Abstract
First-void urine usually contains exfoliated cells of the debris and mucus from the female genital organs and cervix, i.e., high concentration of human papillomavirus deoxyribonucleic acid (HPV DNA). We conducted a meta-analysis of published data and determined an accuracy of HPV detection in first-void urine compared to the women’s cervix. According to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we carried out a comprehensive literature search. Eligible articles published from 2011 until 2021 were gathered by searching Embase, PubMed and Cochrane Library Central databases. The patient selection, index test, standard test, and patient flow were the factors involved in quality evaluation. A meta-analysis of 15 studies (3412 women) based on 5054 potential records was conducted. Pooled sensitivity for high-risk HPV detection in urine of 78% (70–84%) and specificity of 89% (81–94%) were calculated. Any HPV detection in urine of 87% (74–94%) and 91% (83–96%) were pooled sensitivity and specificity, respectively. HPV 16 and 18 had a pooled sensitivity of 77% (76–77%) and specificity of 98% (98–98%). Meta-analysis indicated variations between the pooled specificities and sensitivities. In meta-regression analysis, a heterogeneity in accuracy by using covariates (bias in patient selection, purpose, sample timing, storage temperature and HPV detection method) were not detected. Our meta-analysis demonstrates the accuracy of detection of HPV in urine for the presence of cervical HPV. Although progress is continuously made in urinary HPV detection, further studies are needed to evaluate and to improve the accuracy of the first-void urine test in order to be comparable with other screening methods.
1. Introduction
Is widely known that HPV is the primary cause of cervical cancer [1]. Cervical cancer presents the fourth-most cause of cancer deaths in women worldwide [2]. HPV is detected in almost all cervical cancer biopsies with more than 90% presence in high-grade squamous intraepithelial lesions (HSIL) [3]. More than 200 genotypes of HPV have been identified to date [4]. Of them, HPV16 and HPV18 represent the high-risk oncogenic genotypes, as they cause approximately 70% of nearly all cervical cancer [5,6,7].
A major impediment to controlling cervical cancer is lack of attendance for screening, i.e., in those countries without well-developed screening programs, from 50% to more than 80% of women are not screened [8]. In addition, in countries with well-organised screening programmes, half of all potentially detectable carcinomas are found in women who have not attended screening programmes [9].
There has been a drastic decline in the incidence, as well as the mortality, of cervical cancer worldwide since the introduction of the Pap test [10,11]. However, screening strategies for cervical cytology or Papanicolaou (Pap) tests requires uncomfortable and invasive pelvic examinations. Moreover, healthcare providers find it time-consuming and it cannot be carried out easily in resource-poor settings [12,13]. Additionally, cervical cytology can be susceptible as a result of technical or subjective errors, due to low sensitivity and false negative results [14,15].
There has been a great deal of interest lately in using urine as a liquid biopsy for HPV DNA testing, and this has increased due to observation of high correlations between urine and cervical HPV infections [16,17,18,19,20]. Urine samples are a good option for self-sampling screening since they are cheap, noninvasive and simple to collect [21,22]. The HPV test using urine appears to be an effective method for detecting HPV infection, so there is a possibility that it could be used for women who do not participate in routine screenings [23].
Urine voiding in the first part (first-void urine) usually contains exfoliated cells of the debris and mucus from the female genital organs and cervix, i.e., the first-void urine contains higher concentrations of HPV DNA than midstream urine. According to this theory, the identification of biomarkers in first-void urine, as well as HPV DNA, can be used to screen for (pre)cervical cancer [24].
Therefore, we conducted a systematic review and meta-analysis to determine the accuracy of detection of HPV in first-void urine compared with the cervix in women.
2. Materials and Methods
According to recommended methods, a meta-analysis and systematic review was conducted in compliance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [25].
2.1. Criteria for Search and Eligibility
A literature review for the past 10 years (from January 2011 up to May 2021) in the three databases: Embase and Cochrane library (Title/Keywords/Abstracts) and PubMed (Title/Abstracts) was conducted. In each database, using Boolean logic, we searched for the following terms: (HPV or hrHPV or human papillomavir *) OR (HPV or hrHPV or human papillomavir *) AND (test * or assay * or genotyping or typing or detection or amplification) OR (HPV or hrHPV or human papillomavir *) AND (deoxyribonucleic or ribonucleic or nucleic or DNA or RNA or mRNA) OR (cervical or cervix or cervixes or cervico *) AND (precancer * or cancer * or neoplas * or dysplas * or dyskaryos * or tumor * or tumour * or malignanc * or carcinoma * or adenocarcinoma * or lesion * or squamous or small cell or large cell) OR (cervical intraepithelial neoplasia or CIN or CINII * or CIN2 * or CINIII * or CIN3 * or SIL or HSIL or LSIL or ASCUS or AS-CUS) AND (urin *). We manually searched the relevant publications.
