Effects of Different Intensity Exercise on Glucose Metabolism and Hepatic IRS/PI3K/AKT Pathway in SD Rats Exposed with TCDD
Abstract
:1. Introduction
2. Materials and Methods
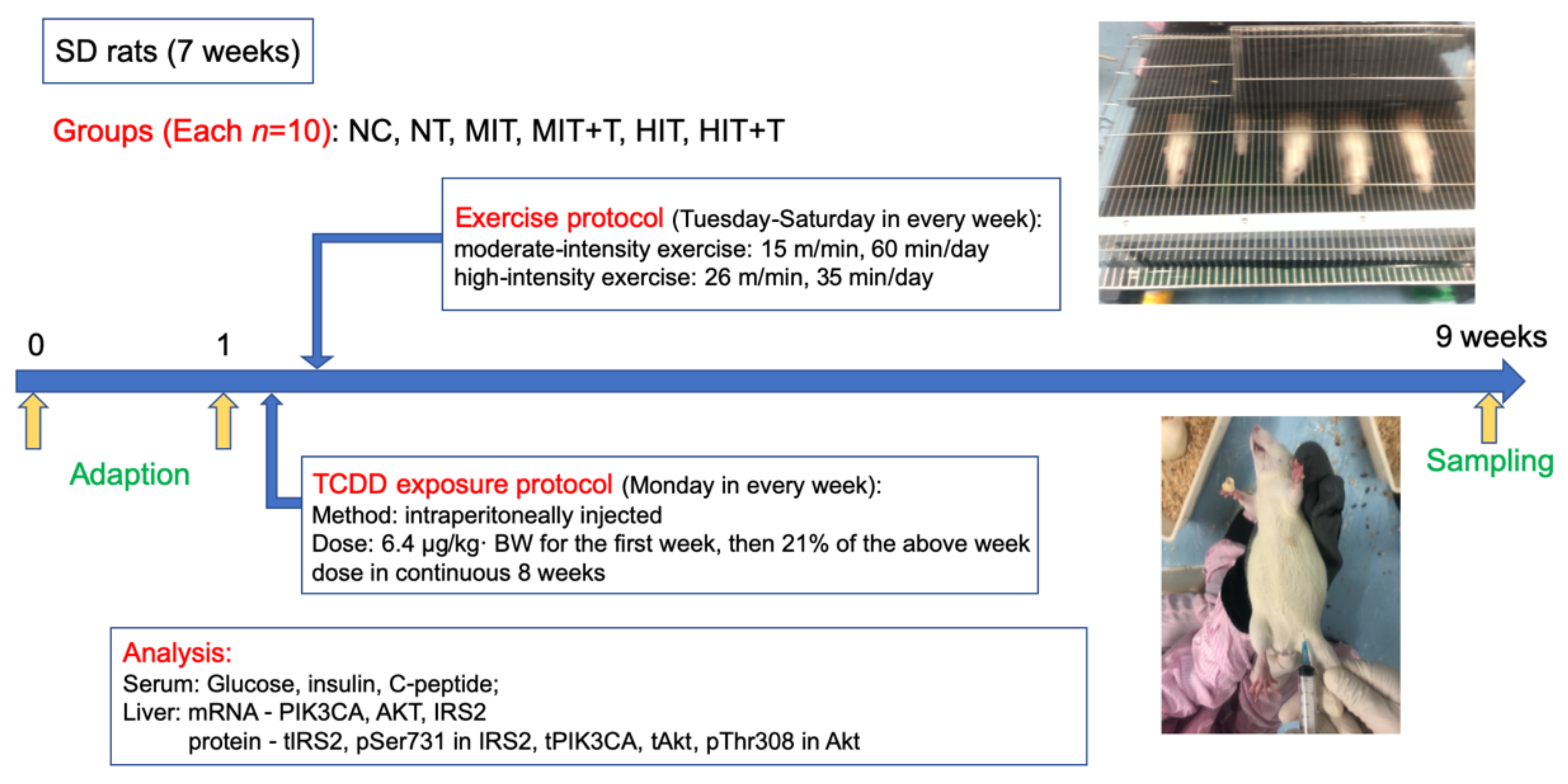
2.1. Subjects
2.2. Experimental Protocol
2.3. Serum and Liver Collection
2.4. Determination of Serum Glucose, Insulin and C-Peptide
2.5. Realtime-PCR
2.6. Western Blot
2.7. Data Analysis
3. Results
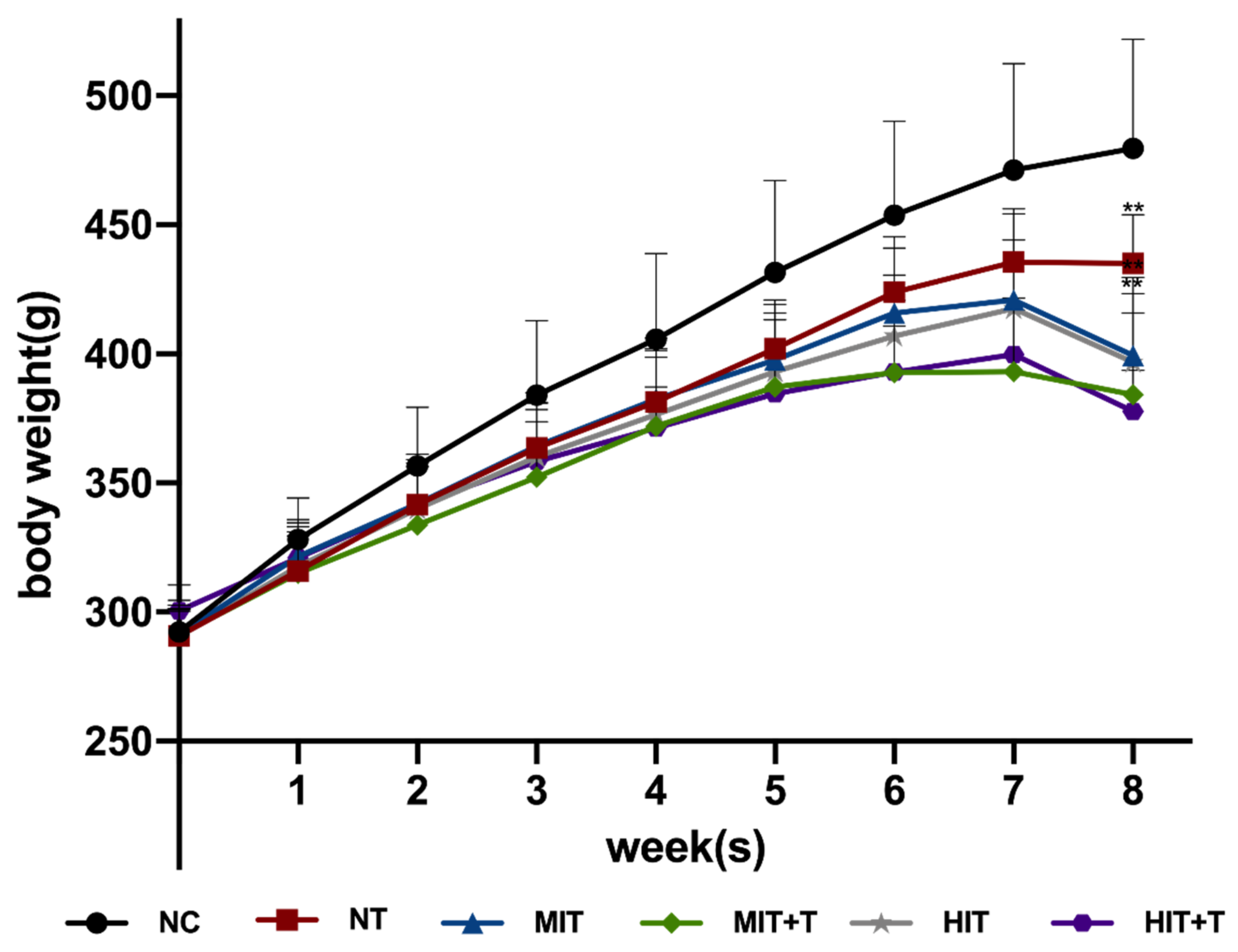
3.1. Body Weight in Rats
3.2. Serum Biochemical Parameters in Rats
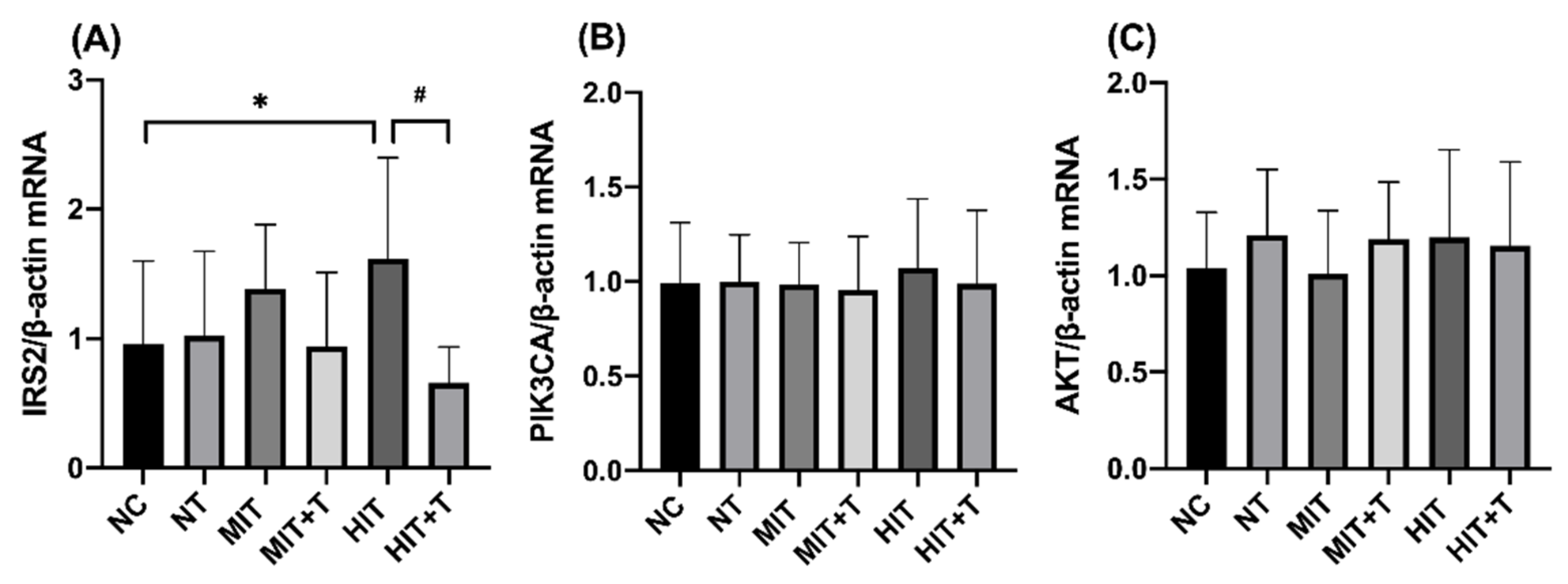
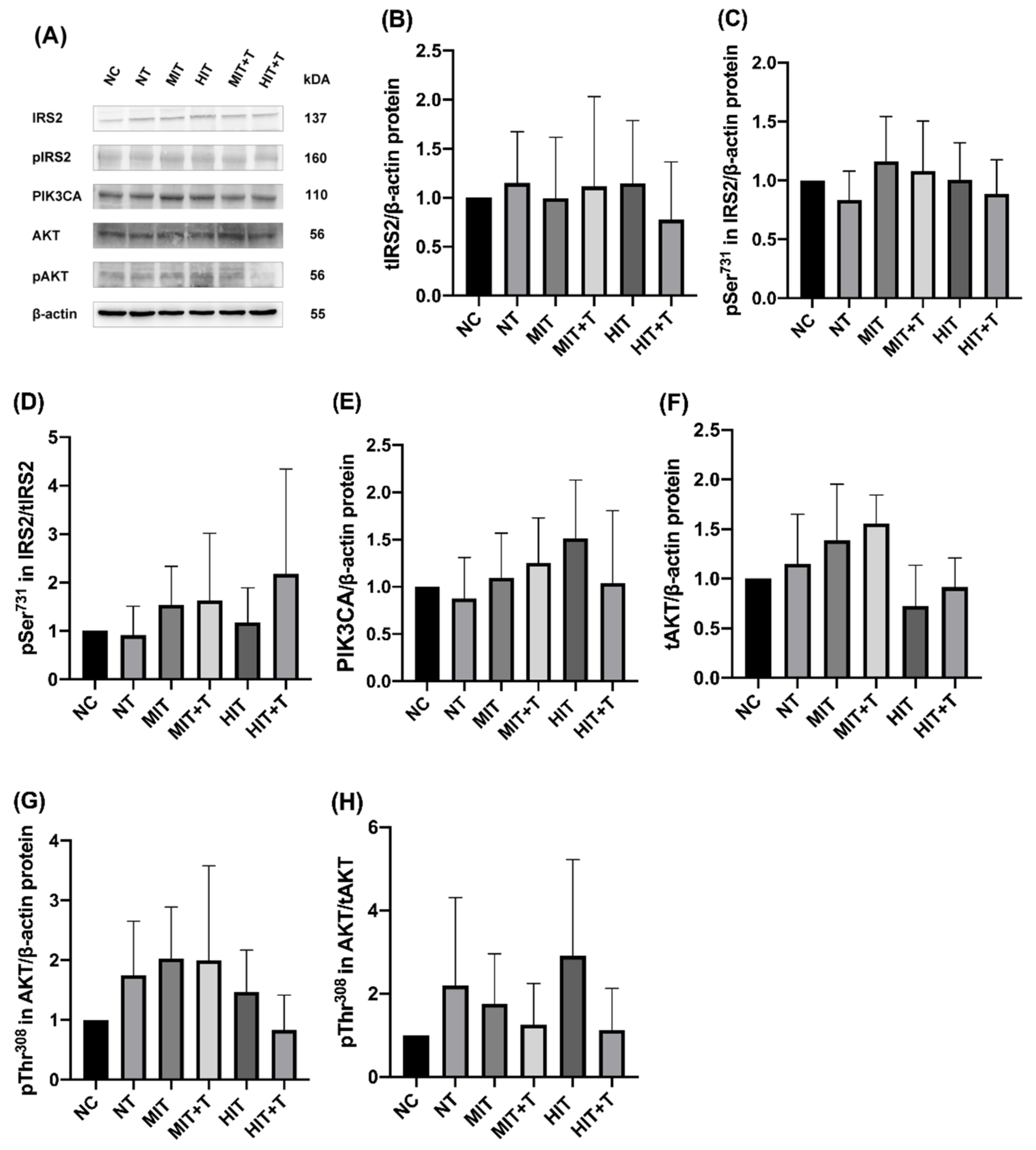
3.3. Hepatic Gene/Protein Expression Profile in Rats
4. Discussion
4.1. Glucose Metabolism
4.2. The Action of the IRS/PI3K/Akt Signaling Pathway on Glucose Metabolism in Rats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Magliano, D.J.; Loh, V.H.Y.; Harding, J.L.; Botton, J.; Shaw, J.E. Persistent organic pollutants and diabetes: A review of the epidemiological evidence. Diabetes Metab. 2014, 40, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.W.; Hong, J.S.; Ohrr, H.; Yi, J.J. Agent Orange Exposure and Disease Prevalence in Korean Vietnam Veterans. Int. J. Epidemiol. 2015, 44 (Suppl. 1), i121–i122. [Google Scholar] [CrossRef]
- Cranmer, M.; Louie, S.; Kennedy, R.H.; Kern, P.A.; Fonseca, V.A. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is associated with hyperinsulinemia and insulin resistance. Toxicol. Sci. 2000, 56, 431–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enan, E.; Liu, P.C.; Matsumura, F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes reduction of glucose transporting activities in the plasma membranes of adipose tissue and pancreas from the guinea pig. J. Biol. Chem. 1992, 267, 19785–19791. [Google Scholar] [CrossRef]
