The Significance of Short Latency in Mesothelioma for Attribution of Causation: Report of a Case with Predisposing Germline Mutations and Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Personal and Family History, Exposure History, and Diagnosis
2.2. Genetic Analysis
3. Results
3.1. Personal and Family History, and Exposure History
3.2. Diagnosis
3.3. Genetic Analysis
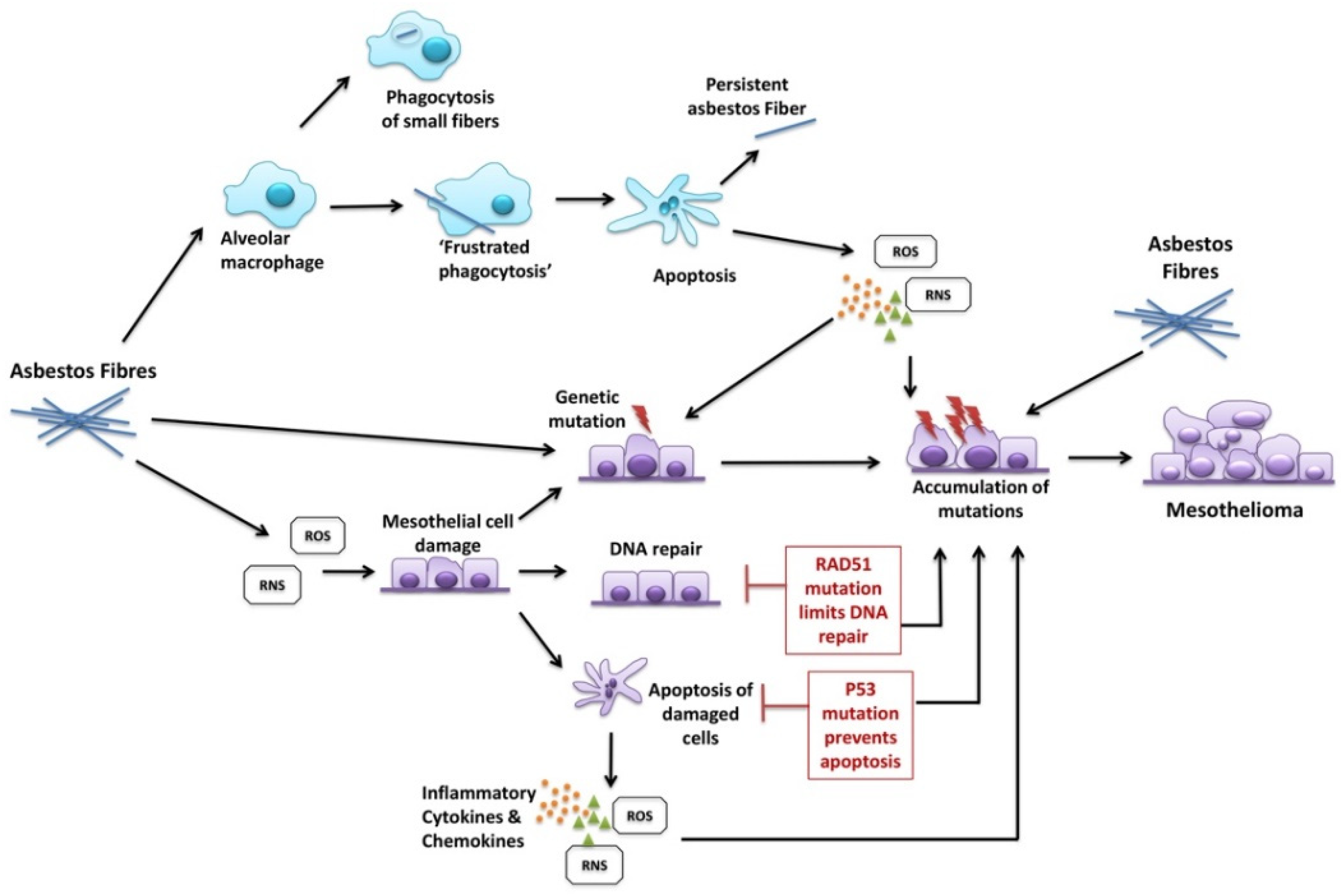
4. Discussion
Toxicology: (i) The inhaled ‘dose’ of asbestos fibres, by way of a no-threshold dose–response relationship—as cumulative asbestos exposure increases, so does the probability and frequency of occurrence of mesothelioma as a consequence;
(ii) Asbestos fibre types: The amphibole forms of asbestos are substantially more potent for mesothelioma induction than white asbestos (chrysotile) on a fibre-for-fibre basis;
| Type of Study | Exposure | Site | Mean/ Median Latency | Latency (Range in Years) | Ref. |
|---|---|---|---|---|---|
| Compensation Board | Mostly Chrysotile | nd | 26.9 | 6–44 | [8] |
| Case report | Amphibole | Pleura | nd | 8 | [14,17] |
| Case report | Chrysotile | Pleura | nd | 7.5 | [15] |
| Registry | Various | nd | nd | 3.5–65 * | [52] |
| Surveillance program | nd | nd | 37.4 | 4–66 # | [53] |
| Registry | nd | nd | 43.9 | 6–77 ^ | [54] |
| Pooled cohort | Various | Pleura | 38.4 | 7.6 | [55] |
| Pooled cohort | Various | Peritoneal | 38.4 years | 7.2 | [55] |
| Registry | Various | nd | nd, 65.7% > 30 | <10–>50 & | [13,56] |
| Cohort study | Mixed | nd | 22.8 all 8.2 peritoneal 2.9 pleural and peritoneal | <10 +—not specified | [9,59] |
| Case report | Mixed | Pleura | 8.5 | 8.5 | [10] |
| Registry | Various | Pleura | 44.6 | 6–84 | [12] |
| Cohort study | Mixed | Pleura | 42.8 | 9.3—not specified | [57] |
| Case–control study | Various | Pleura | 47 | 7–61 | [58] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
References
- Hodgson, J.T.; Darnton, A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann. Occup. Hyg. 2000, 44, 565–601. [Google Scholar] [CrossRef]
- WHO. Environmental Health Criteria 203: Chrysotile Asbestos; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- WTO. World Trade Organization (WTO): European Communities—Measures Concerning Asbestos and Asbestos-Containing Products; WTO: Geneva, Switzerland, 2000. [Google Scholar]
