CrossRef]
- Carobi, C.; Magni, F. Capsaicin-sensitive afferent vagal neurons innervating the rat liver. Neurosci. Lett. 1985, 62, 261–265. [Google Scholar] [CrossRef]
- Hockley, J.R.F.; Smith, E.S.J.; Bulmer, D.C. Human visceral nociception: Findings from translational studies in human tissue. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G464–G472. [Google Scholar] [CrossRef]
- Siri, S.; Maier, F.; Chen, L.; Santos, S.; Pierce, D.M.; Feng, B. Differential biomechanical properties of mouse distal colon and rectum innervated by the splanchnic and pelvic afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G473–G481. [Google Scholar] [CrossRef]
- Kwong, K.; Carr, M.J.; Gibbard, A.; Savage, T.J.; Singh, K.; Jing, J.; Meeker, S.; Undem, B.J. Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs. J. Physiol. 2008, 586, 1321–1336. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sato, Y.; Taniguchi, K. Distribution of TRPV1- and TRPV2-immunoreactive afferent nerve endings in rat trachea. J. Anat. 2007, 211, 775–783. [Google Scholar] [CrossRef]
- Nassenstein, C.; Kwong, K.; Taylor-Clark, T.; Kollarik, M.; Macglashan, D.M.; Braun, A.; Undem, B.J. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J. Physiol. 2008, 586, 1595–1604. [Google Scholar] [CrossRef]
- Tanaka, K.; Hayakawa, T.; Maeda, S.; Kuwahara-Otani, S.; Seki, M. Distribution and ultrastructure of afferent fibers in the parietal peritoneum of the rat. Anat. Rec. 2011, 294, 1736–1742. [Google Scholar] [CrossRef]
- Bentley, G.A.; Newton, S.H.; Starr, J. Evidence for an action of morphine and the enkephalins on sensory nerve endings in the mouse peritoneum. Br. J. Pharmacol. 1981, 73, 325–332. [Google Scholar] [CrossRef]
- Jankowski, M.P.; Rau, K.K.; Ekmann, K.M.; Anderson, C.E.; Koerber, H.R. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J. Neurophysiol. 2013, 109, 2374–2381. [Google Scholar] [CrossRef]
- Taguchi, T.; Yasui, M.; Kubo, A.; Abe, M.; Kiyama, H.; Yamanaka, A.; Mizumura, K. Nociception originating from the crural fascia in rats. Pain 2013, 154, 1103–1114. [Google Scholar] [CrossRef]
- Alhilou, A.M.; Shimada, A.; Svensson, C.I.; Ernberg, M.; Cairns, B.E.; Christidis, N. Density of nerve fibres and expression of substance P, NR2B-receptors and nerve growth factor in healthy human masseter muscle: An immunohistochemical study. J. Oral Rehabil. 2021, 48, 35–44. [Google Scholar] [CrossRef]
- Marchettini, P.; Simone, D.A.; Caputi, G.; Ochoa, J.L. Pain from excitation of identified muscle nociceptors in humans. Brain Res. 1996, 740, 109–116. [Google Scholar] [CrossRef]
- Dauch, J.R.; Lindblad, C.N.; Hayes, J.M.; Lentz, S.I.; Cheng, H.T. Three-dimensional imaging of nociceptive intraepidermal nerve fibers in human skin biopsies. J. Vis. Exp. 2013, 74, e50331. [Google Scholar] [CrossRef]
- Mouraux, A.; Ragé, M.; Bragard, D.; Plaghki, L. Estimation of intraepidermal fiber density by the detection rate of nociceptive laser stimuli in normal and pathological conditions. Neurophysiol. Clin. 2012, 42, 281–291. [Google Scholar] [CrossRef]
- Lauria, G.; Morbin, M.; Lombardi, R.; Capobianco, R.; Camozzi, F.; Pareyson, D.; Manconi, M.; Geppetti, P. Expression of capsaicin receptor immunoreactivity in human peripheral nervous system and in painful neuropathies. J. Peripher. Nerv. Syst. 2006, 11, 262–271. [Google Scholar] [CrossRef]
- Simone, D.A.; Nolano, M.; Johnson, T.; Wendelschafer-Crabb, G.; Kennedy, W.R. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: Correlation with sensory function. J. Neurosci. 1998, 18, 8947–8959. [Google Scholar] [CrossRef] [PubMed]
- Müller, T. Intraepidermal free nerve fiber endings in the hairless skin of the rat as revealed by the zinc iodide-osmium tetroxide technique. Histol. Histopathol. 2000, 15, 493–498. [Google Scholar] [PubMed]
- Kruger, L.; Sampogna, S.L.; Rodin, B.E.; Clague, J.; Brecha, N.; Yeh, Y. Thin-fiber cutaneous innervation and its intraepidermal contribution studied by labeling methods and neurotoxin treatment in rats. Somat. Res. 1985, 2, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Navarro, X.; Verdú, E.; Wendelschafer-Crabb, G.; Kennedy, W.R. Immunohistochemical study of skin reinnervation by regenerative axons. J. Comp. Neurol. 1997, 380, 164–174. [Google Scholar] [CrossRef]
- Navarro, X.; Verdú, E.; Wendelscafer-Crabb, G.; Kennedy, W.R. Innervation of cutaneous structures in the mouse hind paw: A confocal microscopy immunohistochemical study. J. Neurosci. Res. 1995, 41, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Vijay, J.; Gauthier, M.F.; Biswell, R.L.; Louiselle, D.A.; Johnston, J.J.; Cheung, W.A.; Belden, B.; Pramatarova, A.; Biertho, L.; Gibson, M.; et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat. Metab. 2021, 2, 97–109. [Google Scholar] [CrossRef]
- Esteve Ràfols, M. Adipose tissue: Cell heterogeneity and functional diversity. Endocrinol. Nutr. 2014, 61, 100–112. [Google Scholar] [CrossRef]
- Iannitti, T.; Graham, A.; Dolan, S. Adiponectin-Mediated Analgesia and Anti-Inflammatory Effects in Rat. PLoS ONE 2015, 10, e0136819. [Google Scholar]
- Luo, Y.; Liu, M. Adiponectin: A versatile player of innate immunity. J. Mol. Cell Biol. 2016, 8, 120–128. [Google Scholar] [CrossRef]
- Żelechowska, P.; Brzezińska-Błaszczyk, E.; Wiktorska, M.; Różalska, S.; Wawrocki, S.; Kozłowska, E.; Agier, J. Adipocytokines leptin and adiponectin function as mast cell activity modulators. Immunology 2019, 158, 3–18. [Google Scholar] [CrossRef]
- Ma, W.; Chabot, J.G.; Quirion, R. A role for adrenomedullin as a pain-related peptide in the rat. Proc. Natl. Acad. Sci. USA 2006, 103, 16027–16032. [Google Scholar] [CrossRef]
- Shepherd, A.J.; Copits, B.A.; Mickle, A.D.; Karlsson, P.; Kadunganattil, S.; Haroutounian, S.; Tadinada, S.M.; de Kloet, A.D.; Valtcheva, M.V.; McIlvried, L.A.; et al. Angiotensin II Triggers Peripheral Macrophage-to-Sensory Neuron Redox Crosstalk to Elicit Pain. J. Neurosci. 2018, 38, 7032–7057. [Google Scholar] [CrossRef]
- Canpolat, S.; Ozcan, M.; Saral, S.; Kalkan, O.F.; Ayar, A. Effects of apelin-13 in mice model of experimental pain and peripheral nociceptive signaling in rat sensory neurons. J. Recept. Signal Transduct. Res. 2016, 36, 243–247. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, X.; Zhou, Y.; Feng, Y.; Yang, Y.; Wang, X. Intrathecally Administered Apelin-13 Alleviated Complete Freund’s Adjuvant-Induced Inflammatory Pain in Mice. Front. Pharmacol. 2021, 11, 1335. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, X.; Feng, Y.; Zhou, Y.; Cui, B.; Yang, Y.; Wang, X. Intravenous Administration of Pyroglutamyl Apelin-13 Alleviates Murine Inflammatory Pain via the Kappa Opioid Receptor. Front. Neurosci. 2021, 14, 929. [Google Scholar] [CrossRef]
- Xiong, Q.; He, W.; Wang, H.; Zhou, J.; Zhang, Y.; He, J.; Yang, C.; Zhang, B. Effect of the spinal apelin-APJ system on the pathogenesis of chronic constriction injury-induced neuropathic pain in rats. Mol. Med. Rep. 2017, 16, 1223–1231. [Google Scholar] [CrossRef][Green Version]
- Doyle, J.R.; Krishnaji, S.T.; Zhu, G.; Xu, Z.Z.; Heller, D.; Ji, R.R.; Levy, B.D.; Kumar, K.; Kopin, A.S. Development of a membrane-anchored chemerin receptor agonist as a novel modulator of allergic airway inflammation and neuropathic pain. J. Biol. Chem. 2014, 289, 13385–13396. [Google Scholar] [CrossRef]