The eligibility criteria included any test-of-accuracy study comparing HPV DNA detection in urine and cervix samples, in women with concern about infection with HPV or development of cervical cancer. If the reference standard was different or not available, we excluded the study. Meta-analysis included studies with data that could be converted into 2 × 2 table. A test’s diagnostic value can be overestimated by certain factors. Therefore, we excluded case-control studies, i.e., studies testing only cervical cancer patients or non-infected patients from the meta-analysis.
2.2. Study Extraction, Quality and Selection
For relevant studies, we screened all titles and abstracts. Two reviewers (P.B. and J.S.) independently performed a systematic literature search. In addition, P.B. screened the full texts of the included papers and extracted the subsequent data: characteristics of the study (authors, publication year, country, and purpose), characteristics of the patients (median age and range, cytology and histology results), index test characteristics (volume of sample, storage temperature, DNA extraction and amplification method, test timing as compared to the reference standard). To all studies the quality assessment of diagnostic accuracy studies-2 (QUADAS-2) was applied [26]. The patient selection, index test, standard test, and patient flow were the factors involved in quality evaluation.
2.3. Data Synthesis and Statistical Analysis
Upon the detection of any HPV, high-risk HPV, HPV 16 and 18, the 2 × 2 table was made. If the study included more than one method for testing urine HPV, we selected the one with methods closest to those used by other studies. From the estimates, we derived a summary receiver operating characteristic (SROC) curve and the summary accuracy measures with 95% confidence interval (CI) (sensitivity, specificity, likelihood ratio positive and negative (LR+ and LR−)). The shape of a receiver operating characteristic (ROC) curve and the area under the curve (AUC) can help us get a sense of a test’s discriminative power, i.e., AUC presents the measure of diagnostic accuracy. If the curve is located as close as possible to the upper-lefthand corner, and the larger the area under curve, then the test will discriminate better between diseased and healthy individuals. A good indicator of the quality of the test is the area under the curve, which can range from 0 to 1. In a perfect diagnostic test, the AUC is 1, whereas in a nondiscriminating one, the AUC is 0.5 [27]. The forest plots showing the sensitivity and specificity with 95% CI to visualise heterogeneity of studies were generated. In addition, we included the subsequent covariates in meta-regression in order to investigate possible sources of heterogeneity: bias caused by patient selection (high risk versus low risk), purpose (surveillance of HPV versus cervical intraepithelial neoplasia (CIN) and cervical cancer screening), sample timing (urine before versus after cervical tissue collection), storage temperature (more than 0 °C versus less than 0 °C), HPV detection method (conventional PCR versus real time, quantitative polymerase chain reaction (qPCR), DNA microarray, multiplex PCR).
A meta-analysis of diagnostic test accuracy was conducted using an online, freely available interactive web-based tool: MetaDTA, version 2.01 (https://crsu.shinyapps.io/dta_ma/ (Accessed date: 13 December 2021)). The MetaDTA statistical tool pools the sensitivity and specificity estimates for bivariate random-effects models. This model was fitted as a generalized linear mixed-effect model using the glmer function from the package lme4 of the statistical software R with shiny [28]. This approach accounts for potential threshold effects and covariance between sensitivity and specificity. Using the logit estimates of sensitivity and specificity, the diagnostic odds ratios (DORs) were obtained directly. In addition, using parameters estimated from the bivariate model through the equivalence equations of Harbord et al. [29], the SROC plot was rendered.
Meta-regression was performed using Meta-DiSc software (version 1.4). To explore sources of heterogeneity in the studies, we used the Moses–Shapiro–Littenberg method by adding covariates to the model [30]. Meta-regression analysis included the threshold effect, weighted least squares method, the inverse of variance of the log of the DOR, and the random effects between studies using restricted maximum likelihood.
Publication bias was conducted using R Studio (version 1.3.959) with “metafor” package. A p value < 0.05 was considered statistically significant.
3. Results
Identifying and selecting studies is summarized in Figure 1. Of the 5054 potential records, 15 studies (3675 women recruited, 3412 women analysed) were included in the meta-analysis [3,16,17,31,32,33,34,35,36,37,38,39,40,41,42].
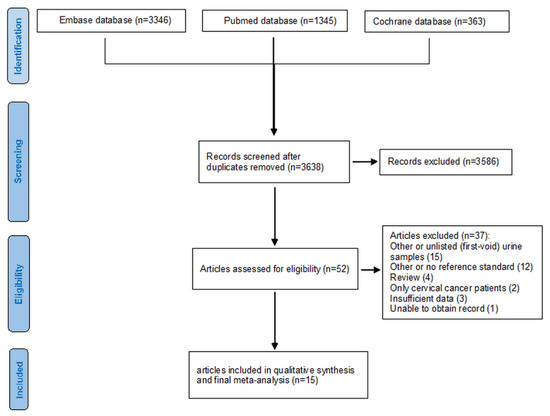
Figure 1.