- Berry, G. Models for mesothelioma incidence following exposure to fibers in terms of timing and duration of exposure and the biopersistence of the fibers. Inhal. Toxicol. 1999, 11, 111–130. [Google Scholar] [CrossRef]
- Berry, G.; de Klerk, N.H.; Reid, A.; Ambrosini, G.L.; Fritschi, L.; Olsen, N.J.; Merler, E.; Musk, A.W. Malignant pleural and peritoneal mesotheliomas in former miners and millers of crocidolite at Wittenoom, Western Australia. Occup. Environ. Med. 2004, 61, e14. Available online: http://www.occenvmed.com/cgi/content/full/61/4/e14 (accessed on 24 August 2021). [CrossRef] [PubMed]
- HEI-AR. Asbestos in Public and Commercial Buildings: A Literature Review and Synthesis of Current Knowledge; Health Effects Institute-Asbestos Research: Cambridge, MA, USA, 1991. [Google Scholar]
- Tossavainen, A. Asbestos, asbestosis, and cancer: The Helsinki criteria for diagnosis and attribution. Scand. J. Work Environ. Health 1997, 23, 311–316. [Google Scholar] [CrossRef]
- Chovil, A.; Stewart, C. Latency period for mesothelioma [letter]. Lancet 1979, 2, 853. [Google Scholar] [CrossRef]
- Frost, G. The latency period of mesothelioma among a cohort of British asbestos workers (1978–2005). Br. J. Cancer 2013, 109, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Bitchatchi, E.; Kayser, K.; Perelman, M.; Richter, E.D. Mesothelioma and asbestosis in a young woman following occupational asbestos exposure: Short latency and long survival: Case Report. Diagn. Pathol. 2010, 5, 81. [Google Scholar] [CrossRef]
- Farioli, A.; Mattioli, S.; Curti, S.; Violante, F.S. Comment on ‘The latency period of mesothelioma among a cohort of British asbestos workers (1978–2005)’: The effect of left censoring. Br. J. Cancer 2014, 111, 2197–2198. [Google Scholar] [CrossRef][Green Version]
- Marinaccio, A.; Binazzi, A.; Cauzillo, G.; Cavone, D.; Zotti, R.D.; Ferrante, P.; Gennaro, V.; Gorini, G.; Menegozzo, M.; Mensi, C.; et al. Analysis of latency time and its determinants in asbestos related malignant mesothelioma cases of the Italian register. Eur. J. Cancer 2007, 43, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Konetzke, G.W.; Bwck, B.; Harold, H.J. Proceedings of the Asbestos-Induced Mesothelioma-Results of a Retrospecyive Study, Prevention of Occupational Cancer. International Symposium, Helsinki, Finland, 21–24 April 1981; International Labour Office, World Health Organization and the International Agency for Research on Cancer: Helsinki, Finland, 1981.