- Enan, E.; Liu, P.C.; Matsumura, F. TCDD (2,3,7,8-tetrachlorodibenzo-P-dioxin) causes reduction in glucose uptake through glucose transporters on the plasma membrane of the guinea pig adipocyte. J. Environ. Sci. Health B 1992, 27, 495–510. [Google Scholar] [CrossRef]
- Novelli, M.; Piaggi, S.; de Tata, V. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced impairment of glucose-stimulated insulin secretion in isolated rat pancreatic islets. Toxicol. Lett. 2005, 156, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Park, J.B.; Woo, M.S.; Lee, S.Y.; Kim, H.Y.; Yoo, Y.H. Persistent Organic Pollutant-Mediated Insulin Resistance. Int. J. Environ. Res. Public Health 2019, 16, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Previs, S.F.; Withers, D.J.; Ren, J.M.; White, M.F.; Shulman, G.I. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo. J. Biol. Chem. 2000, 275, 38990–38994. [Google Scholar] [CrossRef] [Green Version]
- Alessi, D.R.; Cohen, P. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 1998, 8, 55–62. [Google Scholar] [CrossRef]
- Farese, R.V.; Sajan, M.P.; Standaert, M.L. Insulin-sensitive protein kinases (atypical protein kinase C and protein kinase B/Akt): Actions and defects in obesity and type II diabetes. Exp. Biol. Med. 2005, 230, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Sale, E.M.; Sale, G.J. Protein kinase B: Signalling roles and therapeutic targeting. Cell. Mol. Life Sci. 2008, 65, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Calera, M.R.; Martinez, C.; Liu, H.; Jack, A.K.; Birnbaum, M.J.; Pilch, P.F. Insulin increases the association of Akt-2 with Glut4-containing vesicles. J. Biol. Chem. 1998, 273, 7201–7204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 10 November 2021).