Flow diagram of the studies selected for this meta-analysis.
3.1. Studies Description
The characteristics of included studies in this review and meta-analysis are shown in Table 1 and Table 2. We recruited 8 out of 15 populations of studies from gynaecology or colposcopy clinics, 3 from health centres, 1 from genitourinary medicine and 1 from a general practitioner. In most populations of study, cervical cancer screenings were the purpose of the testing (10/15). Those remaining were for CIN follow-up (3/15) or HPV surveillance (2/15).

Table 1.
Qualitative characteristics of included studies.

Table 2.
Quantitative characteristics of included studies.
Results of cytological analysis were recorded for 15 populations, i.e., 51% (1706/3360) women had normal conditions, 25% (848/3360) had atypical squamous cells of undetermined significance (ASCUS), 16% (542/3360) had low-grade squamous intraepithelial lesion (LSIL), 0.42% (14/3360) had atypical squamous cells, possible high-grade lesion (ASC-H), and 7.4% (250/3360) had high-grade squamous intraepithelial lesion (HSIL). From the 9 populations with reported histology results, 33.3% (304/912) of women had normal conditions, 25% (229/912) had CIN1, 14.6% (133/912) had CIN2, 1.2% (11/912) had CIN2+, 25% (229/912) had CIN3, and 0.66% (6/912) had histology proved cervical cancer.
Conventional PCR was used in most studies, but the testing methods used were not uniform. Five of the 15 studies used real-time PCR [31,32,34,40,41], and there was only one PCR-based DNA microarray [37] used out of 15. In one study, real time PCR was evaluated, in the last multiplex PCR. Storage temperatures of urine ranged from −80 °C [33,35,40] to 4 °C [31,32,34,37]. In 13 and 11 studies commercially available amplification platforms and commercial DNA extraction kits, respectively, were used. In all studies, the reference standard of cervical samples for HPV DNA testing were used.
3.2. Quality of Studies
A quality evaluation of the studies is shown in Figure 2. Due to narrow patient spectrums for 6 of the studies, the high-risk of bias for patient selection was recorded: 3 studies focused only on patients with CIN of high grade [31,32,39], 2 studies recorded only young women (18–25 age) [16,17], and 1 study included human immunodeficiency virus (HIV) patients [42]. In most studies, the patient flow and timing reduced the risk of bias; 8/15 analysed all recruited participants, and 7 studies analysed (1.9–23.2%) of recruited participants. In 8 of 15 studies, both tests completed during the same day, and in 8 studies, urine samples were collected prior to taking cervical samples. In all low-risk-of-bias studies, the reference standard was applied. Out of 15 studies, 1 used an index test with in-house methods that did not specify a threshold, i.e., the bias of this study was considered unclear risk [33]. In other studies (14/15), a predetermined threshold of the index test with low risk of bias was used. The publication bias did not appear in this study.
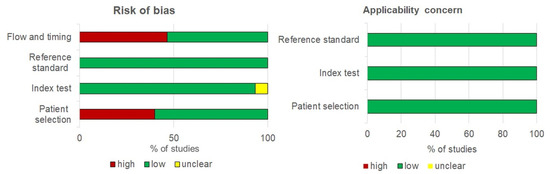
Figure 2.
Qualitative assessment of 15 studies included in the meta-analysis using QADAS-2.
3.3. Meta-Analysis
The heterogeneity of sensitivity and specificity between individual urine detection studies of any HPV (10 studies), high-risk HPV (12 studies), and HPV 16 and 18 (7 studies) is shown in Figure 3. The individual sensitivities and specificities of any HPV detection in urine varied from 54% [37] to 99% [38,42] and from 67% [29] to 99% [38,39], respectively. Individual sensitivities (51% [30] to 92% [33]) and specificities (59% [36] to 98% [35]) for high-risk HPV detection studies in urine were observed. According to analysis conducted on HPV 16 and 18, sensitivities ranged from 27% [32] to 96% [33] and specificities ranged from 92% [37] to 99% [32,35,42] in urine-detection studies.
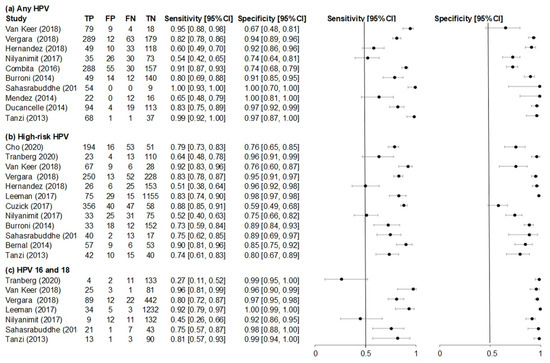
Figure 3.
