Religious Service Attendance and Mortality among Adults in the United States with Chronic Kidney Disease
Abstract
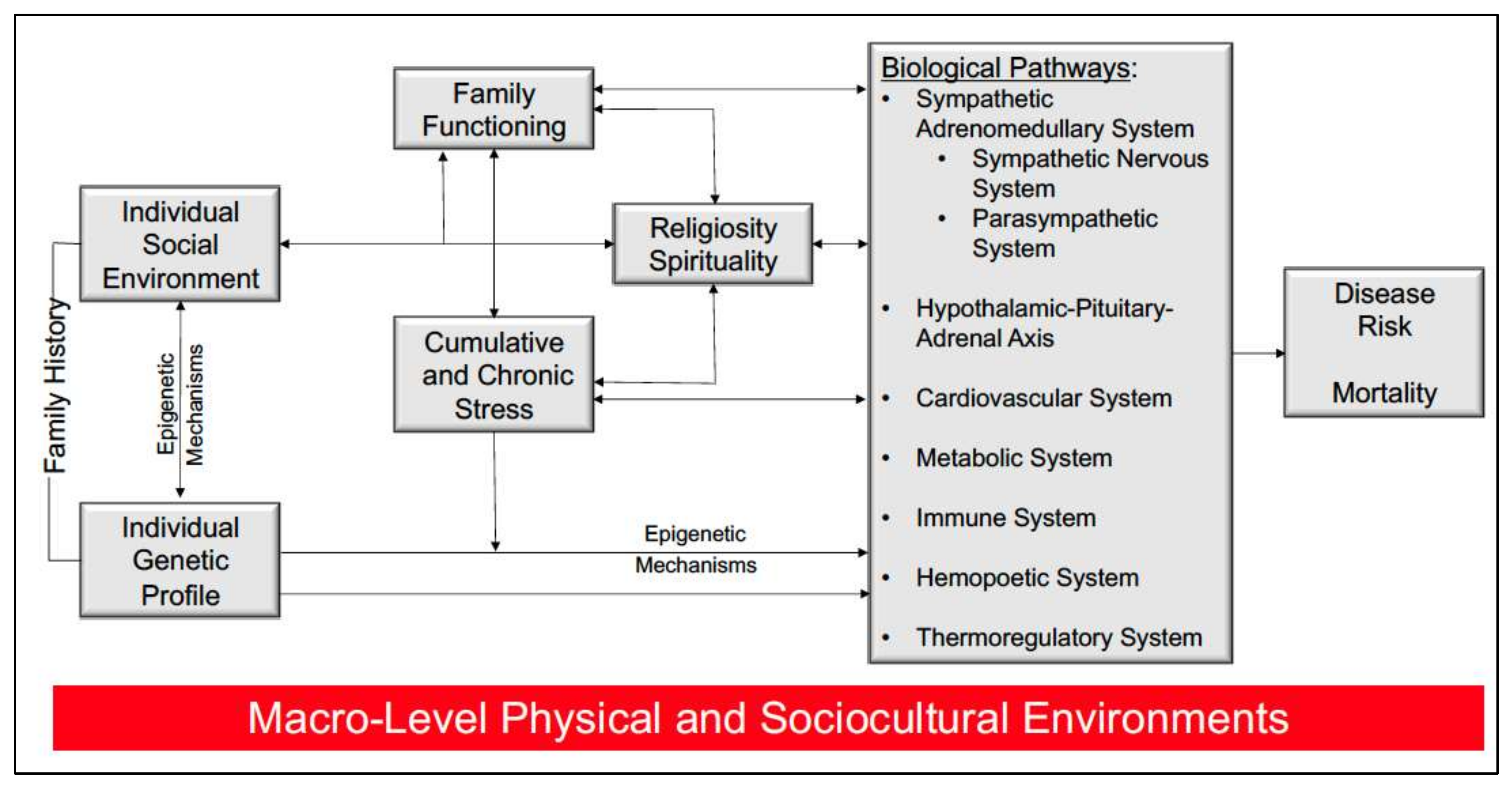
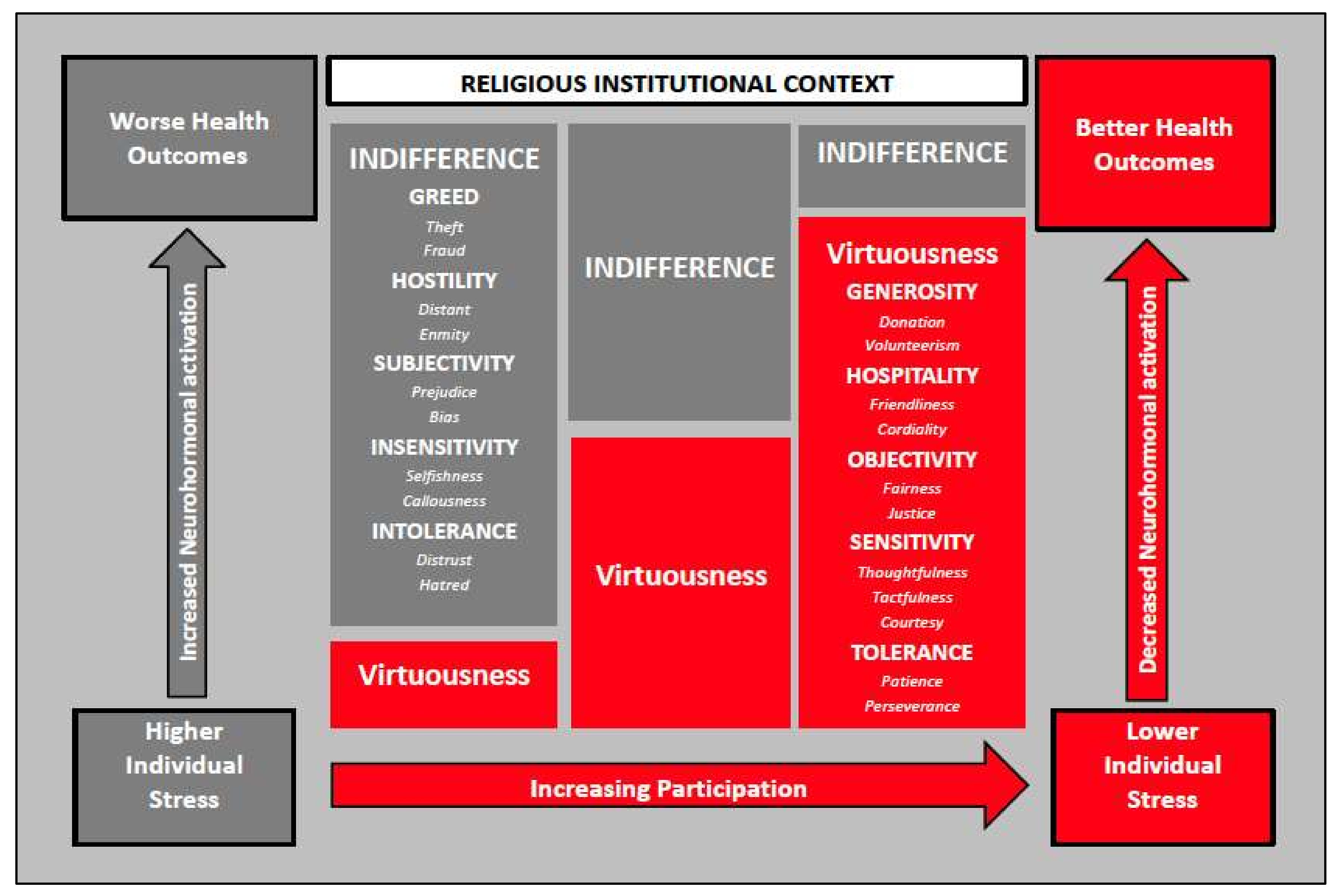
1. Introduction
2. Material and Methods
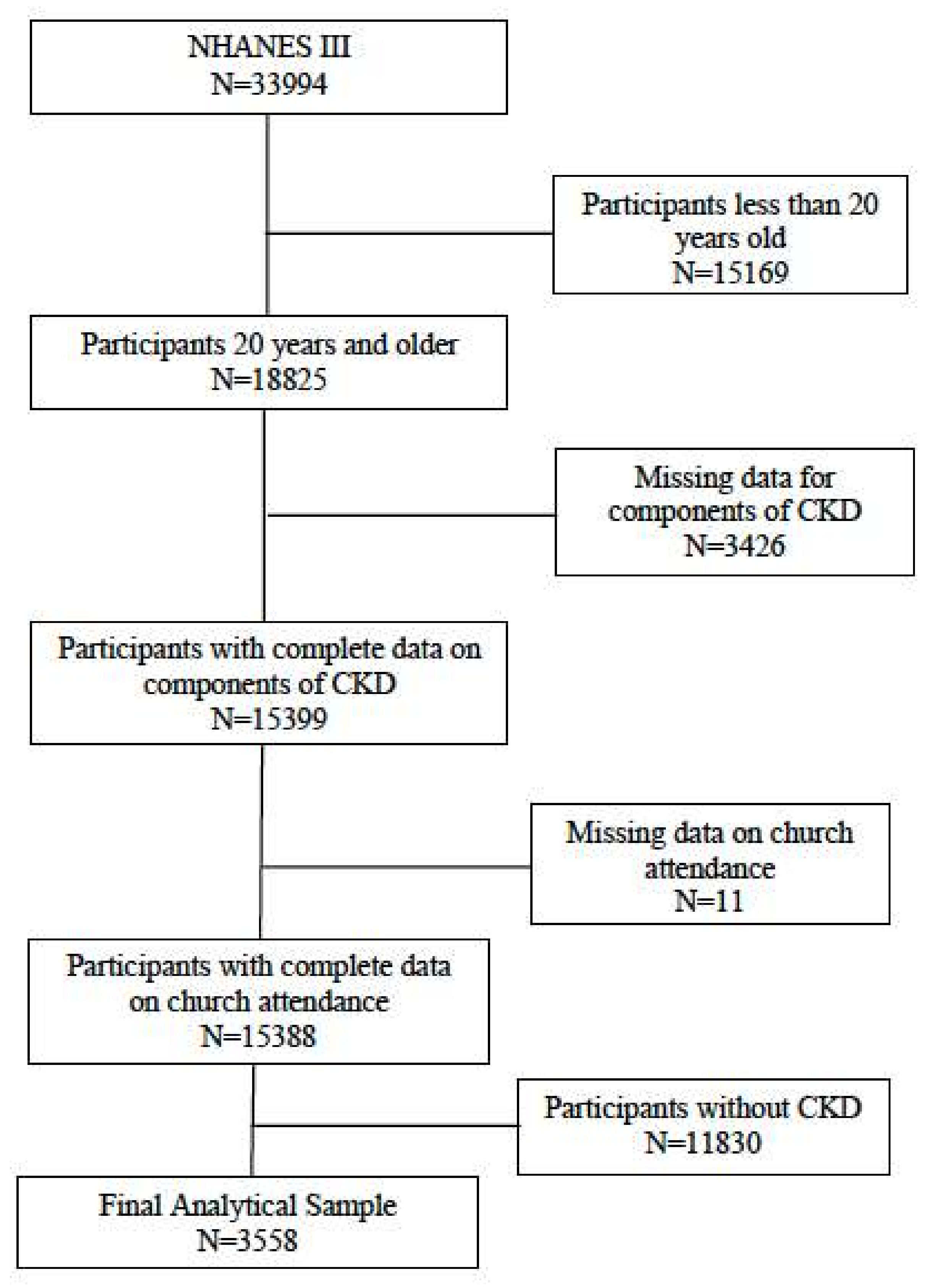
2.1. Data
2.2. Study Variables
2.3. Statistical Analyses
3. Results
3.1. Allostatic Load
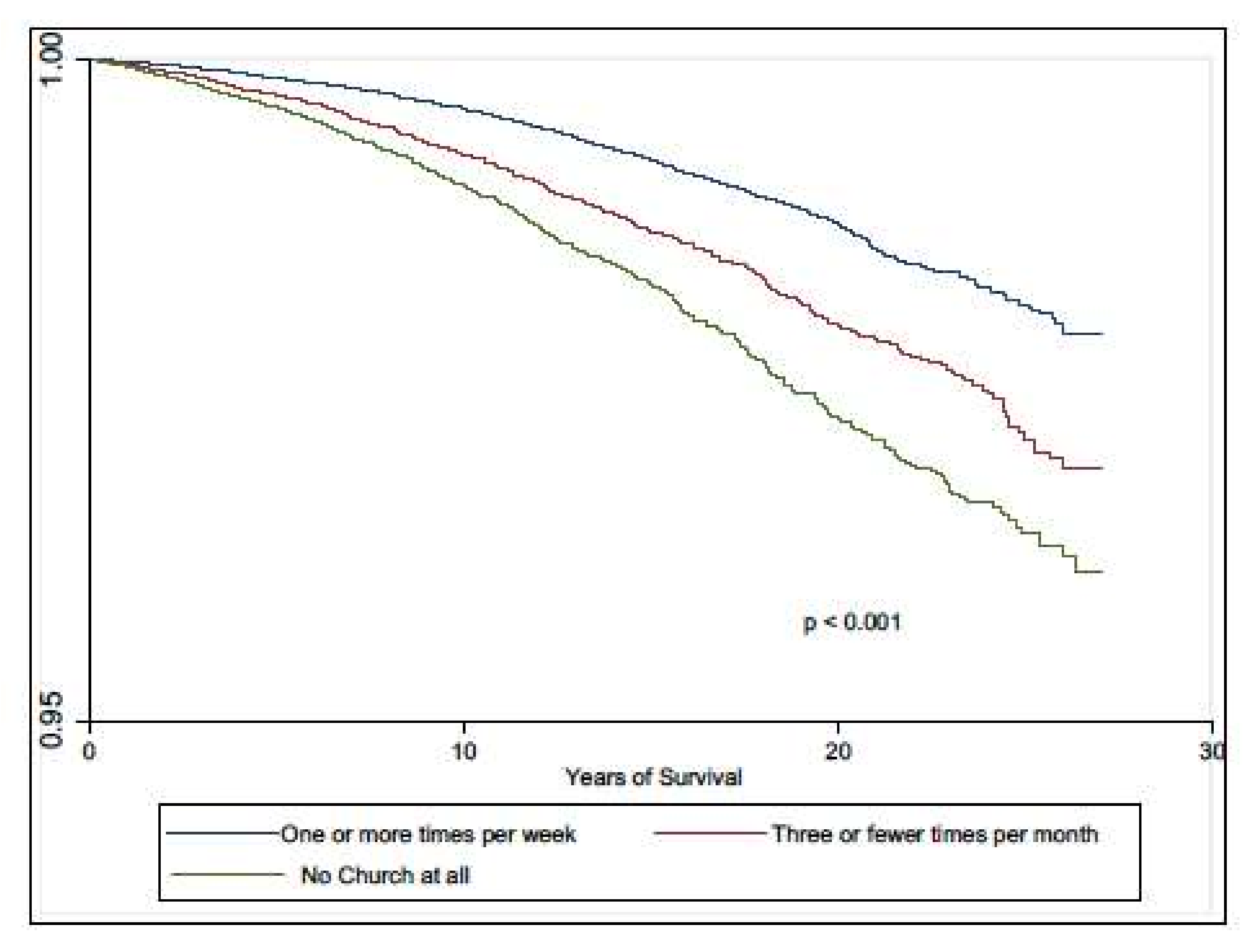
3.2. All-Cause Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crews, D.C.; Bello, A.K.; Saadi, G. 2019 World Kidney Day Editorial—Burden, access, and disparities in kidney disease. J. Bras. Nefrol. 2019, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Al Kibria, G.M.; Crispen, R. Prevalence and trends of chronic kidney disease and its risk factors among US adults: An analysis of NHANES 2003-18. Prev. Med. Rep. 2020, 20, 101193. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.; McCulloch, C.E.; Lin, F.; Banerjee, T.; Bragg-Gresham, J.L.; Eberhardt, M.S.; Morgenstern, H.; Pavkov, M.E.; Saran, R.; Powe, N.R. Trends in prevalence of chronic kidney disease in the United States. Ann. Intern. Med. 2016, 165, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Vart, P.; Powe, N.R.; McCulloch, C.E.; Saran, R.; Gillespie, B.W.; Saydah, S.; Crews, D.C. National trends in the prevalence of chronic kidney disease among racial/ethnic and socioeconomic status groups, 1988–2016. JAMA Netw. Open 2020, 3, e207932. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.A.; Beech, B.M.; Sims, M.; Brown, T.N.; Wyatt, S.B.; Taylor, H.A.; Williams, D.R.; Crook, E. Social environmental stressors, psychological factors, and kidney disease. J. Investig. Med. 2009, 57, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Turin, T.C.; Tonelli, M.; Manns, B.J.; Ravani, P.; Ahmed, S.B.; Hemmelgarn, B.R. Chronic kidney disease and life expectancy. Nephrol. Dial. Transpl. 2012, 27, 3182–3186. [Google Scholar] [CrossRef] [PubMed]
- Tuot, D.S.; Lin, F.; Norris, K.; Gassman, J.; Smogorzewski, M.; Ku, E. Depressive symptoms associates with race and all-cause mortality in patients with CKD. Kidney Int. Rep. 2019, 4, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.; Fischer, M.J.; Brooks, D.; Bruce, M.; Charleston, J.; Cleveland, W.H.; Dowie, D.; Faulkner, M.; Gassman, J.; Greene, T.; et al. Quality of life and psychosocial factors in African Americans with hypertensive chronic kidney disease. Transl. Res. J. Lab. Clin. Med. 2012, 159, 4–11. [Google Scholar] [CrossRef]