- Sampath Kumar, A.; Maiya, A.G.; Shastry, B.A.; Vaishali, K.; Ravishankar, N.; Hazari, A.; Gundmi, S.; Jadhav, R. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Flockhart, M.; Nilsson, L.C.; Tais, S.; Ekblom, B.; Larsen, F.J. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021, 33, 957–970.e6. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.F.; Lu, Y.-F. Effects of long-term regular exercise on expression of fat synthesis-related enzymes in liver of rats exposed to dioxin. J. Environ. Health 2018, 35, 7. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, C.; Du, L.; Song, X.; Ding, X.; Wei, D.; Xu, Z.; Lu, Y. The Effect of Exercise on the Activity of Liver Microsomal Oxidase in 2,3,7,8-Tetrachlorodibenzo-p-dioxin(TCDD)–Exposed Rats. Asian J. Ecotoxicol. 2015, 10, 204–209. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, H.; Lu, Y.; Weng, K.; Wu, T.; Hu, B.; Wang, J.; Liu, Y.; Wei, D.; Xu, Z. Effect of Exercise on the Activity of Liver Antioxidant Ability in 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)–Exposed Rats. Asian J. Ecotoxicol. 2016, 11, 300–306. [Google Scholar] [CrossRef]
- Gilbert, M. Role of skeletal muscle lipids in the pathogenesis of insulin resistance of obesity and type 2 diabetes. J. Diabetes Investig. 2021, 12, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Fujii, J. Emerging connections between oxidative stress, defective proteolysis, and metabolic diseases. Free Radic. Res. 2020, 54, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, O.; Vardi, N.; Ozdemir, I. Effects of quercetin and chrysin on 2,3,7,8-tetrachlorodibenzo-p-dioxin induced hepatotoxicity in rats. Environ. Toxicol. 