- Booth, S.J.; Weaver, E.J. Malignant pleural mesothelioma five years after domestic exposure to blue asbestos. Lancet 1986, 1, 435. [Google Scholar] [CrossRef]
- Scansetti, G.; Mollo, F.; Tiberi, G.; Andrion, A.; Piolatto, G. Pleural mesothelioma after a short interval from first exposure in the wine filter industry. Am. J. Ind. Med. 1984, 5, 335–339. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Buncher, C.R. Latent period for malignant mesothelioma of occupational origin. J. Occup. Med. 1992, 34, 718–721. [Google Scholar] [PubMed]
- Elmes, P.; Browne, K.; Booth, S.J. Mesothelioma shortly after brief exposure to asbestos. Lancet 1986, 327, 746. [Google Scholar] [CrossRef]
- Hjerpe, A.; Abd Own, S.; Dobra, K. Integrative approach to cytologic and molecular diagnosis of malignant pleural mesothelioma. Transl. Lung Cancer Res. 2020, 9, 934–943. [Google Scholar] [CrossRef]
- Klebe, S.; Nakatani, Y.; Dobra, K.; Butnor, K.J.; Roden, A.C.; Nicholson, A.G.; Marchevsky, A.M.; Husain, A.N.; Segal, A.; Walts, A.E.; et al. The concept of mesothelioma in situ, with consideration of its potential impact on cytology diagnosis. Pathology 2021, 53, 446–453. [Google Scholar] [CrossRef]
- Betti, M.; Casalone, E.; Ferrante, D.; Aspesi, A.; Morleo, G.; Biasi, A.; Sculco, M.; Mancuso, G.; Guarrera, S.; Righi, L.; et al. Germline mutations in DNA repair genes predispose asbestos-exposed patients to malignant pleural mesothelioma. Cancer Lett. 2017, 405, 38–45. [Google Scholar] [CrossRef]
- Bertelsen, B.; Tuxen, I.V.; Yde, C.W.; Gabrielaite, M.; Torp, M.H.; Kinalis, S.; Oestrup, O.; Rohrberg, K.; Spangaard, I.; Santoni-Rugiu, E.; et al. High frequency of pathogenic germline variants within homologous recombination repair in patients with advanced cancer. NPJ Genom. Med. 2019, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Panou, V.; Røe, O.D. Inherited Genetic Mutations and Polymorphisms in Malignant Mesothelioma: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 4327. [Google Scholar] [CrossRef] [PubMed]
- Betti, M.; Aspesi, A.; Ferrante, D.; Sculco, M.; Righi, L.; Mirabelli, D.; Napoli, F.; Rondon-Lagos, M.; Casalone, E.; Vignolo Lutati, F.; et al. Sensitivity to asbestos is increased in patients with mesothelioma and pathogenic germline variants in BAP1 or other DNA repair genes. Genes Chromosomes Cancer 2018, 57, 573–583. [Google Scholar] [CrossRef]
- Guazzelli, A.; Meysami, P.; Bakker, E.; Demonacos, C.; Giordano, A.; Krstic-Demonacos, M.; Mutti, L. BAP1 Status Determines the Sensitivity of Malignant Mesothelioma Cells to Gemcitabine Treatment. Int. J. Mol. Sci. 2019, 20, 429. [Google Scholar] [CrossRef]
- Dienstmann, R.; Dong, F.; Borger, D.; Dias-Santagata, D.; Ellisen, L.W.; Le, L.P.; Iafrate, A.J. Standardized decision support in next generation sequencing reports of somatic cancer variants. Mol. Oncol. 2014, 8, 859–873. [Google Scholar] [CrossRef]
- Gaudino, G.; Xue, J.; Yang, H. How asbestos and other fibers cause mesothelioma. Transl. Lung Cancer Res. 2020, 9 (Suppl. 1), S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Ishihara, T.; Lee, W.H.; Ohara, H.; Okazaki, Y.; Okawa, K.; Toyokuni, S. Asbestos surface provides a niche for oxidative modification. Cancer Sci. 2011, 102, 2118–2125. [Google Scholar] [CrossRef]
- Xu, A.; Wu, L.J.; Santella, R.M.; Hei, T.K. Role of oxyradicals in mutagenicity and DNA damage induced by crocidolite asbestos in mammalian cells. Cancer Res. 1999, 59, 5922–5926. [Google Scholar] [PubMed]