- Fischer, M.J.; Xie, D.; Jordan, N.; Kop, W.J.; Krousel-Wood, M.; Kurella Tamura, M.; Kusek, J.W.; Ford, V.; Rosen, L.K.; Strauss, L.; et al. Factors associated with depressive symptoms and use of antidepressant medications among participants in the Chronic Renal Insufficiency Cohort (CRIC) and Hispanic-CRIC Studies. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2012, 60, 27–38. [Google Scholar] [CrossRef]
- Eslami, A.A.; Rabiei, L.; Khayri, F.; Nooshabadi, M.R.R.; Masoudi, R. Sleep quality and spiritual well-being in hemodialysis patients. Iran. Red Crescent Med. J. 2014, 16, e17155. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, P.L.; Emont, S.L.; Newmann, J.M.; Danko, H.; Moss, A.H. ESRD patient quality of life: Symptoms, spiritual beliefs, psychosocial factors, and ethnicity. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2003, 42, 713–721. [Google Scholar] [CrossRef]
- Lucchetti, G.; de Almeida, L.G.C.; Lucchetti, A.L.G. Religiousness, mental health, and quality of life in B razilian dialysis patients. Hemodial. Int. 2012, 16, 89–94. [Google Scholar] [PubMed]
- Saisunantararom, W.; Cheawchanwattana, A.; Kanjanabuch, T.; Buranapatana, M.; Chanthapasa, K. Associations among spirituality, health-related quality of life, and depression in pre-dialysis chronic kidney disease patients: An exploratory analysis in thai buddhist patients. Religions 2015, 6, 1249–1262. [Google Scholar] [CrossRef]
- Siqueira, J.; Fernandes, N.M.; Moreira-Almeida, A. Association between religiosity and happiness in patients with chronic kidney disease on hemodialysis. J. Bras. Nefrol. 2019, 41, 22–28. [Google Scholar] [CrossRef]
- Spinale, J.; Cohen, S.D.; Khetpal, P.; Peterson, R.A.; Clougherty, B.; Puchalski, C.M.; Patel, S.S.; Kimmel, P.L. Spirituality, social support, and survival in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Fetzer Institute; National Institute on Aging Working Group. Multidimentional Measurement of Religiousness Spirituality for Use in Health Research; Fetzer Institute: Kalamazoo, MI, USA, 2003. [Google Scholar]
- Underwood, L.G.; Teresi, J.A. The daily spiritual experience scale: Development, theoretical description, reliability, exploratory factor analysis, and preliminary construct validity using health-related data. Ann. Behav. Med. 2002, 24, 22–33. [Google Scholar] [CrossRef]
- Krause, N. Religion, aging, and health: Current status and future prospects. J. Gerontol. B Psychol. Sci. Soc. Sci. 1997, 52, S291–S293. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Chatters, L.M.; Taylor, R.J. Theory in religion, aging, and health: An overview. J. Relig. Health 2011, 50, 389–406. [Google Scholar] [CrossRef][Green Version]