2013, 28, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Bedford, T.G.; Tipton, C.M.; Wilson, N.C.; Oppliger, R.A.; Gisolfi, C.V. Maximum oxygen consumption of rats and its changes with various experimental procedures. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 47, 1278–1283. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magri, C.J.; Fava, S.; Galea, J. Prediction of insulin resistance in type 2 diabetes mellitus using routinely available clinical parameters. Diabetes Metab. Syndr. 2016, 10, S96–S101. [Google Scholar] [CrossRef] [PubMed]
- Leighton, E.; Sainsbury, C.A.; Jones, G.C. A Practical Review of C-Peptide Testing in Diabetes. Diabetes Ther. 2017, 8, 475–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiston, E.M.; Eichner, N.Z.; Gilbertson, N.M.; Malin, S.K. Exercise improves adiposopathy, insulin sensitivity and metabolic syndrome severity independent of intensity. Exp. Physiol. 2020, 105, 632–640. [Google Scholar] [CrossRef]
- Ryan, B.J.; Schleh, M.W.; Ahn, C.; Ludzki, A.C.; Gillen, J.B.; Varshney, P.; van Pelt, D.W.; Pitchford, L.M.; Chenevert, T.L.; Gioscia-Ryan, R.A.; et al. Moderate-Intensity Exercise and High-Intensity Interval Training Affect Insulin Sensitivity Similarly in Obese Adults. J. Clin. Endocrinol. Metab. 2020, 105, e2941–e2959. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.; Mocarelli, P.; Brambilla, P.; Wesselink, A.; Samuels, S.; Signorini, S.; Eskenazi, B. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: The Seveso women’s health study. Environ. Health Perspect. 2013, 121, 906–911. [Google Scholar] [CrossRef] [Green Version]