- Dickie, A.C.; Torsney, C. The chemerin receptor 23 agonist, chemerin, attenuates monosynaptic C-fibre input to lamina I neurokinin 1 receptor expressing rat spinal cord neurons in inflammatory pain. Mol. Pain 2014, 10, 24. [Google Scholar] [CrossRef]
- Oehler, B.; Mohammadi, M.; Perpina Viciano, C.; Hackel, D.; Hoffmann, C.; Brack, A.; Rittner, H.L. Peripheral Interaction of Resolvin D1 and E1 with Opioid Receptor Antagonists for Antinociception in Inflammatory Pain in Rats. Front. Mol. Neurosci. 2017, 10, 242. [Google Scholar] [CrossRef]
- Hu, Z.J.; Han, W.; Cao, C.Q.; Mao-Ying, Q.L.; Mi, W.L.; Wang, Y.Q. Peripheral Leptin Signaling Mediates Formalin-Induced Nociception. Neurosci. Bull. 2018, 34, 321–329. [Google Scholar] [CrossRef]
- Maeda, T.; Kiguchi, N.; Kobayashi, Y.; Ikuta, T.; Ozaki, M.; Kishioka, S. Leptin derived from adipocytes in injured peripheral nerves facilitates development of neuropathic pain via macrophage stimulation. Proc. Natl. Acad. Sci. USA 2009, 106, 13076–13081. [Google Scholar] [CrossRef]
- Li, X.; Kang, L.; Li, G.; Zeng, H.; Zhang, L.; Ling, X.; Dong, H.; Liang, S.; Chen, H. Intrathecal leptin inhibits expression of the P2 × 2/3 receptors and alleviates neuropathic pain induced by chronic constriction sciatic nerve injury. Mol. Pain 2013, 9, 65. [Google Scholar] [CrossRef]
- Harmon, J.B.; Sanders, A.E.; Wilder, R.S.; Essick, G.K.; Slade, G.D.; Hartung, J.E.; Nackley, A.G. Circulating Omentin-1 and Chronic Painful Temporomandibular Disorders. J. Oral Facial Pain Headache 2016, 30, 203–209. [Google Scholar] [CrossRef]
- Slovacek, H.; Khanna, R.; Poredos, P.; Jezovnik, M.; Hoppensteadt, D.; Fareed, J.; Hopkinson, W. Interrelationship of Osteopontin, MMP-9 and ADAMTS4 in Patients With Osteoarthritis Undergoing Total Joint Arthroplasty. Clin. Appl. Thromb. Hemost. 2021, 26, 1076029620964864. [Google Scholar] [CrossRef]
- Yamaga, M.; Tsuji, K.; Miyatake, K.; Yamada, J.; Abula, K.; Ju, Y.J.; Sekiya, I.; Muneta, T. Osteopontin level in synovial fluid is associated with the severity of joint pain and cartilage degradation after anterior cruciate ligament rupture. PLoS ONE 2012, 7, e49014. [Google Scholar] [CrossRef]
- Marsh, B.C.; Kerr, N.C.; Isles, N.; Denhardt, D.T.; Wynick, D. Osteopontin expression and function within the dorsal root ganglion. Neuroreport 2007, 18, 153–157. [Google Scholar] [CrossRef]
- Sagar, D.R.; Ashraf, S.; Xu, L.; Burston, J.J.; Menhinick, M.R.; Poulter, C.L.; Bennett, A.J.; Walsh, D.A.; Chapman, V. Osteoprotegerin reduces the development of pain behaviour and joint pathology in a model of osteoarthritis. Ann. Rheum. Dis. 2014, 73, 1558–1565. [Google Scholar] [CrossRef]
- Roudier, M.P.; Bain, S.D.; Dougall, W.C. Effects of the RANKL inhibitor, osteoprotegerin, on the pain and histopathology of bone cancer in rats. Clin. Exp. Metastasis. 2006, 23, 167–175. [Google Scholar] [CrossRef]
- Luger, N.M.; Honore, P.; Sabino, M.A.; Schwei, M.J.; Rogers, S.D.; Mach, D.B.; Clohisy, D.R.; Mantyh, P.W. Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res. 2001, 61, 4038–4047. [Google Scholar]
- Fioravanti, A.; Giannitti, C.; Cheleschi, S.; Simpatico, A.; Pascarelli, N.A.; Galeazzi, M. Circulating levels of adiponectin, resistin, and visfatin after mud-bath therapy in patients with bilateral knee osteoarthritis. Int. J. Biometeorol. 2015, 59, 1691–1700. [Google Scholar] [CrossRef]
- Bas, S.; Finckh, A.; Puskas, G.J.; Suva, D.; Hoffmeyer, P.; Gabay, C.; Lübbeke, A. Adipokines correlate with pain in lower limb osteoarthritis: Different associations in hip and knee. Int. Orthop. 2014, 38, 2577–2583. [Google Scholar] [CrossRef] [PubMed]
- Tarabeih, N.; Kalinkovich, A.; Shalata, A.; Livshits, G. Circulating Levels of Visceral Adipose Tissue-Derived Serine Protease Inhibitor (Vaspin) Appear as a Marker of Musculoskeletal Pain Disability. Diagnostics 2021, 10, 797. [Google Scholar] [CrossRef] [PubMed]
- Askari, A.; Arasteh, P.; Homayounfar, R.; Naghizadeh, M.M.; Ehrampoush, E.; Mousavi, S.M.; Alipoor, R. The role of adipose tissue secretion in the creation and pain level in osteoarthritis. Endocr. Regul. 2021, 54, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, L.S.; Xiao, W.H.; Wagner, R.; Myers, R.R. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience 1997, 81, 255–262. [Google Scholar] [CrossRef]
- Hakim, A.W.; Dong, X.D.; Svensson, P.; Kumar, U.; Cairns, B.E. TNFalpha mechanically sensitizes masseter muscle afferent fibers of male rats. J. Neurophysiol. 2009, 102, 1551–1559. [Google Scholar] [CrossRef]
- Brenn, D.; Richter, F.; Schaible, H.G. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: An inflammatory mechanism of joint pain. Arthritis Rheum. 2007, 56, 351–359. [Google Scholar] [CrossRef]
- Fu, L.W.; Longhurst, J.C. Interleukin-1beta sensitizes abdominal visceral afferents of cats to ischaemia and histamine. J. Physiol. 1999, 521, 249–260. [Google Scholar] [CrossRef]
- Ek, M.; Kurosawa, M.; Lundeberg, T.; Ericsson, A. Activation of vagal afferents after intravenous injection of interleukin-1beta: Role of endogenous prostaglandins. J. Neurosci. 1998, 18, 9471–9479. [Google Scholar] [CrossRef]
- Fukuoka, H.; Kawatani, M.; Hisamitsu, T.; Takeshige, C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Res. 1994, 657, 133–140. [Google Scholar] [CrossRef]
- Flatters, S.J.; Fox, A.J.; Dickenson, A.H. Nerve injury alters the effects of interleukin-6 on nociceptive transmission in peripheral afferents. Eur. J. Pharmacol. 2004, 484, 183–191. [Google Scholar] [CrossRef]
- Day, Y.J.; Liou, J.T.; Lee, C.M.; Lin, Y.C.; Mao, C.C.; Chou, A.H.; Liao, C.C.; Lee, H.C. Lack of interleukin-17 leads to a modulated micro-environment and amelioration of mechanical hypersensitivity after peripheral nerve injury in mice. Pain 2014, 155, 1293–1302. [Google Scholar] [CrossRef]
- Liou, J.T.; Mao, C.C.; Ching-Wah Sum, D.; Liu, F.C.; Lai, Y.S.; Li, J.C.; Day, Y.J. Peritoneal administration of Met-RANTES attenuates inflammatory and nociceptive responses in a murine neuropathic pain model. J. Pain 2013, 14, 24–35. [Google Scholar] [CrossRef]
- McNamee, K.E.; Alzabin, S.; Hughes, J.P.; Anand, P.; Feldmann, M.; Williams, R.O.; Inglis, J.J. IL-17 induces hyperalgesia via TNF-dependent neutrophil infiltration. Pain 2011, 152, 1838–1845. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Manchope, M.F.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; Verri, W.A. Targeting IL-33/ST2 signaling: Regulation of immune function and analgesia. Expert Opin. Ther. Targets 2017, 21, 1141–1152. [Google Scholar] [CrossRef]
- Richards, N.; Batty, T.; Dilley, A. CCL2 has similar excitatory effects to TNF-alpha in a subgroup of inflamed C-fiber axons. J. Neurophysiol. 2011, 106, 2838–2848. [Google Scholar] [CrossRef]
- Kao, D.J.; Li, A.H.; Chen, J.C.; Luo, R.S.; Chen, Y.L.; Lu, J.C.; Wang, H.L. CC chemokine ligand 2 upregulates the current density and expression of TRPV1 channels and Nav1.8 sodium channels in dorsal root ganglion neurons. J. Neuroinflammation 2012, 9, 189. [Google Scholar] [CrossRef]
- Bhangoo, S.; Ren, D.; Miller, R.J.; Henry, K.J.; Lineswala, J.; Hamdouchi, C.; Li, B.; Monahan, P.E.; Chan, D.M.; Ripsch, M.S.; et al. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: A mechanism for the development of chronic sensitization of peripheral nociceptors. Mol. Pain 2007, 3, 38. [Google Scholar] [CrossRef]