- Marczynski, B.; Rozynek, P.; Kraus, T.; Schlosser, S.; Raithel, H.J.; Baur, X. Levels of 8-hydroxy-2’-deoxyguanosine in DNA of white blood cells from workers highly exposed to asbestos in Germany. Mutat. Res. 2000, 468, 195–202. [Google Scholar] [CrossRef]
- Liu, G.; Cheresh, P.; Kamp, D.W. Molecular basis of asbestos-induced lung disease. Annu. Rev. Pathol. 2013, 8, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Filiberti, R.; Taioli, E.; Garte, S.; Paracchini, V.; Bolognesi, C.; Canessa, P.A.; Fontana, V.; Ivaldi, G.P.; Verna, A.; et al. Pleural malignant mesothelioma, genetic susceptibility and asbestos exposure. Mutat. Res. 2005, 592, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Ugolini, D.; Dianzani, I.; Gemignani, F.; Landi, S.; Cesario, A.; Magnani, C.; Mutti, L.; Puntoni, R.; Bonassi, S. Genetic susceptibility to malignant pleural mesothelioma and other asbestos-associated diseases. Mutat. Res. 2008, 659, 126–136. [Google Scholar] [CrossRef]
- De Rienzo, A.; Balsara, B.R.; Apostolou, S.; Jhanwar, S.C.; Testa, J.R. Loss of heterozygosity analysis defines a 3-cM region of 15q commonly deleted in human malignant mesothelioma. Oncogene 2001, 20, 6245–6249. [Google Scholar] [CrossRef] [PubMed]
- Tarsounas, M.; Munoz, P.; Claas, A.; Smiraldo, P.G.; Pittman, D.L.; Blasco, M.A.; West, S.C. Telomere maintenance requires the RAD51D recombination/repair protein. Cell 2004, 117, 337–347. [Google Scholar] [CrossRef]
- Braybrooke, J.P.; Spink, K.G.; Thacker, J.; Hickson, I.D. The RAD51 family member, RAD51L3, is a DNA-stimulated ATPase that forms a complex with XRCC2. J. Biol. Chem. 2000, 275, 29100–29106. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Narbutis, M.; Koch, R.; Rana, A. Frequency and prognostic value of mutations associated with the homologous recombination DNA repair pathway in a large pan cancer cohort. Sci. Rep. 2020, 10, 20223. [Google Scholar] [CrossRef] [PubMed]
- Bononi, A.; Goto, K.; Ak, G.; Yoshikawa, Y.; Emi, M.; Pastorino, S.; Carparelli, L.; Ferro, A.; Nasu, M.; Kim, J.-H.; et al. Heterozygous germline BLM mutations increase susceptibility to asbestos and mesothelioma. Proc. Natl. Acad. Sci. USA 2020, 117, 33466–33473. [Google Scholar] [CrossRef]
- Dacic, S.; Roy, S.; Lyons, M.A.; von der Thusen, J.H.; Galateau-Salle, F.; Churg, A. Whole exome sequencing reveals BAP1 somatic abnormalities in mesothelioma in situ. Lung Cancer 2020, 149, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cousineau, I.; Abaji, C.; Belmaaza, A. BRCA1 regulates RAD51 function in response to DNA damage and suppresses spontaneous sister chromatid replication slippage: Implications for sister chromatid cohesion, genome stability, and carcinogenesis. Cancer Res. 2005, 65, 11384–11391. [Google Scholar] [CrossRef]
- Fukuda, T.; Tsuruga, T.; Kuroda, T.; Nishikawa, H.; Ohta, T. Functional Link between BRCA1 and BAP1 through Histone H2A, Heterochromatin and DNA Damage Response. Curr. Cancer Drug Targets 2016, 16, 101–109. [Google Scholar] [CrossRef]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb Perspect Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Carbone, M.; Harbour, J.W.; Brugarolas, J.; Bononi, A.; Pagano, I.; Dey, A.; Krausz, T.; Pass, H.I.; Yang, H.; Gaudino, G. Biological Mechanisms and Clinical Significance of BAP1 Mutations in Human Cancer. Cancer Discov. 2020, 10, 1103–1120. [Google Scholar] [CrossRef]