- Ha, C.H.; Swearingin, B.; Jeon, Y.K.; Lee, M. Effects of combined exercise on HOMA-IR, HOMA β-cell and atherogenic index in Korean obese female. Sport Sci. Health 2015, 11, 49–55. [Google Scholar] [CrossRef]
- Grassiolli, S.; Boschero, A.C.; Carneiro, E.M.; Zoppi, C.C. Exercise-Induced Pancreatic Islet Adaptations in Health and Disease. In Islets of Langerhans; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 1–17. [Google Scholar] [CrossRef]
- Huang, H.H.; Farmer, K.; Windscheffel, J.; Yost, K.; Power, M.; Wright, D.E.; Stehno-Bittel, L. Exercise increases insulin content and basal secretion in pancreatic islets in type 1 diabetic mice. Exp. Diabetes Res. 2011, 2011, 481427. [Google Scholar] [CrossRef] [PubMed]
- Howarth, F.C.; Marzouqi, F.M.; Al Saeedi, A.M.; Hameed, R.S.; Adeghate, E. The effect of a heavy exercise program on the distribution of pancreatic hormones in the streptozotocin-induced diabetic rat. Jop 2009, 10, 485–491. [Google Scholar]
- Wang, S.L.; Chang, Y.C.; Chao, H.R.; Li, C.M.; Li, L.A.; Lin, L.Y.; Päpke, O. Body burdens of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls and their relations to estrogen metabolism in pregnant women. Environ. Health Perspect. 2006, 114, 740–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, P.A.; Dicker-Brown, A.; Said, S.T.; Kennedy, R.; Fonseca, V.A. The stimulation of tumor necrosis factor and inhibition of glucose transport and lipoprotein lipase in adipose cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Metab.-Clin. Exp. 2002, 51, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Biddinger, S.B.; Kahn, C.R. From mice to men: Insights into the insulin resistance syndromes. Annu. Rev. Physiol. 2006, 68, 123–158. [Google Scholar] [CrossRef] [Green Version]