- Taylor, R.J.; Chatters, L.M. Church members as a source of informal social support. Rev. Relig. Res. 1988, 30, 193–203. [Google Scholar] [CrossRef]
- Bruce, M.A.; Martins, D.; Duru, K.; Beech, B.M.; Sims, M.; Harawa, N.; Vargas, R.; Kermah, D.; Nicholas, S.B.; Brown, A.; et al. Church attendance, allostatic load and mortality in middle aged adults. PLoS ONE 2017, 12, e0177618. [Google Scholar] [CrossRef]
- Gillum, R.F.; King, D.E.; Obisesan, T.O.; Koenig, H.G. Frequency of attendance at religious services and mortality in a US national cohort. Ann. Epidemiol. 2008, 18, 124–129. [Google Scholar] [CrossRef][Green Version]
- Koenig, H.G.; Hays, J.C.; Larson, D.B.; George, L.K.; Cohen, H.J.; McCullough, M.E.; Meador, K.G.; Blazer, D.G. Does religious attendance prolong survival? A six-year follow-up study of 3968 older adults. J. Gerontology. Ser. A Biol. Sci. Med Sci. 1999, 54, M370–M376. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.A.; Thorpe, R.J., Jr. Stress, faith, and health among African American middle-age and older men. Annu. Rev. Gerontol. Geriatr. 2019, 39, 123–132. [Google Scholar]
- Seeman, T.E.; Crimmins, E. Social environment effects on health and aging. Ann. New York Acad. Sci. 2001, 954, 88–117. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.A.; Griffith, D.M.; Thorpe, R.J., Jr. Stress and the kidney. Adv Chronic Kidney Dis 2015, 22, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Saban, K.L.; Mathews, H.L.; DeVon, H.A.; Janusek, L.W. Epigenetics and social context: Implications for disparity in cardiovascular disease. Aging Dis. 2014, 5, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Koenig, H.; King, D.; Carson, V.B. Handbook of Religion and Health; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Krause, N.; Hayward, R.D. Church-based social support, functional disability, and change in personal control over time. J. Relig. Health 2014, 53, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Cavanaugh, K.L.; Wallston, K.A.; Mason, O.; Stewart, T.G.; Blot, W.J.; Ikizler, T.A.; Lipworth, L.P. Religion, spirituality, and risk of end-stage kidney disease among adults of low socioeconomic status in the Southeastern United States. J. Health Care Poor Underserved 2020, 31, 1727–1746. [Google Scholar] [CrossRef]
- Pargament, K.I.; Smith, B.W.; Koenig, H.G.; Perez, L. Patterns of positive and negative religious coping with major life stressors. J. Sci. Study Relig. 1998, 37, 710–724. [Google Scholar] [CrossRef]