- Liou, J.T.; Yuan, H.B.; Mao, C.C.; Lai, Y.S.; Day, Y.J. Absence of C-C motif chemokine ligand 5 in mice leads to decreased local macrophage recruitment and behavioral hypersensitivity in a murine neuropathic pain model. Pain 2012, 153, 1283–1291. [Google Scholar] [CrossRef]
- Silva, R.L.; Lopes, A.H.; Guimarães, R.M.; Cunha, T.M. CXCL1/CXCR2 signaling in pathological pain: Role in peripheral and central sensitization. Neurobiol. Dis. 2017, 105, 109–116. [Google Scholar] [CrossRef]
- Sun, Y.; Sahbaie, P.; Liang, D.; Li, W.; Clark, J.D. Opioids enhance CXCL1 expression and function after incision in mice. J. Pain 2014, 15, 856–866. [Google Scholar] [CrossRef]
- Souza, G.R.; Talbot, J.; Lotufo, C.M.; Cunha, F.Q.; Cunha, T.M.; Ferreira, S.H. Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc. Natl. Acad. Sci. USA 2013, 110, 11193–11198. [Google Scholar] [CrossRef]
- Andres, C.; Hasenauer, J.; Ahn, H.S.; Joseph, E.K.; Isensee, J.; Theis, F.J.; Allgöwer, F.; Levine, J.D.; Dib-Hajj, S.D.; Waxman, S.G.; et al. Wound-healing growth factor, basic FGF, induces Erk1/2-dependent mechanical hyperalgesia. Pain 2013, 154, 2216–2226. [Google Scholar] [CrossRef]
- Qiu, C.Y.; Liu, T.T.; Wei, S.; Zhou, Y.M.; Wu, L.; Jin, Y.; Hu, W.P. TGF-beta1 enhances the activity of acid-sensing ion channel in rat primary sensory neurons. J. Neurosci. Res. 2019, 97, 1298–1305. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, X.M.; Duan, K.Z.; Gu, X.Y.; Han, M.; Liu, B.L.; Zhao, Z.Q.; Zhang, Y.Q. Peripheral TGF-beta1 signaling is a critical event in bone cancer-induced hyperalgesia in rodents. J. Neurosci. 2013, 33, 19099–19111. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, Y.; Noë, M.; Li, Q.; Pasricha, P.J. Neuronal Transforming Growth Factor beta Signaling via SMAD3 Contributes to Pain in Animal Models of Chronic Pancreatitis. Gastroenterology 2018, 154, 2252–2265.e2. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Kadowaki, Y.; Fukazawa, Y.; Saika, F.; Kishioka, S. Vascular endothelial growth factor signaling in injured nerves underlies peripheral sensitization in neuropathic pain. J. Neurochem. 2014, 129, 169–178. [Google Scholar] [CrossRef]
- Hulse, R.P.; Beazley-Long, N.; Hua, J.; Kennedy, H.; Prager, J.; Bevan, H.; Qiu, Y.; Fernandes, E.S.; Gammons, M.V.; Ballmer-Hofer, K.; et al. Regulation of alternative VEGF-A mRNA splicing is a therapeutic target for analgesia. Neurobiol. Dis. 2014, 71, 245–259. [Google Scholar] [CrossRef]
- Salvemini, D.; Little, J.W.; Doyle, T.; Neumann, W.L. Roles of reactive oxygen and nitrogen species in pain. Free Radic. Biol. Med. 2011, 51, 951–966. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Schröder, K.; Del Turco, D.; Lu, R.; Kynast, K.; Kosowski, J.; Niederberger, E.; Shah, A.M.; Brandes, R.P.; Geisslinger, G.; et al. NADPH oxidase-4 maintains neuropathic pain after peripheral nerve injury. J. Neurosci. 2012, 32, 10136–10145. [Google Scholar] [CrossRef]
- Holthusen, H.; Arndt, J.O. Nitric oxide evokes pain in humans on intracutaneous injection. Neurosci. Lett. 1994, 165, 71–74. [Google Scholar] [CrossRef]
- Kawabata, A.; Ishiki, T.; Nagasawa, K.; Yoshida, S.; Maeda, Y.; Takahashi, T.; Sekiguchi, F.; Wada, T.; Ichida, S.; Nishikawa, H. Hydrogen sulfide as a novel nociceptive messenger. Pain 2007, 132, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Aoki, Y.; Sekiguchi, F.; Matsunami, M.; Takahashi, T.; Nishikawa, H.; Kawabata, A. Hyperalgesia induced by spinal and peripheral hydrogen sulfide: Evidence for involvement of Cav3.2 T-type calcium channels. Pain 2009, 142, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Jakicic, J.M.; Clark, K.; Coleman, E.; Donnelly, J.E.; Foreyt, J.; Melanson, E.; Volek, J.; Volpe, S.L. American College of Sports Medicine. American College of Sports Medicine position stand. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2001, 33, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2018. [Google Scholar]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H.; Obesity Management Task Force of the European Association for the Study of Obesity. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, G.; Blake, C.; Cunningham, C.; Lennon, O.; Perrotta, C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes. Rev. 2021, 22, e13137. [Google Scholar] [CrossRef]
- Sultana, R.N.; Sabag, A.; Keating, S.E.; Johnson, N.A. The Effect of Low-Volume High-Intensity Interval Training on Body Composition and Cardiorespiratory Fitness: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 1687–1721. [Google Scholar] [CrossRef]
- Sabag, A.; Little, J.P.; Johnson, N.A. Low-volume high-intensity interval training for cardiometabolic health. J. Physiol. 2021, 1–14. [Google Scholar] [CrossRef]
- Ho, S.S.; Dhaliwal, S.S.; Hills, A.P.; Pal, S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health. 2012, 12, 704. [Google Scholar] [CrossRef]
- Stinkens, R.; Brouwers, B.; Jocken, J.W.; Blaak, E.E.; Teunissen-Beekman, K.F.; Hesselink, M.K.; van Baak, M.A.; Schrauwen, P.; Goossens, G.H. Exercise training-induced effects on the abdominal subcutaneous adipose tissue phenotype in humans with obesity. J. Appl. Physiol. 2018, 125, 1585–1593. [Google Scholar] [CrossRef]
- Donges, C.E.; Duffield, R.; Drinkwater, E.J. Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med. Sci. Sports Exerc. 2010, 42, 304–313. [Google Scholar] [CrossRef]
- Amano, Y.; Nonaka, Y.; Takeda, R.; Kano, Y.; Hoshino, D. Effects of electrical stimulation-induced resistance exercise training on white and brown adipose tissues and plasma meteorin-like concentration in rats. Physiol. Rep. 2021, 8, e14540. [Google Scholar] [CrossRef]
- Walton, R.G.; Finlin, B.S.; Mula, J.; Long, D.E.; Zhu, B.; Fry, C.S.; Westgate, P.M.; Lee, J.D.; Bennett, T.; Kern, P.A.; et al. Insulin-resistant subjects have normal angiogenic response to aerobic exercise training in skeletal muscle, but not in adipose tissue. Physiol. Rep. 2015, 3, e12415. [Google Scholar] [CrossRef]
- Van Pelt, D.W.; Guth, L.M.; Horowitz, J.F. Aerobic exercise elevates markers of angiogenesis and macrophage IL-6 gene expression in the subcutaneous adipose tissue of overweight-to-obese adults. J. Appl. Physiol. 2017, 123, 1150–1159. [Google Scholar] [CrossRef]
- De Glisezinski, I.; Crampes, F.; Harant, I.; Berlan, M.; Hejnova, J.; Langin, D.; Rivière, D.; Stich, V. Endurance training changes in lipolytic responsiveness of obese adipose tissue. Am. J. Physiol. 1998, 275, E951–E956. [Google Scholar] [CrossRef]
- Lakhdar, N.; Denguezli, M.; Zaouali, M.; Zbidi, A.; Tabka, Z.; Bouassida, A. Six months training alone or combined with diet alters HOMA-AD, HOMA-IR and plasma and adipose tissue adiponectin in obese women. Neuro. Endocrinol. Lett. 2014, 35, 373–379. [Google Scholar]
- Oh, S.; So, R.; Shida, T.; Matsuo, T.; Kim, B.; Akiyama, K.; Isobe, T.; Okamoto, Y.; Tanaka, K.; Shoda, J. High-Intensity Aerobic Exercise Improves Both Hepatic Fat Content and Stiffness in Sedentary Obese Men with Nonalcoholic Fatty Liver Disease. Sci. Rep. 2017, 7, 43029. [Google Scholar] [CrossRef]