- Ospina, D.; Villegas, V.E.; Rodriguez-Leguizamon, G.; Rondon-Lagos, M. Analyzing biological and molecular characteristics and genomic damage induced by exposure to asbestos. Cancer Manag. Res. 2019, 11, 4997–5012. [Google Scholar] [CrossRef]
- Taghizadeh, H.; Zochbauer-Muller, S.; Mader, R.M.; Mullauer, L.; Klikovits, T.; Bachleitner-Hofmann, T.; Hoda, M.A.; Prager, G.W. Gender differences in molecular-guided therapy recommendations for metastatic malignant mesothelioma. Thorac. Cancer 2020, 11, 1979–1988. [Google Scholar] [CrossRef]
- Hassan, R.; Morrow, B.; Thomas, A.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Gadiraju, M.; Panou, V.; Gao, S.; Mian, I.; et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc. Natl. Acad. Sci. USA 2019, 116, 9008–9013. [Google Scholar] [CrossRef] [PubMed]
- Arulananda, S.; Thapa, B.; Walkiewicz, M.; Zapparoli, G.V.; Williams, D.S.; Dobrovic, A.; John, T. Mismatch Repair Protein Defects and Microsatellite Instability in Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Panou, V.; Gadiraju, M.; Wolin, A.; Weipert, C.M.; Skarda, E.; Husain, A.N.; Patel, J.D.; Rose, B.; Zhang, S.R.; Weatherly, M.; et al. Frequency of Germline Mutations in Cancer Susceptibility Genes in Malignant Mesothelioma. J. Clin. Oncol. 2018, 36, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Marinaccio, A.; Binazzi, A.; Marzio, D.D.; Scarselli, A.; Verardo, M.; Mirabelli, D.; Gennaro, V.; Mensi, C.; Riboldi, L.; Merler, E.; et al. Pleural malignant mesothelioma epidemic: Incidence, modalities of asbestos exposure and occupations involved from the Italian National Register. Int. J. Cancer 2012, 130, 2146–2154. [Google Scholar] [CrossRef]
- Mowé, G.; Gylseth, B.; Hartveit, F.; Skaug, V. Occupational asbestos exposure, lung-fiber concentration and latency time in malignant mesothelioma. Scand. J. Work Environ. Health 1984, 10, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Giarelli, L.; Grandi, G.; Brollo, A.; Ramani, L.; Zuch, C. Latency periods in asbestos-related mesothelioma of the pleura. Eur. J. Cancer Prev. 1997, 6, 162–166. [Google Scholar] [PubMed]
- Wolff, H.; Vehmas, T.; Oksa, P.; Rantanen, J.; Vainio, H. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: Recommendations. Scand. J. Work Environ. Health 2015, 41, 5–15. [Google Scholar] [CrossRef]
- Greenberg, M.; Davies, T. Mesothelioma register 1967–68. Br. J. Ind. Med. 1974, 31, 91–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferguson, D.A.; Berry, G.; Jelihovsky, T.; Andreas, S.B.; Rogers, A.J.; Fung, S.C.; Grimwood, A.; Thompson, R. The Australian Mesothelioma Surveillance Program 1979–1985. Med. J. Aust. 1987, 147, 166–172. [Google Scholar] [CrossRef]
- Driscoll, T.; Leigh, J. Preparing an Estimate of the National Pattern of Exposure to Asebstos in Cases of Malignamt Mesothelioma; Australian Safety and Compensation Council, Commwealth of Australia: Canberra, Australia, 2008. [Google Scholar]
- Reid, A.; de Klerk, N.H.; Magnani, C.; Ferrante, D.; Berry, G.; Musk, A.W.; Merler, E. Mesothelioma risk after 40 years since first exposure to asbestos: A pooled analysis. Thorax 2014, 69, 843–850. [Google Scholar] [CrossRef]
- Konetzke, G.W.; Beck, B. [Risk factor asbestos (author’s transl)]. Arch. Fur Geschwulstforsch. 1981, 51, 567–574. (In Germany) [Google Scholar]