Forest plots of (a) any HPV, (b) high-risk HPV and (c) HPV 16 and 18 sensitivity and specificity for studies evaluating accuracy of first-void urine human papillomavirus (HPV) detection compared to cervical HPV.
A SROC plot for pooled sensitivity and specificity for the three groups, (a) any HPV, (b) high-risk HPV and (c) HPV 16 and 18 is shown in Figure 4. Pooled sensitivity for high-risk HPV detection in urine of 78% (70% to 84%) and specificity of 89% (81% to 94%) were calculated. For any HPV detection in urine of 87% (74% to 94%) and 89% (81% to 93%) sensitivity and specificity, respectively, were pooled. HPV 16 and 18 had a pooled sensitivity of 77% (76% to 77%) and specificity of 98% (98% to 98%). The whole upper-left quadrant in Figure 4 represents the 95% prediction region for the SROC plots, i.e., between studies was heterogeneity. For any HPV detection, the 95% prediction region covers the largest portion of the plot, i.e., it had the most heterogeneity between studies (Figure 4a). For any HPV detection, the LR+ was 15.62 (95% CI 4.60 to 53.05) and the LR− was 0.14 (95% CI 0.08 to 0.24). For high-risk HPV detection, the LR+ was 6.81 (4.07 to 11.41) and the LR− was 0.25 (0.18 to 0.34). For HPV 16 and 18 detection, the LR+ was 39.73 (39.33 to 40.14) and the LR− was 0.24 (0.24 to 0.24).
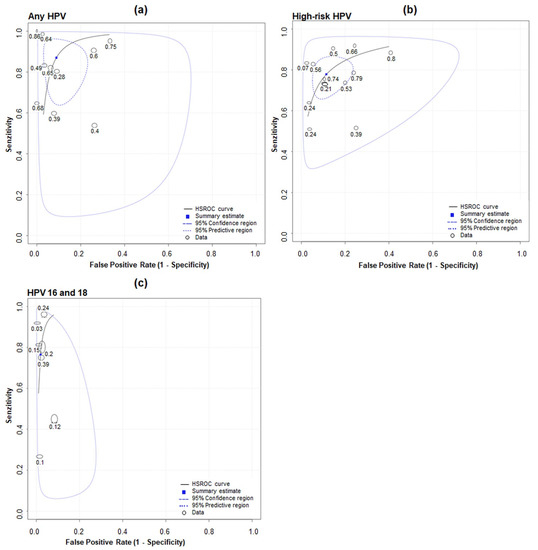
Figure 4.
SROC plot for studies evaluating accuracy of detecting (a) any HPV, (b) high-risk HPV and (c) HPV 16 and 18 in first-void urine compared with in cervix.
3.4. Meta-Regression Analyses
A meta-regression with the following covariates (bias in patient selection, purpose, sample timing, storage temperature and HPV detection method) was conducted to identify the possible sources of heterogeneity. Using the Cochran’s Q test, likelihood ratios and diagnostic odds ratios were tested for homogeneity between studies. Heterogeneity and variation between studies were not confirmed using the covariates listed above (Table 3).

Table 3.
Multivariate meta-regression results for characteristics with backward regression analysis.
3.5. Publication Bias
We investigated the potential publication bias by using Deek´s funnel plot asymmetry test, as shown in Figure 5. The regression test showed no significant publication bias (p = 0.19).
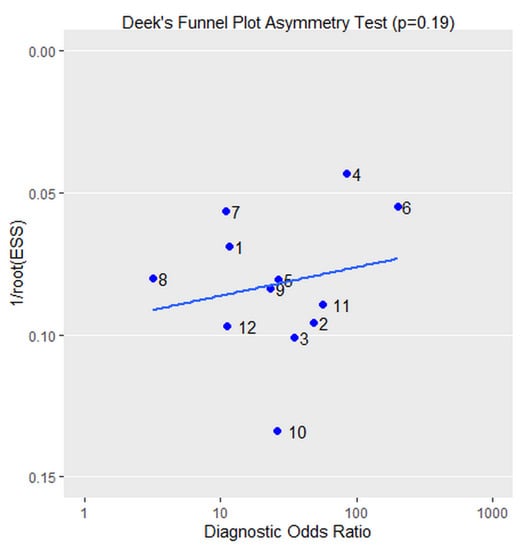
Figure 5.
Deek’s funnel plot. The regression test showed no significant publication bias (p = 0.19).
