Systematic Review and Meta-Analysis of the Relationship between Actual Exercise Intensity and Rating of Perceived Exertion in the Overweight and Obese Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Eligibility Criteria
- The study sample included the overweight and/or obese population without other diseases and had no age or sex limitations.
- The study included an assessment of AEI. There were no restrictions on exercise protocol, type or interval time, or intensity.
- The study included an assessment of RPE. There were no restrictions on the scale (e.g., Borg scale (0–10), OMNI scale, and Borg scale (6–20)).
- The study analysis reported on the assessment of the relationship between AEI and RPE.
- The study had to include randomized controlled trials of NW and OW or OB individuals.
- To ensure the quality of the included articles, the impact factor of the articles had to be no less than 2.
2.4. Data Extraction
2.5. Methodological Quality and Risk of Bias Assessment
2.6. Data Analyses
3. Results
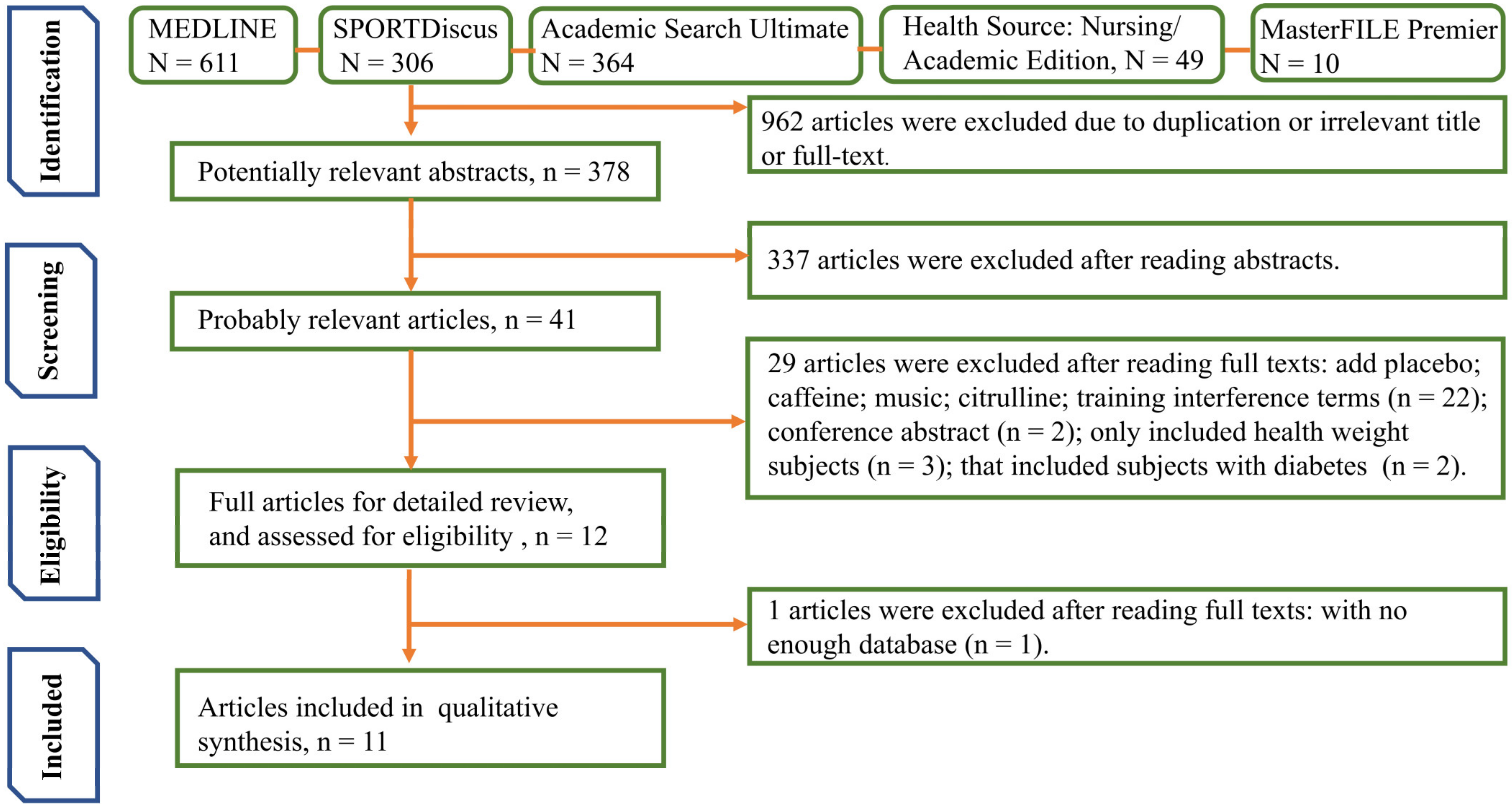