- Merlo, D.F.; Bruzzone, M.; Bruzzi, P.; Garrone, E.; Puntoni, R.; Maiorana, L.; Ceppi, M. Mortality among workers exposed to asbestos at the shipyard of Genoa, Italy: A 55 years follow-up. Environ. Health A Glob. Access Sci. Source 2018, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Lacourt, A.; Leveque, E.; Guichard, E.; Gilg Soit Ilg, A.; Sylvestre, M.P.; Leffondre, K. Dose-time-response association between occupational asbestos exposure and pleural mesothelioma. Occup. Environ. Med. 2017, 74, 691–697. [Google Scholar] [CrossRef]
- Frost, G. Response to comment on ‘The latency period of mesothelioma among a cohort of British asbestos workers (1978–2005)’. Br. J. Cancer 2014, 111, 2198–2199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mowé, G.; Gylseth, B. Occupational exposure and regional variation of malignant mesothelioma in Norway, 1970–1979. Am. J. Ind. Med. 1986, 9, 323–332. [Google Scholar] [CrossRef]
- Consonni, D.; Barone-Adesi, F.; Mensi, C. Comment on ‘The latency period of mesothelioma among a cohort of British asbestos workers (1978–2005)’: Methodological problems with case-only survival analysis. Br. J. Cancer 2014, 111, 1674. [Google Scholar] [CrossRef][Green Version]
- Hilliard, A.K.; Lovett, J.K.; McGavin, C.R. The rise and fall in incidence of malignant mesothelioma from a British Naval Dockyard, 1979–1999. Occup. Med. 2003, 53, 209–212. [Google Scholar] [CrossRef] [PubMed]
- D’Agostin, F.; De Michieli, P.; Chermaz, C.; Negro, C. Pleural and peritoneal mesotheliomas in the Friuli Venezia Giulia register: Data analysis from 1995 to 2015 in Northeastern Italy. J. Thorac. Dis. 2017, 9, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Neumann, V.; Gunthe, S.; Muller, K.M.; Fischer, M. Malignant mesothelioma—German mesothelioma register 1987–1999. Int. Arch. Occup. Environ. Health 2001, 74, 383–395. [Google Scholar] [CrossRef]
- Dragani, T.A.; Colombo, F.; Pavlisko, E.N.; Roggli, V.L. Malignant mesothelioma diagnosed at a younger age is associated with heavier asbestos exposure. Carcinogenesis 2018, 39, 1151–1156. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klebe, S.; Hocking, A.J.; Soeberg, M.; Leigh, J. The Significance of Short Latency in Mesothelioma for Attribution of Causation: Report of a Case with Predisposing Germline Mutations and Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 13310. https://doi.org/10.3390/ijerph182413310
Klebe S, Hocking AJ, Soeberg M, Leigh J. The Significance of Short Latency in Mesothelioma for Attribution of Causation: Report of a Case with Predisposing Germline Mutations and Review of the Literature. International Journal of Environmental Research and Public Health. 2021; 18(24):13310. https://doi.org/10.3390/ijerph182413310
Chicago/Turabian StyleKlebe, Sonja, Ashleigh J. Hocking, Matthew Soeberg, and James Leigh. 2021. "The Significance of Short Latency in Mesothelioma for Attribution of Causation: Report of a Case with Predisposing Germline Mutations and Review of the Literature" International Journal of Environmental Research and Public Health 18, no. 24: 13310. https://doi.org/10.3390/ijerph182413310
APA StyleKlebe, S., Hocking, A. J., Soeberg, M., & Leigh, J. (2021). The Significance of Short Latency in Mesothelioma for Attribution of Causation: Report of a Case with Predisposing Germline Mutations and Review of the Literature. International Journal of Environmental Research and Public Health, 18(24), 13310. https://doi.org/10.3390/ijerph182413310