4. Discussion
The purpose of diagnostic tests in healthcare settings is to confirm or exclude diagnoses. Assessment of accuracy is determined by comparing the diagnostic test results with the “gold standard” according to which individuals’ true diagnosis can be determined. In our study, the HPV DNA in cervix samples represented the gold standard test, to compare with the HPV DNA in first-void urine samples.
In Pathak’s review, accuracy of urinary HPV testing for cervical human papillomavirus was investigated through meta-analysis. There was only one source of heterogeneity identified, which was urine sampling, i.e., the accuracy of samples collected as random or midstream, as opposed to first-void samples, decreased by more than 22 times [23]. The first-void urine contains higher levels of high-risk HPV as expected, i.e., 4.8–160 times higher in comparison to the other fraction [24]. The first-void urine can produce more HPV DNA-positive results than paired cervical samples when using sensitive HPV DNA assays [43,44,45]. Therefore, in our meta-analysis we used studies with first-void urine samples.
To evaluate the performance of a diagnostic test, we synthesized sensitivity and specificity from a meta-analysis of diagnostic test accuracy studies. In our meta-analysis, a heterogeneity between the pooled sensitivities and specificities was detected, i.e., pooled sensitivity for high-risk HPV detection in urine was 78% (70% to 84%) and specificity was 89% (81% to 94%). For any HPV detection in urine of 87% (74% to 94%) and 91% (83% to 96%), we pooled sensitivity and specificity, respectively. HPV 16 and 18 had a pooled sensitivity of 77% (76% to 77%) and a specificity of 98% (98% to 98%).
The bivariate model has been shown to be mathematically identical to the HSROC model when covariates are not included. The HSROC parameters were estimated using parameters of the bivariate model and the equivalence equations of Harbord et al. The SROC plot was drawn using the resulting HSROC parameters [29], and it shows the relationship between sensitivity (y-axis) and 1-specificity (x-axis), illustrating variations in sensitivity and specificity for different thresholds of a test. The whole upper-left quadrant in Figure 4 represents the 95% prediction region for the SROC plots, i.e., between studies there was heterogeneity. For any HPV detection, the 95% prediction region covers the largest portion of the plot, i.e., it had the most heterogeneity between studies (Figure 4a). Regarding the method used in the present meta-analysis, we acknowledge as a limitation that hierarchical models (such as the bivariate model) used in this meta-analysis are likely to be vulnerable when the number of studies is small and also when sample sizes are highly variable, which is partly the case of the present data [46].
The estimates of logit sensitivity and logit specificity were used to calculate LR+ and LR-. In our study, higher values of the positive likelihood ratio were detected, i.e., for any HPV detection, the LR+ was 15.62 (95% CI 4.60 to 53.05) and the LR- was 0.14 (95% CI 0.08 to 0.24). For high-risk HPV detection, the LR+ was 6.81 (4.07 to 11.41) and the LR- was 0.25 (0.18 to 0.34). For HPV 16 and 18 detection, the LR+ was 39.73 (39.33 to 40.14) and the LR- was 0.24 (0.24 to 0.24).
QUADAS-2 was used as a revised tool for the quality assessment of diagnostic accuracy studies [26]. The patient selection, index test, standard test, and patient flow were the factors involved in quality evaluation. Generally, these studies had a high quality, i.e., an appropriate patient spectrum and a consecutive or random recruitment of participants were used, the majority of recruited participants were included in analyses and all of them used the same reference standard. However, the main weakness in some studies was that they included only patients with CIN2+ [31,32,39], young women (18–25) [16,17] and HIV patients [42]. In addition to resulting in a high prevalence, these factors could also lead to a biased evaluation of test accuracy [47,48].
To determine whether these differences in testing methods influenced results, a meta-regression was used. In the meta-regression analysis, the variation in accuracy was not seen by using covariates (bias in patient selection, purpose, sample timing, storage temperature, and HPV detection method). However, a heterogeneity between the pooled sensitivities and specificities, and higher values of the positive likelihood ratio were detected. These factors could have a significant impact on the probability of infection in HPV-positive women. Therefore, the false positive results could lead to unnecessary invasive examination and costs, which is the advantage of the urine-testing method. However, the high specificity of our test suggests that this scenario is less likely to occur. For these reasons, our results should be interpreted cautiously because there is always the risk of over- or underestimating data. Testing methods need to be more consistent and reproducible if the test is to be successfully implemented in current practice. Therefore, we recommend standardizing urine testing methods, i.e., before incorporating urine testing for HPV into cervical cancer screening guidelines, it is important to minimise variation.