3.1. Included Studies
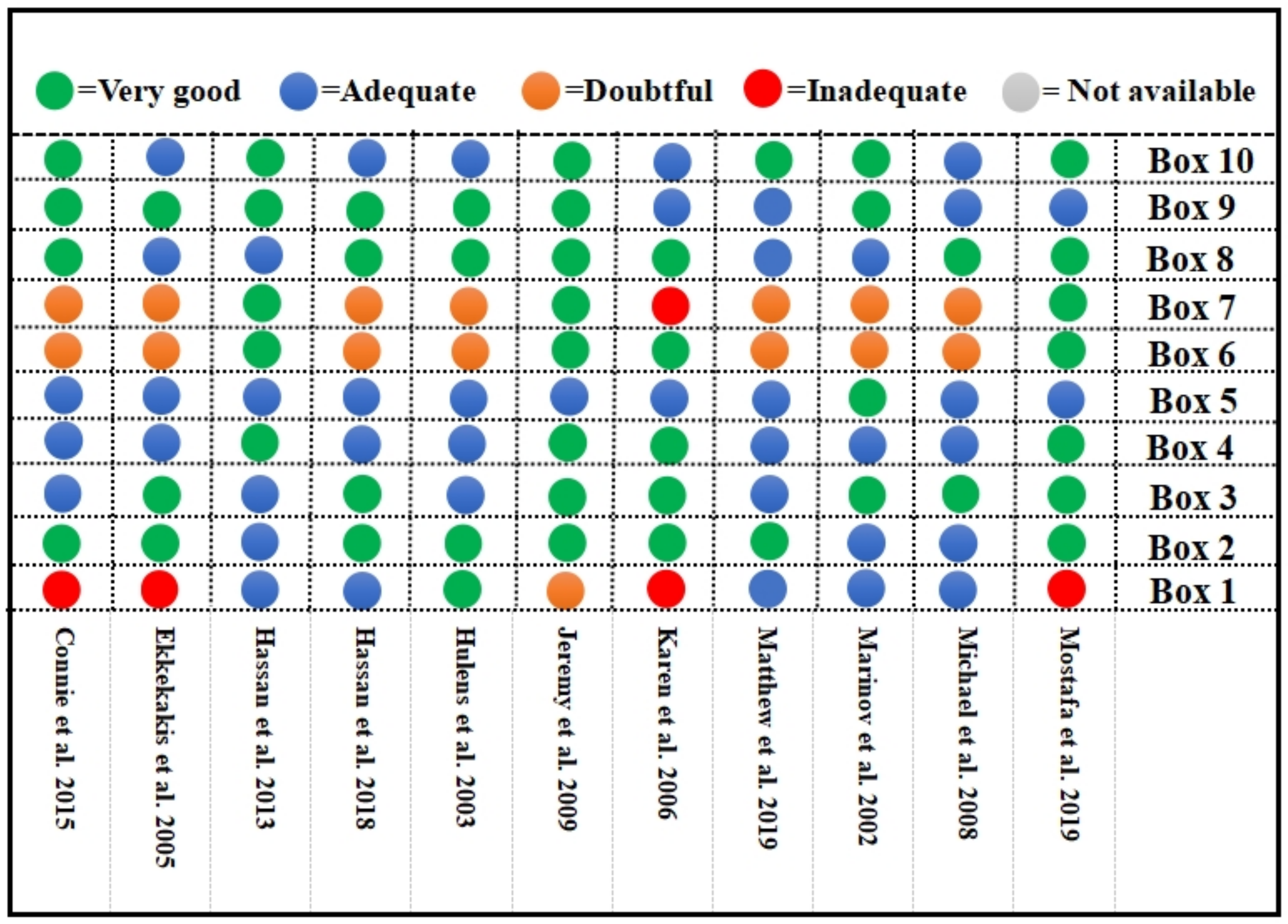
3.2. Bias Risk Assessment
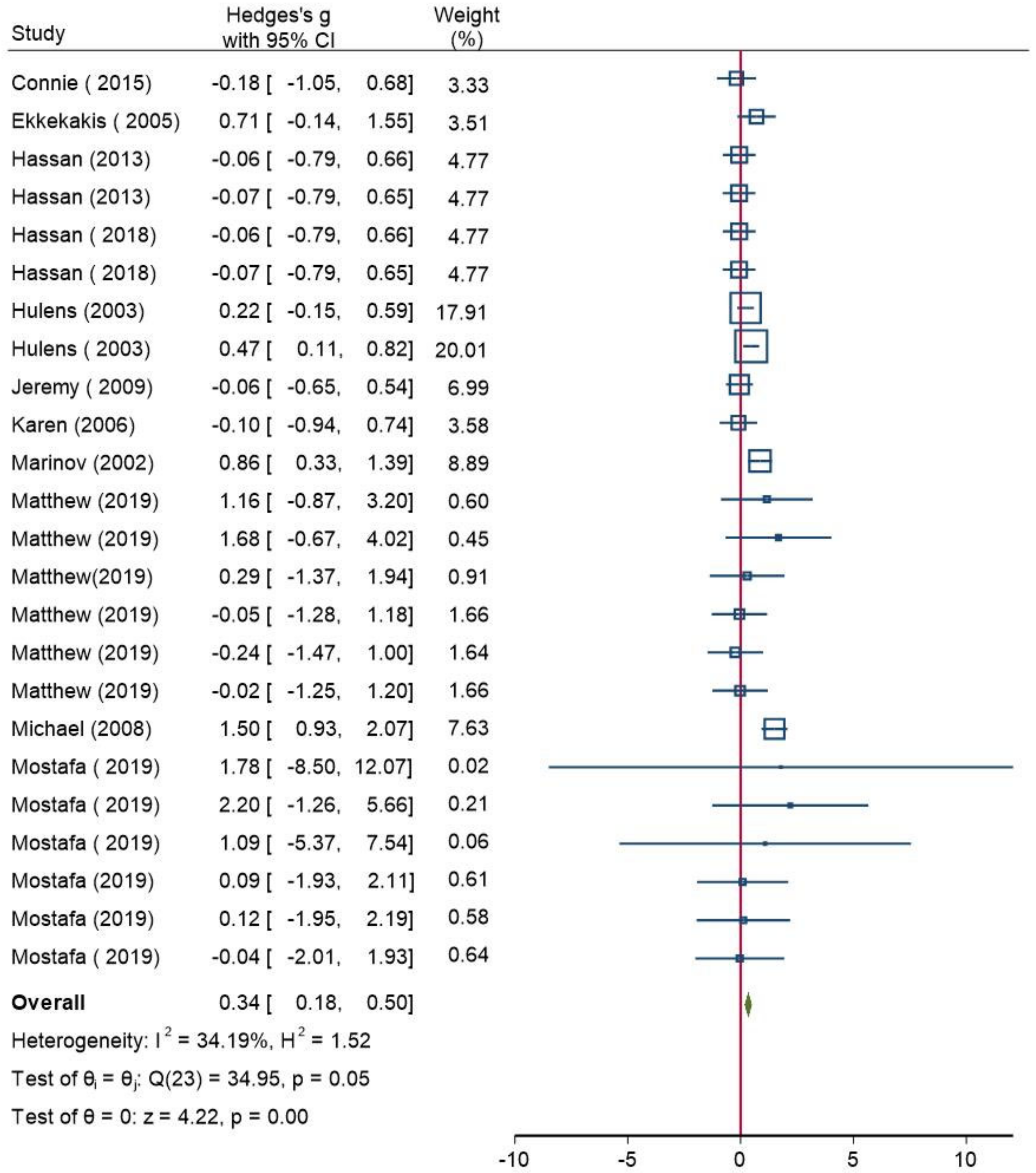
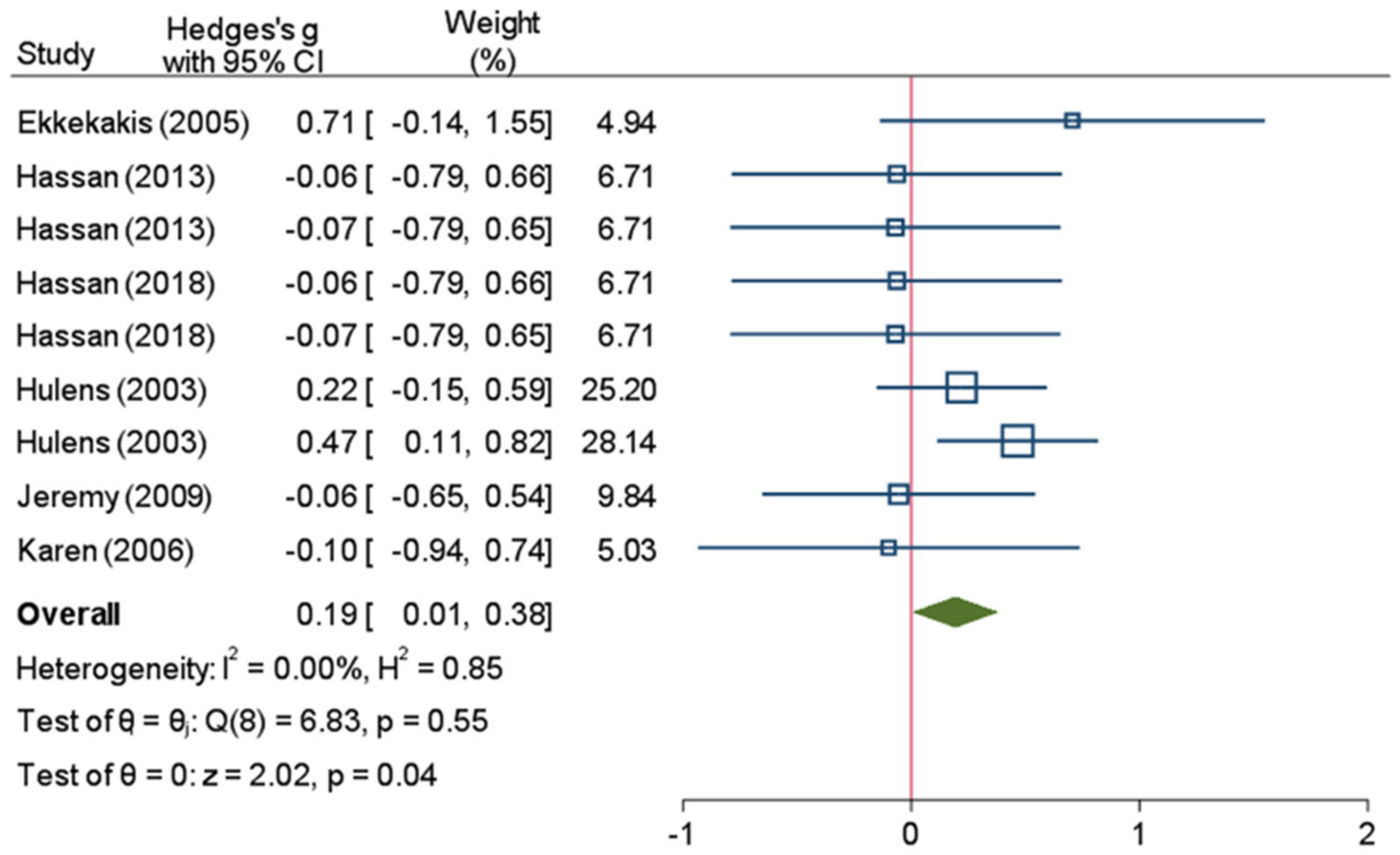
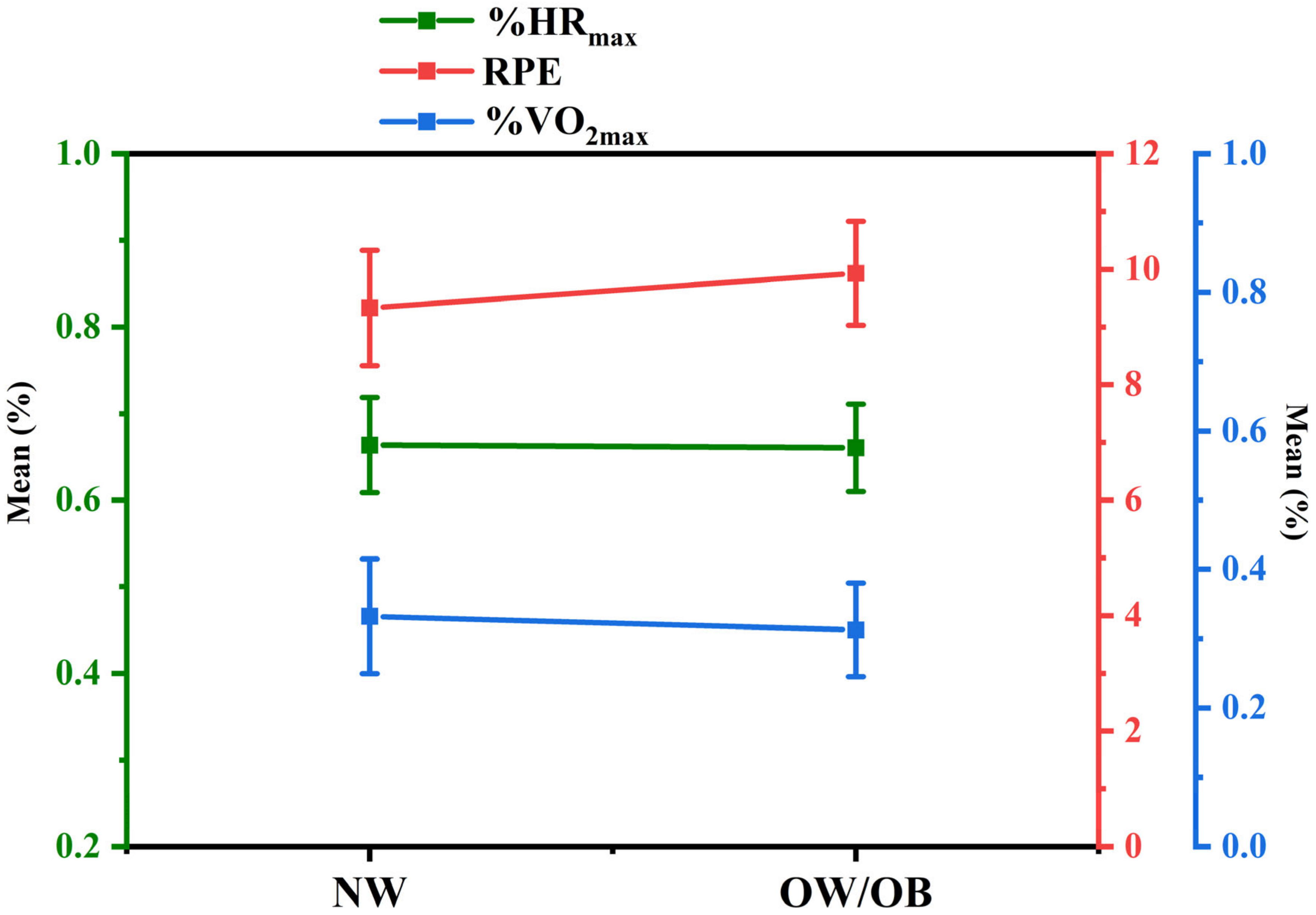