- Langleite, T.M.; Jensen, J.; Norheim, F.; Gulseth, H.L.; Tangen, D.S.; Kolnes, K.J.; Heck, A.; Storås, T.; Grøthe, G.; Dahl, M.A.; et al. Insulin sensitivity, body composition and adipose depots following 12 w combined endurance and strength training in dysglycemic and normoglycemic sedentary men. Arch. Physiol. Biochem. 2016, 122, 167–179. [Google Scholar] [CrossRef]
- Jamka, M.; Mądry, E.; Krzyżanowska-Jankowska, P.; Skrypnik, D.; Szulińska, M.; Mądry, R.; Lisowska, A.; Batyrova, G.; Duś-Żuchowska, M.; Gotz-Więckowska, A.; et al. The effect of endurance and endurance-strength training on body composition and cardiometabolic markers in abdominally obese women: A randomised trial. Sci. Rep. 2021, 11, 12339. [Google Scholar] [CrossRef]
- Costa, J.S.R.; Fonseca, G.F.A.C.; Ottone, N.C.D.S.; Silva, P.A.; Antonaccio, R.F.; Silva, G.; Rocha, M.D.S.A.; Coimbra, C.C.; Esteves, E.A.; Mang, Z.A.; et al. Strength training improves insulin resistance and differently affects mitochondria in skeletal muscle and visceral adipose tissue in high-fat fed mice. Life Sci. 2021, 278, 119639. [Google Scholar] [CrossRef]
- Lee, S.; Norheim, F.; Gulseth, H.L.; Langleite, T.M.; Kolnes, K.J.; Tangen, D.S.; Stadheim, H.K.; Gilfillan, G.D.; Holen, T.; Birkeland, K.I.; et al. Interaction between plasma fetuin-A and free fatty acids predicts changes in insulin sensitivity in response to long-term exercise. Physiol. Rep. 2017, 5, e13183. [Google Scholar] [CrossRef]
- Donges, C.E.; Duffield, R.; Guelfi, K.J.; Smith, G.C.; Adams, D.R.; Edge, J.A. Comparative effects of single-mode vs. duration-matched concurrent exercise training on body composition, low-grade inflammation, and glucose regulation in sedentary, overweight, middle-aged men. Appl. Physiol. Nutr. Metab. 2013, 38, 779–788. [Google Scholar] [CrossRef]
- Mauriège, P.; Prud’Homme, D.; Marcotte, M.; Yoshioka, M.; Tremblay, A.; Després, J.P. Regional differences in adipose tissue metabolism between sedentary and endurance-trained women. Am. J. Physiol. 1997, 273, E497–E506. [Google Scholar] [CrossRef]
- Lamarche, B.; Després, J.P.; Moorjani, S.; Nadeau, A.; Lupien, P.J.; Tremblay, A.; Thériault, G.; Bouchard, C. Evidence for a role of insulin in the regulation of abdominal adipose tissue lipoprotein lipase response to exercise training in obese women. Int. J. Obes. Relat. Metab. Disord. 1993, 17, 255–261. [Google Scholar]
- Nordby, P.; Auerbach, P.L.; Rosenkilde, M.; Kristiansen, L.; Thomasen, J.R.; Rygaard, L.; Groth, R.; Brandt, N.; Helge, J.W.; Richter, E.A.; et al. Endurance training per se increases metabolic health in young, moderately overweight men. Obesity 2012, 20, 2202–2212. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P.; Bouchard, C.; Savard, R.; Tremblay, A.; Marcotte, M.; Thériault, G. The effect of a 20-week endurance training program on adipose-tissue morphology and lipolysis in men and women. Metabolism 1984, 33, 235–239. [Google Scholar] [CrossRef]
- Moghadasi, M.; Mohebbi, H.; Rahmani-Nia, F.; Hassan-Nia, S.; Noroozi, H.; Pirooznia, N. High-intensity endurance training improves adiponectin mRNA and plasma concentrations. Eur. J. Appl. Physiol. 2012, 112, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Eijnde, B.O.; Roelants, M.; Broekmans, T.; Rummens, J.L.; Hensen, K.; Daniels, A.; Van Erum, M.; Bonné, K.; Reyckers, I.; et al. Clinical benefits of the addition of lower extremity low-intensity resistance muscle training to early aerobic endurance training intervention in patients with coronary artery disease: A randomized controlled trial. J. Rehabil. Med. 2011, 43, 800–807. [Google Scholar] [CrossRef]
- Halverstadt, A.; Phares, D.A.; Wilund, K.R.; Goldberg, A.P.; Hagberg, J.M. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism 2007, 56, 444–450. [Google Scholar] [CrossRef]
- Couillard, C.; Després, J.P.; Lamarche, B.; Bergeron, J.; Gagnon, J.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H.; Bouchard, C. Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides: Evidence from men of the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) Family Study. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1226–1232. [Google Scholar] [CrossRef]
- Bladbjerg, E.M.; Skov, J.; Nordby, P.; Stallknecht, B. Endurance exercise per se reduces the cardiovascular risk marker t-PA antigen in healthy, younger, overweight men. Thromb. Res. 2017, 152, 69–73. [Google Scholar] [CrossRef]
- Tjønna, A.E.; Leinan, I.M.; Bartnes, A.T.; Jenssen, B.M.; Gibala, M.J.; Winett, R.A.; Wisløff, U. Low- and high-volume of intensive endurance training significantly improves maximal oxygen uptake after 10-weeks of training in healthy men. PLoS ONE 2013, 8, e65382. [Google Scholar]
- Polak, J.; Moro, C.; Klimcakova, E.; Hejnova, J.; Majercik, M.; Viguerie, N.; Langin, D.; Lafontan, M.; Stich, V.; Berlan, M. Dynamic strength training improves insulin sensitivity and functional balance between adrenergic alpha 2A and beta pathways in subcutaneous adipose tissue of obese subjects. Diabetologia 2005, 48, 2631–2640. [Google Scholar] [CrossRef]
- Klimcakova, E.; Polak, J.; Moro, C.; Hejnova, J.; Majercik, M.; Viguerie, N.; Berlan, M.; Langin, D.; Stich, V. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J. Clin. Endocrinol. Metab. 2006, 91, 5107–5112. [Google Scholar] [CrossRef]
- Avila, J.J.; Gutierres, J.A.; Sheehy, M.E.; Lofgren, I.E.; Delmonico, M.J. Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. Eur. J. Appl. Physiol. 2010, 109, 517–525. [Google Scholar] [CrossRef]
- Cottell, K.E.; Dorfman, L.R.; Straight, C.R.; Delmonico, M.J.; Lofgren, I.E. The effects of diet education plus light resistance training on coronary heart disease risk factors in community-dwelling older adults. J. Nutr. Health Aging 2011, 15, 762–767. [Google Scholar] [CrossRef]
- Falk, B.; Sadres, E.; Constantini, N.; Zigel, L.; Lidor, R.; Eliakim, A. The association between adiposity and the response to resistance training among pre- and early-pubertal boys. J. Pediatr. Endocrinol. Metab. 2002, 15, 597–606. [Google Scholar] [CrossRef]
- Kordi, R.; Dehghani, S.; Noormohammadpour, P.; Rostami, M.; Mansournia, M.A. Effect of abdominal resistance exercise on abdominal subcutaneous fat of obese women: A randomized controlled trial using ultrasound imaging assessments. J. Manip. Physiol. Ther. 2015, 38, 203–209. [Google Scholar] [CrossRef]
- Larsen, S.; Danielsen, J.H.; Søndergård, S.D.; Søgaard, D.; Vigelsoe, A.; Dybboe, R.; Skaaby, S.; Dela, F.; Helge, J.W. The effect of high-intensity training on mitochondrial fat oxidation in skeletal muscle and subcutaneous adipose tissue. Scand. J. Med. Sci. Sports 2015, 25, e59–e69. [Google Scholar] [CrossRef]
- Dohlmann, T.L.; Hindsø, M.; Dela, F.; Helge, J.W.; Larsen, S. High-intensity interval training changes mitochondrial respiratory capacity differently in adipose tissue and skeletal muscle. Physiol. Rep. 2018, 6, e13857. [Google Scholar] [CrossRef]
- Devries, M.C.; Samjoo, I.A.; Hamadeh, M.J.; McCready, C.; Raha, S.; Watt, M.J.; Steinberg, G.R.; Tarnopolsky, M.A. Endurance training modulates intramyocellular lipid compartmentalization and morphology in skeletal muscle of lean and obese women. J. Clin. Endocrinol. Metab. 2013, 98, 4852–4862. [Google Scholar] [CrossRef]
- Booth, F.W.; Ruegsegger, G.N.; Toedebusch, R.G.; Yan, Z. Endurance Exercise and the Regulation of Skeletal Muscle Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 129–151. [Google Scholar]