Based on the above-mentioned facts, it is necessary to optimise the HPV DNA detection in first-void urine in order to minimise variation of the first-void urine test (sensitivity and specificity) for the presence cervical HPV in women. Optimised HPV DNA detection in urine should include the following: (1) use of the first-void urine (morning or later during the day) captured with a urine collection device [49]; (2) immediately mix first-void urine with a conservation medium to prevent HPV DNA degradation during extraction and storage; (3) provide sufficient first-void urine volume for subsequent sample concentration; (4) recover cell-associated HPV DNA as well as cell-free DNA [43]; (5) use of HPV tests meeting the criteria for primary cervical cancer screening [50]; (6) not cleaning the genital area before collecting the sample [21]; and (7) collect the first-void urine samples before cervical samples since this may reduce mucus and debris [51].
5. Conclusions
Our meta-analysis demonstrates the accuracy of detection of HPV in urine for the presence of cervical HPV. Although progress is continuously made in urinary HPV detection, further studies are needed to evaluate and to improve the accuracy of the first-void urine test in order to be comparable with other screening methods. Different testing platforms and conditions were used in these studies. Therefore, all results should be interpreted carefully, as they may have been over- or underestimated.
Author Contributions
Conceptualisation, P.B. and J.S.; methodology, P.B.; software, P.B.; validation, J.S.; writing—original draft preparation, P.B.; writing—review and editing, J.S. and P.F.; visualisation, P.B.; supervision, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovak Research and Development Agency, under project: APVV-19-0476.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bosch, F.X.; Lorincz, A.; Munoz, N.; Meijer, C.J.L.M.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2014, 136, E359–E386. [Google Scholar] [CrossRef]
- Vergara, N.; Balanda, M.; Hidalgo, W.; San Martín, H.; Aceituno, A.; Roldán, F.; Villalón, T.; Hott, M.; Espinoza, G.; Quiero, A.; et al. Detection and genotyping of HPV in urine samples from Chilean women attending primary health care centers. Med. Microbiol. Immunol. 2017, 207, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- Clifford, G.M.; Smith, J.S.; Aguado, T.; Franceschi, S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: A meta-analysis. Br. J. Cancer 2003, 89, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Sanjose, S. de Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Greig, J.M.; Ellis, C.J. Occupational Hygiene of Chemical and Biological Agents, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 2008; Volume 100, pp. 344–359. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Gómez, D.; Muñoz, J.; Bosch, F.; de Sanjosé, S. Human Papillomavirus and Related Diseases in the World-Summary Rep; ICO/IARC Inf Cent HPV Cancer (HPV Inf Centre): Geneva, Switzerland, 2019; p. 307. [Google Scholar]
- Peto, J.; Gilham, C.; Fletcher, O.; Matthews, F.E. The cervical cancer epidemic that screening has prevented in the UK. Lancet 2004, 364, 249–256. [Google Scholar] [CrossRef]
- Safaeian, M.; Solomon, D.; Castle, P.E. Cervical Cancer Prevention—Cervical Screening: Science in Evolution. Obstet. Gynecol. Clin. N. Am. 2007, 34, 739–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasieni, P.; Adams, J. Effect of screening on cervical cancer mortality in England and Wales: Analysis of trends with an age period cohort model. BMJ 1999, 318, 1244–1245. [Google Scholar] [CrossRef] [Green Version]
- Louie, K.S.; Silvia de Sanjose, P.M. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: A comprehensive review. Trop. Med. Int. Heal. 2009, 14, 1287–1302. [Google Scholar] [CrossRef]
- Cronjé, H.S. Cervical screening strategies in resourced and resource-constrained countries. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Monsonego, J.; Bosch, F.X.; Coursaget, P.; Cox, J.T.; Franco, E.; Frazer, I.; Sankaranarayanan, R.; Schiller, J.; Singer, A.; Wright, T.; et al. Cervical cancer control, priorities and new directions. Int. J. Cancer 2003, 108, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.W.; Sikon, A.; Yen-Lieberman, B. Cervical cancer screening: Less testing, smarter testing. Cleve. Clin. J. Med. 2011, 78, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Burroni, E.; Bonanni, P.; Sani, C.; Lastrucci, V.; Carozzi, F. Human papillomavirus prevalence in paired urine and cervical samples in women invited for cervical cancer screening. J. Med. Virol. 2014, 87, 508–515. [Google Scholar] [CrossRef]