- Bowie, J.V.; Bell, C.N.; Ewing, A.; Kinlock, B.; Ezema, A.; Thorpe, R.J., Jr.; LaVeist, T.A. Religious coping and types and sources of information used in making prostate cancer treatment decisions. Am. J. Mens Health 2017, 11, 1237–1246. [Google Scholar] [CrossRef]
- Tsevat, J. Spirituality/religion and quality of life in patients with HIV/AIDS. J. Gen. Intern. Med. 2006, 21, S1–S2. [Google Scholar] [CrossRef][Green Version]
- Bruce, M.A.; Skrine Jeffers, K.; King Robinson, J.; Norris, K.C. Contemplative practices: A strategy to improve health and reduce disparities. Int. J. Environ. Res. Public Health 2018, 15, 2253. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Block, G.; Humphreys, M.H.; Kopple, J.D. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003, 63, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Rhee, C.M.; Chou, J.; Ahmadi, S.F.; Park, J.; Chen, J.L.; Amin, A.N. The obesity paradox in kidney disease: How to reconcile it with obesity management. Kidney Int. Rep. 2017, 2, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Anderson, J.E. Reverse epidemiology in patients with chronic kidney disease who are not yet on dialysis. Semin. Dial. 2007, 20, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Plan and operation of the third national health and nutrition examination survey, 1988–1994: Series 1: Programs and collection procedures. Vital Health Stat. 1994, 32, 1–407. [Google Scholar]
- National Center for Health Statistics; Centers for Disease Control and Prevention. Plan, and Operation of the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994): Reference Manuals and Reports: Weighting and Estimation Methodology Report; U.S. Department of Health & Human Services, Public Health Service, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 1998. [Google Scholar]
- Thorpe, R.J., Jr.; Wilson-Frederick, S.M.; Bowie, J.V.; Coa, K.; Clay, O.J.; LaVeist, T.A.; Whitfield, K.E. Health behaviors and all-cause mortality in African American men. Am. J. Men’s Health 2013, 7, 8S–18S. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2010, 55, 622–627. [Google Scholar] [CrossRef]
- Mattix, H.J.; Hsu, C.Y.; Shaykevich, S.; Curhan, G. Use of the albumin/creatinine ratio to detect microalbuminuria: Implications of sex and race. J. Am. Soc. Nephrol. 2002, 13, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics; Office of Analysis and Epidemiology. The Linkage of National Center for Health Statistics Survey Data to the National Death Index—2015 Linked Mortality File (LMF): Methodology Overview and Analytic Considerations, March 2019. Available online: https://www.cdc.gov/nchs/data-linkage/mortality-methods.htm (accessed on 15 October 2021).