- Seip, R.L.; Semenkovich, C.F. Skeletal muscle lipoprotein lipase: Molecular regulation and physiological effects in relation to exercise. Exerc. Sport Sci. Rev. 1998, 26, 191–218. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2021, 9, 1970. [Google Scholar] [CrossRef]
- Sullivan, B.P.; Weiss, J.A.; Nie, Y.; Garner, R.T.; Drohan, C.J.; Kuang, S.; Stout, J.; Gavin, T.P. Skeletal muscle IGF-1 is lower at rest and after resistance exercise in humans with obesity. Eur. J. Appl. Physiol. 2021, 120, 2835–2846. [Google Scholar] [CrossRef]
- Kras, K.A.; Hoffman, N.; Roust, L.R.; Benjamin, T.R.; De Filippis, E.A.; Katsanos, C.S. Adenosine Triphosphate Production of Muscle Mitochondria after Acute Exercise in Lean and Obese Humans. Med. Sci. Sports Exerc. 2019, 51, 445–453. [Google Scholar] [CrossRef]
- Berggren, J.R.; Boyle, K.E.; Chapman, W.H.; Houmard, J.A. Skeletal muscle lipid oxidation and obesity: Influence of weight loss and exercise. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E726–E732. [Google Scholar] [CrossRef]
- Louche, K.; Badin, P.M.; Montastier, E.; Laurens, C.; Bourlier, V.; de Glisezinski, I.; Thalamas, C.; Viguerie, N.; Langin, D.; Moro, C. Endurance exercise training up-regulates lipolytic proteins and reduces triglyceride content in skeletal muscle of obese subjects. J. Clin. Endocrinol. Metab. 2013, 98, 4863–4871. [Google Scholar] [CrossRef]
- Hansen, D.; Meeusen, R.; Mullens, A.; Dendale, P. Effect of acute endurance and resistance exercise on endocrine hormones directly related to lipolysis and skeletal muscle protein synthesis in adult individuals with obesity. Sports Med. 2012, 42, 415–431. [Google Scholar] [CrossRef]
- Leon, B.; Jenkins, S.; Pepin, K.; Chaudhry, H.; Smith, K.; Zalos, G.; Miller, B.V., 3rd; Chen, K.Y.; Remaley, A.T.; Waclawiw, M.A.; et al. Insulin and extremity muscle mass in overweight and obese women. Int. J. Obes. 2013, 37, 1560–1564. [Google Scholar] [CrossRef][Green Version]
- Jensen, J.; Tantiwong, P.; Stuenæs, J.T.; Molina-Carrion, M.; DeFronzo, R.A.; Sakamoto, K.; Musi, N. Effect of acute exercise on glycogen synthase in muscle from obese and diabetic subjects. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E82–E89. [Google Scholar] [CrossRef]
- Shojaa, M.; Von Stengel, S.; Schoene, D.; Kohl, M.; Barone, G.; Bragonzoni, L.; Dallolio, L.; Marini, S.; Murphy, M.H.; Stephenson, A.; et al. Effect of Exercise Training on Bone Mineral Density in Post-menopausal Women: A Systematic Review and Meta-Analysis of Intervention Studies. Front. Physiol. 2021, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Schwab, P.; Scalapino, K. Exercise for bone health: Rationale and prescription. Curr. Opin. Rheumatol. 2011, 23, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.P.; Sale, C.; Greeves, J.P.; Casey, A.; Dutton, J.; Fraser, W.D. The role of exercise intensity in the bone metabolic response to an acute bout of weight-bearing exercise. J. Appl. Physiol. 2011, 110, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, I.; Kemppainen, J.; Kaskinoro, K.; Langberg, H.; Knuuti, J.; Boushel, R.; Kjaer, M.; Kalliokoski, K.K. Bone blood flow and metabolism in humans: Effect of muscular exercise and other physiological perturbations. J. Bone Miner. Res. 2013, 28, 1068–1074. [Google Scholar] [CrossRef]
- Fujimura, R.; Ashizawa, N.; Watanabe, M.; Mukai, N.; Amagai, H.; Fukubayashi, T.; Hayashi, K.; Tokuyama, K.; Suzuki, M. Effect of resistance exercise training on bone formation and resorption in young male subjects assessed by biomarkers of bone metabolism. J. Bone Min. Res. 1997, 12, 656–662. [Google Scholar] [CrossRef]
- Li, K.C.; Zernicke, R.F.; Barnard, R.J.; Li, A.F. Differential response of rat limb bones to strenuous exercise. J. Appl. Physiol. 1991, 70, 554–560. [Google Scholar] [CrossRef]
- Suniaga, S.; Rolvien, T.; Vom Scheidt, A.; Fiedler, I.A.K.; Bale, H.A.; Huysseune, A.; Witten, P.E.; Amling, M.; Busse, B. Increased mechanical loading through controlled swimming exercise induces bone formation and mineralization in adult zebrafish. Sci. Rep. 2018, 8, 3646. [Google Scholar] [CrossRef]
- Gleeson, M. Immune function in sport and exercise. J. Appl. Physiol. 2007, 103, 693–699. [Google Scholar] [CrossRef]
- Brolinson, P.G.; Elliott, D. Exercise and the immune system. Clin. Sports Med. 2007, 26, 311–319. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef]
- Yoon, J.R.; Ha, G.C.; Ko, K.J.; Kang, S.J. Effects of exercise type on estrogen, tumor markers, immune function, antioxidant function, and physical fitness in postmenopausal obese women. J. Exerc. Rehabil. 2018, 14, 1032–1040. [Google Scholar] [CrossRef]
- Nieman, D.C.; Nehlsen-Cannarella, S.L.; Henson, D.A.; Butterworth, D.E.; Fagoaga, O.R.; Warren, B.J.; Rainwater, M.K. Immune response to obesity and moderate weight loss. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 353–360. [Google Scholar]
- Tanaka, S.; Inoue, S.; Isoda, F.; Waseda, M.; Ishihara, M.; Yamakawa, T.; Sugiyama, A.; Takamura, Y.; Okuda, K. Impaired immunity in obesity: Suppressed but reversible lymphocyte responsiveness. Int. J. Obes. Relat. Metab. Disord. 1993, 17, 631–636. [Google Scholar]
- Kimura, M.; Tanaka, S.; Isoda, F.; Sekigawa, K.; Yamakawa, T.; Sekihara, H. T lymphopenia in obese diabetic (db/db) mice is nonselective and thymus independent. Life Sci. 1998, 62, 1243–1250. [Google Scholar] [CrossRef]
- Plotkin, B.J.; Paulson, D.; Chelich, A.; Jurak, D.; Cole, J.; Kasimos, J.; Burdick, J.R.; Casteel, N. Immune responsiveness in a rat model for type II diabetes (Zucker rat, fa/fa): Susceptibility to Candida albicans infection and leucocyte function. J. Med. Microbiol. 1996, 44, 277–283. [Google Scholar] [CrossRef][Green Version]
- Lee, B.S.; Kim, K.A.; Kim, J.K.; Nho, H. Augmented Hemodynamic Responses in Obese Young Men during Dynamic Exercise: Role of the Muscle Metaboreflex. Int. J. Environ. Res. Public Health 2021, 17, 7321. [Google Scholar] [CrossRef]
- Hambrecht, R.; Adams, V.; Erbs, S.; Linke, A.; Kränkel, N.; Shu, Y.; Baither, Y.; Gielen, S.; Thiele, H.; Gummert, J.F.; et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 2003, 107, 3152–3158. [Google Scholar] [CrossRef]
- Thijssen, D.H.; Maiorana, A.J.; O’Driscoll, G.; Cable, N.T.; Hopman, M.T.; Green, D.J. Impact of inactivity and exercise on the vasculature in humans. Eur. J. Appl. Physiol. 2010, 108, 845–875. [Google Scholar] [CrossRef]
- Evans, D.L. Cardiovascular adaptations to exercise and training. Vet. Clin. N. Am. Equine Pract. 1985, 1, 513–531. [Google Scholar] [CrossRef]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the Cardiovascular System. Clinical Science and Cardiovascular Outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef]
- Weiner, R.B.; Baggish, A.L. Exercise-induced cardiac remodeling. Prog. Cardiovasc. Dis. 2012, 54, 380–386. [Google Scholar] [CrossRef]
- Delp, M.D. Differential effects of training on the control of skeletal muscle perfusion. Med. Sci. Sports Exerc. 1998, 30, 361–374. [Google Scholar] [CrossRef]
- Hogan, T.S. Exercise-induced reduction in systemic vascular resistance: A covert killer and an unrecognised resuscitation challenge? Med. Hypotheses 2009, 73, 479–484. [Google Scholar] [CrossRef]