- Rector, R.S.; Morris, E.M.; Ridenhour, S.; Meers, G.M.; Hsu, F.F.; Turk, J.; Ibdah, J.A. Selective hepatic insulin resistance in a murine model heterozygous for a mitochondrial trifunctional protein defect. Hepatology 2013, 57, 2213–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udelhoven, M.; Leeser, U.; Freude, S.; Hettich, M.M.; Laudes, M.; Schnitker, J.; Krone, W.; Schubert, M. Identification of a region in the human irs2 promoter essential for stress induced transcription depending on sp1, nfi binding and erk activation in hepg2 cells. J. Mol. Endocrinol. 2010, 44, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Chibalin, A.V.; Yu, M.; Ryder, J.W.; Song, X.M.; Galuska, D.; Krook, A.; Wallberg-Henriksson, H.; Zierath, J.R. Exercise-induced changes in expression and activity of proteins involved in insulin signal transduction in skeletal muscle: Differential effects on insulin-receptor substrates 1 and 2. Proc. Natl. Acad. Sci. USA 2000, 97, 38–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polito, R.; Monaco, M.L.; Mallardo, M.; Elce, A.; Daniele, A.; Nigro, E. Treatment with sera from water polo athletes activates ampkα and acc proteins in hepg2 hepatoma cell line. Sport Sci. Health 2021, 17, 745–752. [Google Scholar] [CrossRef]

| Groups | Grade | Speed (m/min) | Time (min/day) | Distance (m) | VO2 |
|---|---|---|---|---|---|
| MIT | 5% | 15 | 60 | 900 | 60%VO2max |
| MIT+T | 5% | 15 | 60 | 900 | 60%VO2max |