- Duru, O.K.; Harawa, N.T.; Kermah, D.; Norris, K.C. Allostatic load burden and racial disparities in mortality. J. Natl. Med Assoc. 2012, 104, 89–95. [Google Scholar] [CrossRef]
- Geronimus, A.T.; Hicken, M.; Keene, D.; Bound, J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am. J. Public Health 2006, 96, 826–833. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services; National Center for Health Statistics. Analytic and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988–1994); Centers for Disease Control and Prevention: Hyattsville, MD, USA, 1996. [Google Scholar]
- Breland-Noble, A.M.; Wong, M.J.; Childers, T.; Hankerson, S.; Sotomayor, J. Spirituality and religious coping in African American youth with depressive illness. Ment. Health Relig. Cult. 2015, 18, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Dill, L.J. “Wearing My Spiritual Jacket”: The role of spirituality as a coping mechanism among African American youth. Health Educ. Behav. 2017, 44, 696–704. [Google Scholar] [CrossRef]
- Godbolt, D.; Vaghela, P.; Burdette, A.M.; Hill, T.D. Religious attendance and body mass: An examination of variations by race and gender. J. Relig. Health 2017, 57, 2140–2152. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.N.; Jhangri, G.S. Existential and supportive care needs among patients with chronic kidney disease. J. Pain Symptom. Manage 2010, 40, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Kop, W.J.; Seliger, S.L.; Fink, J.C.; Katz, R.; Odden, M.C.; Fried, L.F.; Rifkin, D.E.; Sarnak, M.J.; Gottdiener, J.S. Longitudinal association of depressive symptoms with rapid kidney function decline and adverse clinical renal disease outcomes. Clin. J. Am. Soc. Nephrol. 2011, 6, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, S.S.; Minhajuddin, A.T.; Afshar, M.; Toto, R.D.; Trivedi, M.H.; Rush, A.J. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. Jama 2010, 303, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.; Kimmel, P.L.; Greene, T.; Gassman, J.J.; Wang, X.; Brooks, D.H.; Charleston, J.; Dowie, D.; Thornley-Brown, D.; Cooper, L.A.; et al. Elevated depressive affect is associated with adverse cardiovascular outcomes among African Americans with chronic kidney disease. Kidney Int. 2011, 80, 670–678. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hill, T.D.; Ellison, C.G.; Burdette, A.M.; Taylor, J.; Friedman, K.L. Dimensions of religious involvement and leukocyte telomere length. Soc. Sci. Med. 2016, 163, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.D.; Vaghela, P.; Ellison, C.G.; Rote, S. Processes linking religious involvement and telomere length. Biodemography Soc. Biol. 2017, 63, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Hummer, R.A.; Rogers, R.G.; Nam, C.B.; Ellison, C.G. Religious involvement and US adult mortality. Demography 1999, 36, 273–285. [Google Scholar] [CrossRef]