- Cómbita, A.L.; Gheit, T.; González, P.; Puerto, D.; Murillo, R.H.; Montoya, L.; Vorsters, A.; Van Keer, S.; Van Damme, P.; Tommasino, M.; et al. Comparison between Urine and Cervical Samples for HPV DNA Detection and Typing in Young Women in Colombia. Cancer Prev. Res. 2016, 9, 766–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, S.; Umulisa, M.C.; Tshomo, U.; Gheit, T.; Baussano, I.; Tenet, V.; Tshokey, T.; Gatera, M.; Ngabo, F.; Van Damme, P.; et al. Urine testing to monitor the impact of HPV vaccination in Bhutan and Rwanda. Int. J. Cancer 2016, 139, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorsters, A.; Micalessi, I.; Bilcke, J.; Ieven, M.; Bogers, J.; Damme, P. Detection of human papillomavirus DNA in urine. A review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 31, 627–640. [Google Scholar] [CrossRef]
- Vorsters, A.; Van Keer, S.; Biesmans, S.; Hens, A.; De Coster, I.; Goossens, H.; Ieven, M.; Damme, P. Van Long-Term Follow-up of {HPV} Infection Using Urine and Cervical Quantitative HPV DNA Testing. Int. J. Mol. Sci. 2016, 17, 750. [Google Scholar] [CrossRef] [Green Version]
- Pattyn, J.; Van Keer, S.; Téblick, L.; Van Damme, P.; Vorsters, A. HPV DNA detection in urine samples of women: `An efficacious and accurate alternative to cervical samples? Expert Rev. Anti. Infect. Ther. 2019, 17, 755–757. [Google Scholar] [CrossRef]
- Lefeuvre, C.; Pivert, A.; Guillou-Guillemette, H.L.; Lunel-Fabiani, F.; Veillon, P.; Le Duc-Banaszuk, A.-S.; Ducancelle, A. Urinary HPV DNA testing as a tool for cervical cancer screening in women who are reluctant to have a Pap smear in France. J. Infect. 2020, 81, 248–254. [Google Scholar] [CrossRef]
- Pathak, N.; Dodds, J.; Zamora, J.; Khan, K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: Systematic review and meta-analysis. BMJ 2014, 349, g5264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorsters, A.; Van Damme, P.; Clifford, G. Urine testing for HPV: Rationale for using first void. BMJ 2014, 349, g6252. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Whiting, P.F. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529. [Google Scholar] [CrossRef]
- Šimundič, A.M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. [Google Scholar]
- Chu, H.; Cole, S.R. Bivariate meta-analysis of sensitivity and specificity with sparse data: A generalized linear mixed model approach. J. Clin. Epidemiol. 2006, 59, 1331–1332. [Google Scholar] [CrossRef]
- Harbord, R.M.; Deeks, J.J.; Egger, M.; Whiting, P.; Sterne, J.A.C. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2006, 8, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Lijmer, J.G.; Patrick, M.M.; Bossuyt, H.; Siem, H. Heisterkamp2, Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Statist. Med. 2002, 21, 1525–1537. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-W.; Hong, J.H.; Min, K.J.; Ouh, Y.-T.; Seong, S.J.; Moon, J.H.; Cho, S.H.; Lee, J.K. Performance and Diagnostic Accuracy of Human Papillomavirus Testing on Self-Collected Urine and Vaginal Samples in a Referral Population. Cancer Res. Treat. 2021, 53, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Tranberg, M.; Jensen, J.S.; Bech, B.H.; Andersen, B. Urine collection in cervical cancer screening–analytical comparison of two HPV DNA assays. BMC Infect. Dis. 2020, 20, 926. [Google Scholar] [CrossRef]
- Van Keer, S.; Tjalma, W.A.A.; Pattyn, J.; Biesmans, S.; Pieters, Z.; Van Ostade, X.; Ieven, M.; Van Damme, P.; Vorsters, A. Human papillomavirus genotype and viral load agreement between paired first-void urine and clinician-collected cervical samples. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 859–869. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, B.Y.; Tareg, A.C.; Reichhardt, M.; Agapito, A.; Zhu, X.; Sy, A.; Yuji, A.; Killeen, J.; Chan, O.; Buenconsejo-Lum, L.E. Randomized controlled trial evaluating the utility of urine HPV DNA for cervical cancer screening in a Pacific Island population. J. Glob. Heal. Rep. 2018, 2. [Google Scholar] [CrossRef]
- Leeman, A.; del Pino, M.; Molijn, A.; Rodriguez, A.; Torné, A.; de Koning, M.; Ordi, J.; van Kemenade, F.; Jenkins, D.; Quint, W. HPV testing in first-void urine provides sensitivity for CIN2+ detection comparable with a smear taken by a clinician or a brush-based self-sample: Cross-sectional data from a triage population. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1356–1363. [Google Scholar] [CrossRef]