- Breisch, E.A.; White, F.C.; Nimmo, L.E.; McKirnan, M.D.; Bloor, C.M. Exercise-induced cardiac hypertrophy: A correlation of blood flow and microvasculature. J. Appl. Physiol. 1986, 60, 1259–1267. [Google Scholar] [CrossRef]
- Wisloff, U.; Loennechen, J.P.; Currie, S.; Smith, G.L.; Ellingsen, O. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc. Res. 2002, 54, 162–174. [Google Scholar] [CrossRef]
- Natali, A.J.; Wilson, L.A.; Peckham, M.; Turner, D.L.; Harrison, S.M.; White, E. Different regional effects of voluntary exercise on the mechanical and electrical properties of rat ventricular myocytes. J. Physiol. 2002, 541, 863–875. [Google Scholar] [CrossRef]
- Kemi, O.J.; Wisloff, U. Mechanisms of exercise-induced improvements in the contractile apparatus of the mammalian myocardium. Acta Physiol. 2010, 199, 425–439. [Google Scholar] [CrossRef]
- Hallen, J. K+ balance in humans during exercise. Acta Physiol. Scand. 1996, 156, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Radegran, G.; Calbet, J.A. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiol. Scand. 2001, 171, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sarelius, I.; Pohl, U. Control of muscle blood flow during exercise: Local factors and integrative mechanisms. Acta Physiol. 2010, 199, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, H.C.; Dempsey, J.A.; Miller, J.D.; Romer, L.M.; Eldridge, M.W. Physiologic response to exercise. In Physiological Basis of Respiratory Disease, 1st ed.; Hamid, Q., Shannon, J., Martin, J., Eds.; BC Decker, Inc.: Hamilton, ON, Canada, 2005; pp. 525–540. [Google Scholar]
- Chlif, M.; Chaouachi, A.; Ahmaidi, S. Effect of Aerobic Exercise Training on Ventilatory Efficiency and Respiratory Drive in Obese Subjects. Respir. Care 2017, 62, 936–946. [Google Scholar] [CrossRef]
- Chlif, M.; Temfemo, A.; Keochkerian, D.; Choquet, D.; Chaouachi, A.; Ahmaidi, S. Advanced Mechanical Ventilatory Constraints During Incremental Exercise in Class III Obese Male Subjects. Respir. Care 2015, 60, 549–560. [Google Scholar] [CrossRef]
- Wang, L.Y.; Cerny, F.J. Ventilatory response to exercise in simulated obesity by chest loading. Med. Sci. Sports Exerc. 2004, 36, 780–786. [Google Scholar] [CrossRef]
- Li, A.M.; Chan, D.; Wong, E.; Yin, J.; Nelson, E.A.; Fok, T.F. The effects of obesity on pulmonary function. Arch. Dis. Child. 2003, 88, 361–363. [Google Scholar] [CrossRef]
- Koenig, S.M. Pulmonary complications of obesity. Am. J. Med. Sci. 2001, 321, 249–279. [Google Scholar] [CrossRef]
- Faria, A.G.; Ribeiro, M.A.; Marson, F.A.; Schivinski, C.I.; Severino, S.D.; Ribeiro, J.D.; Barros Filho, A.A. Effect of exercise test on pulmonary function of obese adolescents. J. Pediatr. 2014, 90, 242–249. [Google Scholar] [CrossRef]
- Torchio, R.; Gobb, A.; Gulotta, C.; Dellacà, R.; Tinivella, M.; Hyatt, R.E.; Brusasco, V.; Pellegrino, R. Mechanical effects of obesity on airway responsiveness in otherwise healthy humans. J. Appl. Physiol. 2009, 107, 408–416. [Google Scholar] [CrossRef]
- Moses, F.M. The effect of exercise on the gastrointestinal tract. Sports Med. 1990, 9, 159–172. [Google Scholar] [CrossRef]
- De Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal complaints during exercise: Prevalence, etiology, and nutritional recommendations. Sports Med. 2014, 44, S79–S85. [Google Scholar] [CrossRef]
- Sari, R.; Balci, N.; Balci, M.K. Effects of exercise on gallbladder volume and motility in obese women. J. Clin. Ultrasound 2005, 33, 218–222. [Google Scholar] [CrossRef]
- Wilund, K.R.; Feeney, L.A.; Tomayko, E.J.; Weiss, E.P.; Hagberg, J.M. Effects of endurance exercise training on markers of cholesterol absorption and synthesis. Physiol. Res. 2009, 58, 545–552. [Google Scholar] [CrossRef]
- Devries, M.C.; Samjoo, I.A.; Hamadeh, M.J.; Tarnopolsky, M.A. Effect of endurance exercise on hepatic lipid content, enzymes, and adiposity in men and women. Obesity 2008, 16, 2281–2288. [Google Scholar] [CrossRef]
- Skrypnik, D.; Ratajczak, M.; Karolkiewicz, J.; Mądry, E.; Pupek-Musialik, D.; Hansdorfer-Korzon, R.; Walkowiak, J.; Jakubowski, H.; Bogdański, P. Effects of endurance and endurance-strength exercise on biochemical parameters of liver function in women with abdominal obesity. Biomed. Pharmacother. 2016, 80, 1–7. [Google Scholar] [CrossRef]
- Hackney, A.C.; Lane, A.R. Exercise and the Regulation of Endocrine Hormones. Prog. Mol. Biol. Transl. Sci. 2015, 135, 293–311. [Google Scholar]
- Cano Sokoloff, N.; Misra, M.; Ackerman, K.E. Exercise, Training, and the Hypothalamic-Pituitary-Gonadal Axis in Men and Women. Front. Horm. Res. 2016, 47, 27–43. [Google Scholar] [PubMed]
- Mastorakos, G.; Pavlatou, M.; Diamanti-Kandarakis, E.; Chrousos, G.P. Exercise and the stress system. Hormones 2005, 4, 73–89. [Google Scholar] [PubMed]
- Slentz, C.A.; Tanner, C.J.; Bateman, L.A.; Durheim, M.T.; Huffman, K.M.; Houmard, J.A.; Kraus, W.E. Effects of exercise training intensity on pancreatic beta-cell function. Diabetes Care 2009, 32, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.M.; Thorup, A.C.; Overgaard, K.; Jeppesen, P.B. High Intensity Interval Training Improves Glycaemic Control and Pancreatic beta Cell Function of Type 2 Diabetes Patients. PLoS ONE 2015, 10, e0133286. [Google Scholar] [CrossRef]
- Heiskanen, M.A.; Motiani, K.K.; Mari, A.; Saunavaara, V.; Eskelinen, J.J.; Virtanen, K.A.; Koivumäki, M.; Löyttyniemi, E.; Nuutila, P.; Kalliokoski, K.K.; et al. Exercise training decreases pancreatic fat content and improves beta cell function regardless of baseline glucose tolerance: A randomised controlled trial. Diabetologia 2018, 61, 1817–1828. [Google Scholar] [CrossRef]
- Huang, H.H.; Farmer, K.; Windscheffel, J.; Yost, K.; Power, M.; Wright, D.E.; Stehno-Bittel, L. Exercise increases insulin content and basal secretion in pancreatic islets in type 1 diabetic mice. Exp. Diabetes Res. 2011, 2011, 481427. [Google Scholar] [CrossRef]
- Malin, S.K.; Francois, M.E.; Eichner, N.Z.M.; Gilbertson, N.M.; Heiston, E.M.; Fabris, C.; Breton, M. Impact of short-term exercise training intensity on beta-cell function in older obese adults with prediabetes. J. Appl. Physiol. 2018, 125, 1979–1986. [Google Scholar] [CrossRef]
- Morgan, J.A.; Corrigan, F.; Baune, B.T. Effects of physical exercise on central nervous system functions: A review of brain region specific adaptations. J. Mol. Psychiatry 2015, 3, 3. [Google Scholar] [CrossRef]
- Dishman, R.K.; Berthoud, H.R.; Booth, F.W.; Cotman, C.W.; Edgerton, V.R.; Fleshner, M.R.; Gandevia, S.C.; Gomez-Pinilla, F.; Greenwood, B.N.; Hillman, C.H.; et al. Neurobiology of exercise. Obesity 2006, 14, 345–356. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef]
- Díaz, B.B.; González, D.A.; Gannar, F.; Pérez, M.C.R.; de León, A.C. Myokines, physical activity, insulin resistance and autoimmune diseases. Immunol. Lett. 2018, 203, 1–5. [Google Scholar] [CrossRef]
- Sholl-Franco, A.; da Silva, A.G.; Adão-Novaes, J. Interleukin-4 as a neuromodulatory cytokine: Roles and signaling in the nervous system. Ann. N. Y. Acad. Sci. 2009, 1153, 65–75. [Google Scholar] [CrossRef]
- Plata-Salamán, C.R.; Borkoski, J.P. Interleukin-8 modulates feeding by direct action in the central nervous system. Am. J. Physiol. 1993, 265, R877–R882. [Google Scholar] [CrossRef]