- Li, S.; Stampfer, M.J.; Williams, D.R.; VanderWeele, T.J. Association of religious service attendance with mortality among women. JAMA Intern. Med. 2016, 176, 777–785. [Google Scholar] [CrossRef]
- Koenig, H.G. Religion, spirituality, and health: The research and clinical implications. ISRN Psychiatry 2012, 2012, 278730. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.E.; Sabate, J.; Beeson, W.L.; Strahan, T.M. A possible protective effect of nut consumption on risk of coronary heart disease. The adventist health study. Arch. Intern. Med. 1992, 152, 1416–1424. [Google Scholar] [CrossRef]
- Russell, R.D. A joust with Obie: Some comments on convictions held by Delbert Oberteuffer about health and health education. Health Educ. 1984, 15, 3–7. [Google Scholar] [CrossRef]
- Bellingham, R.; Cohen, B.; Jones, T.; Spaniol, L.R. Connectedness: Some skills for spiritual health. Am. J. Health Promot. AJHP 1989, 4, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Banks, R. Health and the spiritual dimension: Relationships and implications for professional preparation programs. J. Sch. Health 1980, 50, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Brown, I. Exploring the spiritual dimension of school health education. Eta Sigma Gamman 1978, 10, 12–16. [Google Scholar]
- Chapman, L.S. Developing a useful perspective on spiritual health: Love, joy, peace and fulfillment. Am. J. Health Promot. AJHP 1987, 2, 12–17. [Google Scholar] [CrossRef]
- Sprecher, S.; Fehr, B. Enhancement of mood and self-esteem as a result of giving and receiving compassionate love. Curr. Res. Soc. Psychol. 2006, 11, 227–242. [Google Scholar]
- Davison, S.N.; Jhangri, G.S. Existential and religious dimensions of spirituality and their relationship with health-related quality of life in chronic kidney disease. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 3558) | One or More Times per Week (n = 1548) | Less Than Once per Week (n = 809) | No Religious Service Attendance (n = 1201) | p-Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (Mean (SE)) | 58 (0.7) | 61 (0.7) | 51 (1.2) | 59 (1.1) | <0.001 |
| Race/Ethnicity (%) | |||||
| White Black Hispanic Other | 77.2 12.5 3.9 6.4 | 80.0 11.2 3.6 5.2 | 72.2 17.2 4.1 6.5 | 84.4 8.2 2.0 5.4 | <0.001 |
| Sex (%) | <0.001 | ||||