- Cuzick, J.; Cadman, L.; Ahmad, A.S.; Ho, L.; Terry, G.; Kleeman, M.; Lyons, D.; Austin, J.; Stoler, M.H.; Vibat, C.R.T.; et al. Performance and Diagnostic Accuracy of a Urine-Based Human Papillomavirus Assay in a Referral Population. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1053–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilyanimit, P.; Chansaenroj, J.; Karalak, A.; Laowahutanont, P.; Junyangdikul, P.; Poovorawan, Y. Comparison of human papillomavirus (HPV) detection in urine and cervical swab samples using the HPV GenoArray Diagnostic assay. PeerJ 2017, 5, e3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahasrabuddhe, V.V.; Gravitt, P.E.; Dunn, S.T.; Brown, D.; Allen, R.A.; Eby, Y.J.; Smith, K.; Zuna, R.E.; Zhang, R.R.; Gold, M.A.; et al. Comparison of Human Papillomavirus Detections in Urine, Vulvar, and Cervical Samples from Women Attending a Colposcopy Clinic. J. Clin. Microbiol. 2013, 52, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Mendez, K.; Romaguera, J.; Ortiz, A.P.; López, M.; Steinau, M.; Unger, E.R. Urine-based human papillomavirus DNA testing as a screening tool for cervical cancer in high-risk women. Int. J. Gynecol. Obstet. 2013, 124, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Ducancelle, A.; Legrand, M.C.; Pivert, A.; Veillon, P.; Le Guillou-Guillemette, H.; De Brux, M.A.; Beby-Defaux, A.; Agius, G.; Hantz, S.; Alain, S.; et al. Interest of Human Papillomavirus DNA quantification and genotyping in paired cervical and urine samples to detect cervical lesions. Arch. Gynecol. Obstet. 2014, 290, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Bernal, S.; Palomares, J.C.; Artura, A.; Parra, M.; Cabezas, J.L.; Robles, A.; Mazuelos, E.M. Comparison of urine and cervical samples for detecting human papillomavirus (HPV) with the Cobas 4800 HPV test. J. Clin. Virol. 2014, 61, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, E.; Bianchi, S.; Fasolo, M.M.; Frati, E.R.; Mazza, F.; Martinelli, M.; Colzani, D.; Beretta, R.; Zappa, A.; Orlando, G. High performance of a new PCR-based urine assay for HPV-DNA detection and genotyping. J. Med. Virol. 2012, 85, 91–98. [Google Scholar] [CrossRef]
- Vorsters, A.; Van den Bergh, J.; Micalessi, I.; Biesmans, S.; Bogers, J.; Hens, A.; De Coster, I.; Ieven, M.; Van Damme, P. Optimization of HPV DNA detection in urine by improving collection, storage, and extraction. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2005–2014. [Google Scholar] [CrossRef]
- Tshomo, U.; Franceschi, S.; Tshokey, T.; Tobgay, T.; Baussano, I.; Tenet, V.; Snijders, P.J.F.; Gheit, T.; Tommasino, M.; Vorsters, A.; et al. Evaluation of the performance of Human Papillomavirus testing in paired urine and clinician-collected cervical samples among women aged over 30~years in Bhutan. Virol. J. 2017, 14, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, G.M.; Vaccarella, S.; Franceschi, S.; Tenet, V.; Umulisa, M.C.; Tshomo, U.; Dondog, B.; Vorsters, A.; Tommasino, M.; Heideman, D.A.M.; et al. Comparison of Two Widely Used Human Papillomavirus Detection and Genotyping Methods, GP5+/6+-Based PCR Followed by Reverse Line Blot Hybridization and Multiplex Type-Specific E7-Based PCR. J. Clin. Microbiol. 2016, 54, 2031–2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, C.B. Meta-analysis methods for diagnostic accuracy. J. Clin. Epidemiol. 2008, 61, 1081–1082. [Google Scholar] [CrossRef] [PubMed]
- Lijmer, J.G. Empirical Evidence of Design-Related Bias in Studies of Diagnostic Tests. JAMA 1999, 282, 1061. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, M.M.G.; Bossuyt, P.M.M.; Irwig, L. Diagnostic test accuracy may vary with prevalence: Implications for evidence-based diagnosis. J. Clin. Epidemiol. 2009, 62, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Van Keer, S.; Biesmans, S.; Ieven, M.; Vanderborght, C.; Beyers, K.; Vankerckhoven, V.; Bruyndonckx, R.; Van Damme, P.; Vorsters, A. Human papillomavirus detection in urine: Effect of a first-void urine collection device and timing of collection. J. Virol. Methods 2019, 264, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Snijders, P.J.; Meijer, C.J.; Berkhof, J.; Cuschieri, K.; Kocjan, B.J.; Poljak, M. Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening? Clin. Microbiol. Infect. 2015, 21, 817–826. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, A.; Gupta, S.; Parashari, A.; Sodhani, P.; Singh, V. Urine HPV-DNA detection for cervical cancer screening: Prospects and prejudices. J. Obstet. Gynaecol. 2009, 29, 583–589. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).