| HIT | 5% | 26 | 35 | 910 | 75%VO2max |
| HIT+T | 5% | 26 | 35 | 910 | 75%VO2max |
| Gene | Primer Sequence | Product Size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| IRS2 | CTGGACAGAGGACTGAGGAGAGG | AGGCAGAGGAAGGCTGAGGAAC | 82 |
| PIK3CA | AGGATGCCCAACTTGATGCTGATG | CCGTTCATATAGGGTGTCGCTGTG | 119 |
| AKT | CAGGAGGAGGAGAGATGGACTTC | CACACGGTGCTTGGGCTTGG | 99 |
| β-actin | CCCAGGCATTGCTGACAGGATG | TGCTGGAAGGTGGACAGTGAGG | 144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, J.; Zhu, Y.; Yan, H.; Lu, Y. Effects of Different Intensity Exercise on Glucose Metabolism and Hepatic IRS/PI3K/AKT Pathway in SD Rats Exposed with TCDD. Int. J. Environ. Res. Public Health 2021, 18, 13141. https://doi.org/10.3390/ijerph182413141
Wang H, Wang J, Zhu Y, Yan H, Lu Y. Effects of Different Intensity Exercise on Glucose Metabolism and Hepatic IRS/PI3K/AKT Pathway in SD Rats Exposed with TCDD. International Journal of Environmental Research and Public Health. 2021; 18(24):13141. https://doi.org/10.3390/ijerph182413141
Chicago/Turabian StyleWang, Huohuo, Juanjuan Wang, Yihua Zhu, Huiping Yan, and Yifan Lu. 2021. "Effects of Different Intensity Exercise on Glucose Metabolism and Hepatic IRS/PI3K/AKT Pathway in SD Rats Exposed with TCDD" International Journal of Environmental Research and Public Health 18, no. 24: 13141. https://doi.org/10.3390/ijerph182413141
APA StyleWang, H., Wang, J., Zhu, Y., Yan, H., & Lu, Y. (2021). Effects of Different Intensity Exercise on Glucose Metabolism and Hepatic IRS/PI3K/AKT Pathway in SD Rats Exposed with TCDD. International Journal of Environmental Research and Public Health, 18(24), 13141. https://doi.org/10.3390/ijerph182413141