| Male Female | 46.0 54.0 | 41.0 59.0 | 43.9 56.1 | 53.4 46.6 | |
| Never Married | 9.3 | 7.7 | 10.1 | 10.7 | 0.562 |
| Socio-Economic Factors | |||||
| Education (%) | 0.004 | ||||
| <9 years 9–12 years >12 years | 21.6 47.4 31.0 | 20.7 45.5 33.8 | 19.1 44.0 36.9 | 24.4 51.8 23.7 | |
| Poor (poverty-income ratio < 2) (%) | 41.0 | 39.2 | 40.4 | 43.6 | 0.292 |
| No health insurance (%) | 8.0 | 6.1 | 10.2 | 8.7 | 0.037 |
| Major Cardio-Renal Comorbid Conditions | |||||
| Hypertension (%) | 53.1 | 56.9 | 44.0 | 54.7 | 0.004 |
| Diabetes (%) | 21.2 | 19.8 | 22.0 | 22.4 | 0.515 |
| Congestive Heart Failure (%) | 6.8 | 6.4 | 6.7 | 7.2 | 0.754 |
| eGFR (CKD-EPI) ml/min/1.73 m2 (Mean (SE)) | 81.5 (1.5) | 76.6 (1.6) | 90.8 (3.2) | 81.3 (2.4) | <0.001 |
| UACR (mg/g) (Mean (SE)) | 126.3 (8.3) | 99.6 (9.8) | 138.3 (16.8) | 150.7 (15.5) | 0.005 |
| Mean (SE) allostatic load score (range 0–9) a | 2.9 (0.1) | 2.8 (0.1) | 2.7 (0.1) | 3.0 (0.1) | 0.07 |
| Comorbid conditions (non-ckd related) | |||||
| Lung disease (%) | 12.5 | 13.2 | 8.9 | 14.1 | 0.024 |
| Cancer (%) | 7.2 | 7.0 | 7.4 | 7.4 | 0.943 |
| Thyroid disease (%) | 8.4 | 10.8 | 5.8 | 7.3 | 0.022 |
| Rheumatoid arthritis (%) | 7.0 | 6.0 | 6.8 | 8.2 | 0.131 |
| Asthma (%) | 8.3 | 7.7 | 9.6 | 8.0 | 0.433 |
| Health Behaviors | |||||
| Tobacco Use | <0.001 | ||||
| Never smokers (%) Former smokers (%) Current smokers (%) | 42.7 34.7 22.6 | 49.0 36.8 14.2 | 43.8 30.2 26.0 | 34.7 35.1 30.1 | |
| Physically active (%) | 70.8 | 72.5 | 75.0 | 66.0 | 0.002 |
| Alcohol Use | 0.056 | ||||
| Non-drinkers (%) ≤1 alcoholic drink/day (%) >1 alcoholic drink/day (%) | 56.1 36.9 7.0 | 62.1 31.6 6.3 | 50.5 43.6 5.9 | 52.9 38.6 8.5 | |
| Social Support Measures (Mean (Se)) | |||||
| Number of phone calls with family/friends/neighbors per week. | 7.9 (1.0) | 7.7 (1.1) | 8.6 (1.1) | 7.5 (1.1) | 0.37 |
| Number of home visits with friends or relatives per year. | 72 (1.0) | 75 (1.1) | 81 (1.1) | 65 (1.1) | 0.058 |
| Number of home visits with neighbors per year. | 66 (1.1) | 65 (1.1) | 55 (1.1) | 77 (1.1) | 0.039 |
| Total (n = 3558) | One or More Times per Week (n = 1548) | Less Than Once per Week (n = 809) | No Religious Service Attendance (n = 1201) | p-Value | |
|---|---|---|---|---|---|
| Allostatic Load Components (% of each subgroup with “high risk” values) a | |||||
| Systolic blood pressure (mmHg) | 1563 (37.2) | 715 (39.6) | 310 (28.0) | 538 (40.5) | 0.002 |
| Diastolic blood pressure (mmHg) | 381 (10.3) | 155 (9.4) | 109 (10.0) | 117 (11.5) | 0.58 |
| Waist/hip ratio | 2667 (79.9) | 1206 (80.6) | 608 (75.0) | 853 (82.4) | 0.08 |