- Pan, W.; Wu, X.; He, Y.; Hsuchou, H.; Huang, E.Y.K.; Mishra, P.K.; Kastin, A.J. Brain interleukin-15 in neuroinflammation and behavior. Neurosci. Biobehav. Rev. 2013, 37, 184–192. [Google Scholar] [CrossRef]
- Dyer, A.H.; Vahdatpour, C.; Sanfeliu, A.; Tropea, D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience 2016, 325, 89–99. [Google Scholar] [CrossRef]
- Von Holstein-Rathlou, S.; Gillum, M.P. Fibroblast growth factor 21: An endocrine inhibitor of sugar and alcohol appetite. J. Physiol. 2019, 597, 3539–3548. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W. Can irisin be a linker between physical activity and brain function? Biomol. Concepts 2016, 7, 253–258. [Google Scholar] [CrossRef]
- Genc, S.; Koroglu, T.F.; Genc, K. Erythropoietin and the nervous system. Brain Res. 2004, 1000, 19–31. [Google Scholar] [CrossRef]
- Buemi, M.; Cavallaro, E.; Floccari, F.; Sturiale, A.; Aloisi, C.; Trimarchi, M.; Corica, F.; Frisina, N. The pleiotropic effects of erythropoietin in the central nervous system. J. Neuropathol. Exp. Neurol. 2003, 62, 228–236. [Google Scholar] [CrossRef]
- Nagai, N.; Moritani, T. Effect of physical activity on autonomic nervous system function in lean and obese children. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 27–33. [Google Scholar] [CrossRef]
- Amano, M.; Kanda, T.; Ue, H.; Moritani, T. Exercise training and autonomic nervous system activity in obese individuals. Med. Sci. Sports Exerc. 2001, 33, 1287–1291. [Google Scholar] [CrossRef]
- Cornier, M.A.; Melanson, E.L.; Salzberg, A.K.; Bechtell, J.L.; Tregellas, J.R. The effects of exercise on the neuronal response to food cues. Physiol. Behav. 2012, 105, 1028–1034. [Google Scholar] [CrossRef]
- Cho, S.Y.; Roh, H.T. Effects of aerobic exercise training on peripheral brain-derived neurotrophic factor and eotaxin-1 levels in obese young men. J. Phys. Ther. Sci. 2016, 28, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Araya, A.V.; Orellana, X.; Godoy, D.; Soto, L.; Fiedler, J. Effect of exercise on circulating levels of brain-derived neurotrophic factor (BDNF) in overweight and obese subjects. Horm. Metab. Res. 2013, 45, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.L.; Whitehurst, M.; Fico, B.G.; Dodge, K.M.; Ferrandi, P.J.; Pena, G.; Adelman, A.; Huang, C. Acute high-intensity interval exercise induces greater levels of serum brain-derived neurotrophic factor in obese individuals. Exp. Biol. Med. 2018, 243, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Pate, R.R.; O’Neill, J.R.; Lobelo, F. The evolving definition of “sedentary”. Exerc. Sport Sci. Rev. 2008, 36, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Sedentary Behaviour and Research Network. Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl. Physiol. Nutr. Metab. 2012, 37, 540–542. [Google Scholar] [CrossRef]
- Chau, J.Y.; Grunseit, A.C.; Chey, T.; Stamatakis, E.; Brown, W.J.; Matthews, C.E.; Bauman, A.E.; van der Ploeg, H.P. Daily sitting time and all-cause mortality: A meta-analysis. PLoS ONE 2013, 8, e80000. [Google Scholar]
- Ching, P.L.; Willett, W.C.; Rimm, E.B.; Colditz, G.A.; Gortmaker, S.L.; Stampfer, M.J. Activity level and risk of overweight in male health professionals. Am. J. Public Health 1996, 86, 25–30. [Google Scholar] [CrossRef]
- Bowman, S.A. Television-viewing characteristics of adults: Correlations to eating practices and overweight and health status. Prev. Chronic. Dis. 2006, 3, A38. [Google Scholar]
- Thomson, M.; Spence, J.C.; Raine, K.; Laing, L. The association of television viewing with snacking behavior and body weight of young adults. Am. J. Health Promot. 2008, 22, 329–335. [Google Scholar] [CrossRef]
- Frank, L.D.; Andresen, M.A.; Schmid, T.L. Obesity relationships with community design, physical activity, and time spent in cars. Am. J. Prev. Med. 2004, 27, 87–96. [Google Scholar] [CrossRef]
- Lopez-Zetina, J.; Lee, H.; Friis, R. The link between obesity and the built environment. Evidence from an ecological analysis of obesity and vehicle miles of travel in California. Health Place 2006, 12, 656–664. [Google Scholar] [CrossRef]
- Dunton, G.F.; Berrigan, D.; Ballard-Barbash, R.; Graubard, B.; Atienza, A.A. Joint associations of physical activity and sedentary behaviors with body mass index: Results from a time use survey of US adults. Int. J. Obes. 2009, 33, 1427–1436. [Google Scholar] [CrossRef]
- Stefansdottir, R.; Gudmundsdottir, S.L. Sedentary behavior and musculoskeletal pain: A five-year longitudinal Icelandic study. Public Health 2017, 149, 71–73. [Google Scholar] [CrossRef]
- Santos, M.C.S.; de Andrade, S.M.; González, A.D.; Dias, D.F.; Mesas, A.E. Association Between Chronic Pain and Leisure Time Physical Activity and Sedentary Behavior in Schoolteachers. Behav. Med. 2018, 44, 335–343. [Google Scholar] [CrossRef]
- Nijs, J.; D’Hondt, E.; Clarys, P.; Deliens, T.; Polli, A.; Malfliet, A.; Coppieters, I.; Willaert, W.; Tumkaya Yilmaz, S.; Elma, Ö.; et al. Lifestyle and Chronic Pain across the Lifespan: An Inconvenient Truth? PM&R 2020, 12, 410–419. [Google Scholar]
- Zhaoyang, R.; Martire, L.M.; Darnall, B.D. Daily pain catastrophizing predicts less physical activity and more sedentary behavior in older adults with osteoarthritis. Pain 2020, 161, 2603–2610. [Google Scholar] [CrossRef]
- Lee, S.H.; Son, C.; Yeo, S.; Ha, I.H. Cross-sectional analysis of self-reported sedentary behaviors and chronic knee pain among South Korean adults over 50 years of age in KNHANES 2013–2015. BMC Public Health 2019, 19, 1375. [Google Scholar] [CrossRef]
- Mendonça, C.R.; Noll, M.; Rodrigues, A.P.D.S.; Vitorino, P.V.O.; Mendes, M.A.; Silveira, E.A. Association of Pain, Severe Pain, and Multisite Pain with the Level of Physical Activity and Sedentary Behavior in Severely Obese Adults: Baseline Data from the DieTBra Trial. Int. J. Environ. Res. Public Health 2020, 17, 4478. [Google Scholar] [CrossRef]
- O’Leary, H.; Larkin, L.; Murphy, G.M.; Quinn, K. Relationship Between Pain and Sedentary Behavior in Rheumatoid Arthritis Patients: A Cross-Sectional Study. Arthritis Care Res. 2021, 73, 990–997. [Google Scholar] [CrossRef]
- Segura-Jiménez, V.; Borges-Cosic, M.; Soriano-Maldonado, A.; Estévez-López, F.; Álvarez-Gallardo, I.C.; Herrador-Colmenero, M.; Delgado-Fernández, M.; Ruiz, J.R. Association of sedentary time and physical activity with pain, fatigue, and impact of fibromyalgia: The al-Andalus study. Scand. J. Med. Sci. Sports 2017, 27, 83–92. [Google Scholar] [CrossRef]
- Baena-Beato, P.Á.; Artero, E.G.; Arroyo-Morales, M.; Robles-Fuentes, A.; Gatto-Cardia, M.C.; Delgado-Fernández, M. Aquatic therapy improves pain, disability, quality of life, body composition and fitness in sedentary adults with chronic low back pain. A controlled clinical trial. Clin. Rehabil. 2014, 28, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Tu, Y.; Chen, X.; Hu, K.; Tu, X.; Lin, M.; Xie, G.; Chen, S.; Huang, J.; et al. Different exercise modalities relieve pain syndrome in patients with knee osteoarthritis and modulate the dorsolateral prefrontal cortex: A multiple mode MRI study. Brain Behav. Immun. 2019, 82, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Knauf, M.T.; Koltyn, K.F. Exercise-induced modulation of pain in adults with and without painful diabetic neuropathy. J. Pain 2014, 15, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Gowans, S.E.; deHueck, A.; Voss, S.; Richardson, M. A randomized, controlled trial of exercise and education for individuals with fibromyalgia. Arthritis Care Res. 1999, 12, 120–128. [Google Scholar] [CrossRef]