| Total cholesterol/HDL ratio | 1307 (39.6) | 598 (38.5) | 267 (36.9) | 442 (42.7) | 0.04 |
| Glycated hemoglobin (%) | 1589 (37.0) | 722 (35.9) | 346 (36.5) | 521 (38.7) | 0.64 |
| Heart Rate (beats/min) | 150 (6.0) | 59 (4.1) | 29 (6.7) | 62 (8.1) | 0.01 |
| Albumin (g/dL) | 689 (16.2) | 280 (15.2) | 163 (15.6) | 246 (17.8) | 0.35 |
| C-reactive protein (mg/L) | 1633 (42.8) | 681 (40.5) | 364 (44.2) | 588 (44.6) | 0.32 |
| Body Mass Index (kg/m2) | 987 (28.9) | 412 (26.6) | 248 (30.1) | 327 (30.9) | 0.33 |
| Mean (SE) allostatic load score (range 0–9) b | 2.9 (0.1) | 2.8 (0.1) | 2.7 (0.1) | 3.0 (0.1) | 0.07 |
| Unadjusted | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| No religious service attendance (n = 1201) | Reference | Reference | Reference | Reference |
| Less than once per week (n = 809) | 0.59 (0.49–0.70) | 0.84 (0.72–0.99) | 0.90 (0.76–1.06) | 0.90 (0.73–1.10) |
| One or more times per week (n = 1548) | 0.88 (0.78–0.99) | 0.72 (0.64–0.81) | 0.82 (0.72–0.92) | 0.79 (0.64–0.98) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruce, M.A.; Thorpe, R.J., Jr.; Kermah, D.; Shen, J.; Nicholas, S.B.; Beech, B.M.; Tuot, D.S.; Ku, E.; Waterman, A.D.; Duru, K.; et al. Religious Service Attendance and Mortality among Adults in the United States with Chronic Kidney Disease. Int. J. Environ. Res. Public Health 2021, 18, 13179. https://doi.org/10.3390/ijerph182413179
Bruce MA, Thorpe RJ Jr., Kermah D, Shen J, Nicholas SB, Beech BM, Tuot DS, Ku E, Waterman AD, Duru K, et al. Religious Service Attendance and Mortality among Adults in the United States with Chronic Kidney Disease. International Journal of Environmental Research and Public Health. 2021; 18(24):13179. https://doi.org/10.3390/ijerph182413179
Chicago/Turabian StyleBruce, Marino A., Roland J. Thorpe, Jr., Dulcie Kermah, Jenny Shen, Susanne B. Nicholas, Bettina M. Beech, Delphine S. Tuot, Elaine Ku, Amy D. Waterman, Kenrik Duru, and et al. 2021. "Religious Service Attendance and Mortality among Adults in the United States with Chronic Kidney Disease" International Journal of Environmental Research and Public Health 18, no. 24: 13179. https://doi.org/10.3390/ijerph182413179
APA StyleBruce, M. A., Thorpe, R. J., Jr., Kermah, D., Shen, J., Nicholas, S. B., Beech, B. M., Tuot, D. S., Ku, E., Waterman, A. D., Duru, K., Brown, A., & Norris, K. C. (2021). Religious Service Attendance and Mortality among Adults in the United States with Chronic Kidney Disease. International Journal of Environmental Research and Public Health, 18(24), 13179. https://doi.org/10.3390/ijerph182413179