- Jentoft, E.S.; Kvalvik, A.G.; Mengshoel, A.M. Effects of pool-based and land-based aerobic exercise on women with fibromyalgia/chronic widespread muscle pain. Arthritis Rheum. 2001, 45, 42–47. [Google Scholar] [CrossRef]
- Dönmez, A.; Karagülle, M.Z.; Tercan, N.; Dinler, M.; Işsever, H.; Karagülle, M.; Turan, M. SPA therapy in fibromyalgia: A randomised controlled clinic study. Rheumatol. Int. 2005, 26, 168–172. [Google Scholar] [CrossRef]
- Jones, K.D.; Adams, D.; Winters-Stone, K.; Burckhardt, C.S. A comprehensive review of 46 exercise treatment studies in fibromyalgia (1988–2005). Health Qual. Life Outcomes 2006, 4, 67. [Google Scholar] [CrossRef]
- Palandi, J.; Bobinski, F.; de Oliveira, G.M.; Ilha, J. Neuropathic pain after spinal cord injury and physical exercise in animal models: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 108, 781–795. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Ding, J.; Wang, S.; Dong, C.; Wu, Q. Exercise training modulates glutamic acid decarboxylase-65/67 expression through TrkB signaling to ameliorate neuropathic pain in rats with spinal cord injury. Mol. Pain 2021, 16, 1744806920924511. [Google Scholar] [CrossRef]
- Detloff, M.R.; Smith, E.J.; Quiros Molina, D.; Ganzer, P.D.; Houlé, J.D. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp. Neurol. 2014, 255, 38–48. [Google Scholar] [CrossRef]
- Dugan, E.A.; Sagen, J. An Intensive Locomotor Training Paradigm Improves Neuropathic Pain following Spinal Cord Compression Injury in Rats. J. Neurotrauma 2015, 32, 622–632. [Google Scholar] [CrossRef]
- Nees, T.A.; Tappe-Theodor, A.; Sliwinski, C.; Motsch, M.; Rupp, R.; Kuner, R.; Weidner, N.; Blesch, A. Early-onset treadmill training reduces mechanical allodynia and modulates calcitonin gene-related peptide fiber density in lamina III/IV in a mouse model of spinal cord contusion injury. Pain 2016, 157, 687–697. [Google Scholar] [CrossRef]
- Dugan, E.A.; Schachner, B.; Jergova, S.; Sagen, J. Intensive Locomotor Training Provides Sustained Alleviation of Chronic Spinal Cord Injury-Associated Neuropathic Pain: A Two-Year Pre-Clinical Study. J. Neurotrauma 2021, 38, 789–802. [Google Scholar] [CrossRef]
- Chen, Y.W.; Li, Y.T.; Chen, Y.C.; Li, Z.Y.; Hung, C.H. Exercise training attenuates neuropathic pain and cytokine expression after chronic constriction injury of rat sciatic nerve. Anesth. Analg. 2012, 114, 1330–1337. [Google Scholar] [CrossRef]
- Lopes, B.C.; Medeiros, L.F.; Silva de Souza, V.; Cioato, S.G.; Medeiros, H.R.; Regner, G.G.; Lino de Oliveira, C.; Fregni, F.; Caumo, W.; Torres, I.L.S. Transcranial direct current stimulation combined with exercise modulates the inflammatory profile and hyperalgesic response in rats subjected to a neuropathic pain model: Long-term effects. Brain Stimul. 2021, 13, 774–782. [Google Scholar] [CrossRef]
- Huang, P.C.; Tsai, K.L.; Chen, Y.W.; Lin, H.T.; Hung, C.H. Exercise Combined With Ultrasound Attenuates Neuropathic Pain in Rats Associated With Downregulation of IL-6 and TNF-alpha, but With Upregulation of IL-10. Anesth. Analg. 2017, 124, 2038–2044. [Google Scholar] [CrossRef]
- Farzad, B.; Rajabi, H.; Gharakhanlou, R.; Allison, D.J.; Hayat, P.; Jameie, S.B. Swimming Training Attenuates Allodynia and Hyperalgesia Induced by Peripheral Nerve Injury in an Adult Male Rat Neuropathic Model: Effects on Irisin and GAD65. Pain Med. 2018, 19, 2236–2245. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, J.E.; Seo, T.B. Effect of treadmill exercise on pain-related Wnt/beta-catenin signaling pathway in dorsal root ganglion neurons at the early phase regeneration of the injured sciatic nerve. J. Exerc. Rehabil. 2021, 17, 96–102. [Google Scholar] [CrossRef]
- Almeida, C.; DeMaman, A.; Kusuda, R.; Cadetti, F.; Ravanelli, M.I.; Queiroz, A.L.; Sousa, T.A.; Zanon, S.; Silveira, L.R.; Lucas, G. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain 2015, 156, 504–513. [Google Scholar] [CrossRef]
- Kami, K.; Taguchi Ms, S.; Tajima, F.; Senba, E. Improvements in impaired GABA and GAD65/67 production in the spinal dorsal horn contribute to exercise-induced hypoalgesia in a mouse model of neuropathic pain. Mol. Pain 2016, 12, 1744806916629059. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Palstam, A.; Löfgren, M.; Ernberg, M.; Bjersing, J.; Bileviciute-Ljungar, I.; Gerdle, B.; Kosek, E.; Mannerkorpi, K. Resistance exercise improves muscle strength.; health status and pain intensity in fibromyalgia—A randomized controlled trial. Arthritis Res. Ther. 2015, 17, 161. [Google Scholar] [CrossRef]
- Toprak Celenay, S.; Mete, O.; Akan, S.; Un Yildirim, N.; Erten, S. Comparison of the effects of stabilization exercise plus kinesio taping and stabilization exercise alone on pain and well-being in fibromyalgia. Complement. Ther. Clin. Pract. 2021, 38, 101076. [Google Scholar] [CrossRef] [PubMed]
- Munguía-Izquierdo, D.; Legaz-Arrese, A. Exercise in warm water decreases pain and improves cognitive function in middle-aged women with fibromyalgia. Clin. Exp. Rheumatol. 2007, 25, 823–830. [Google Scholar] [PubMed]
- Bjersing, J.L.; Dehlin, M.; Erlandsson, M.; Bokarewa, M.I.; Mannerkorpi, K. Changes in pain and insulin-like growth factor 1 in fibromyalgia during exercise: The involvement of cerebrospinal inflammatory factors and neuropeptides. Arthritis Res. Ther. 2012, 14, R162. [Google Scholar] [CrossRef]
- Ferro Moura Franco, K.; Lenoir, D.; Dos Santos Franco, Y.R.; Jandre Reis, F.J.; Nunes Cabral, C.M.; Meeus, M. Prescription of exercises for the treatment of chronic pain along the continuum of nociplastic pain: A systematic review with meta-analysis. Eur. J. Pain 2021, 25, 51–70. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Obata, K.; Noguchi, K. BDNF in sensory neurons and chronic pain. Neurosci. Res. 2006, 55, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, W.; Qian, Z.; Liu, X.; Wang, H.; Gong, S.; Sun, Y.G.; Snutch, T.P.; Jiang, X.; Tao, J. Peripheral pain is enhanced by insulin-like growth factor 1 through a G protein-mediated stimulation of T-type calcium channels. Sci. Signal 2014, 7, ra94. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef]
- Dameni, S.; Janzadeh, A.; Yousefifard, M.; Nasirinezhad, F. The effect of intrathecal injection of irisin on pain threshold and expression rate of GABAB receptors in peripheral neuropathic pain model. J. Chem. Neuroanat. 2018, 91, 17–26. [Google Scholar] [CrossRef]
- Huang, L.; Yan, S.; Luo, L.; Yang, L. Irisin regulates the expression of BDNF and glycometabolism in diabetic rats. Mol. Med. Rep. 2019, 19, 1074–1082. [Google Scholar] [CrossRef]
- Groover, A.L.; Ryals, J.M.; Guilford, B.L.; Wilson, N.M.; Christianson, J.A.; Wright, D.E. Exercise-mediated improvements in painful neuropathy associated with prediabetes in mice. Pain 2013, 154, 2658–2667. [Google Scholar] [CrossRef]
- Coradini, J.G.; Kunz, R.I.; Kakihata, C.M.; Errero, T.K.; Bonfleur, M.L.; Ribeiro Lde, F.; Brancalhão, R.M.; Bertolini, G.R. Swimming does not alter nociception threshold in obese rats submitted to median nerve compression. Neurol. Res. 2015, 37, 1118–1124. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).