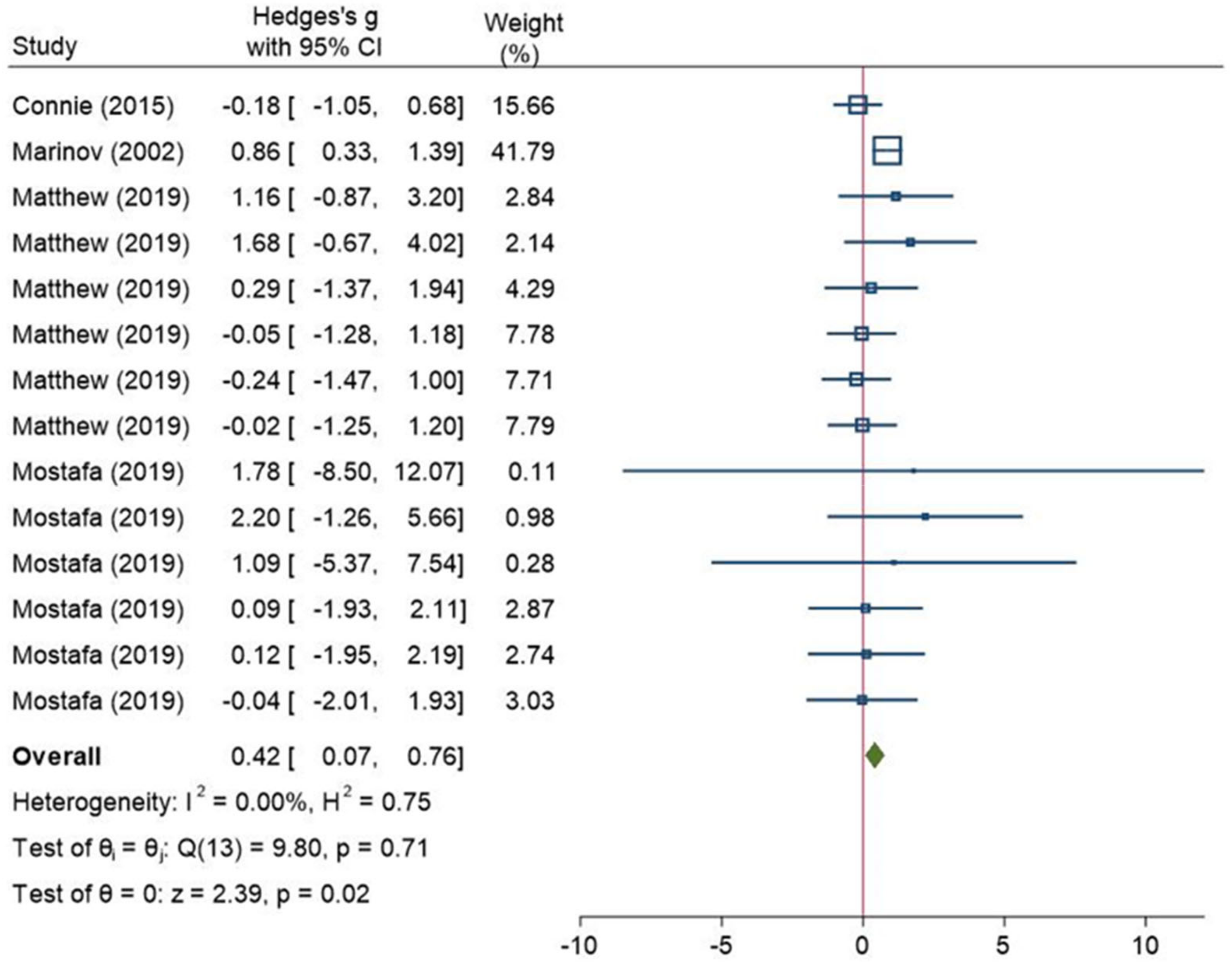
3.3. Meta-Analysis
3.3.1. Heterogeneity Test and Pooled Results
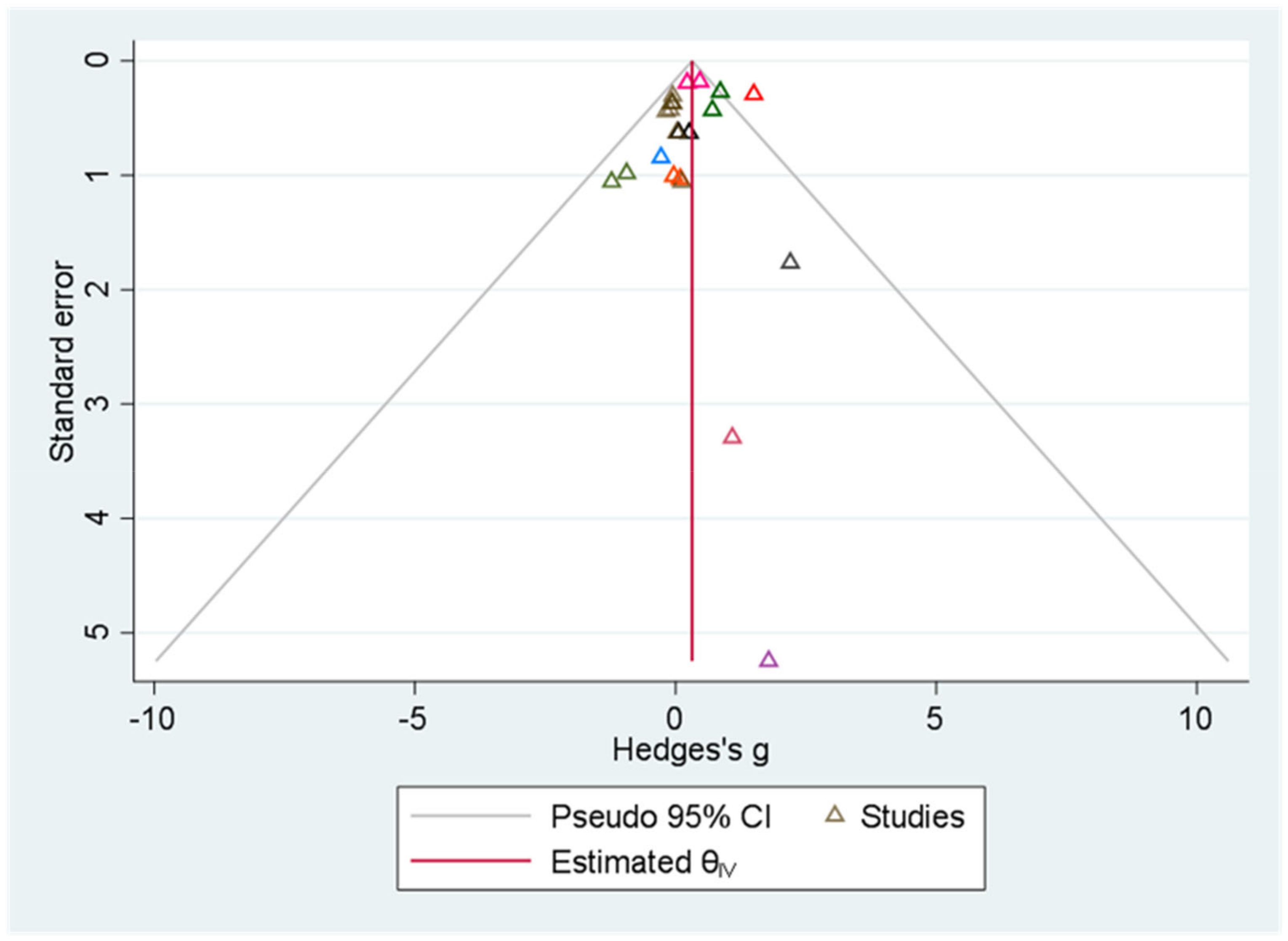
3.3.2. Sensitivity Analysis and Publication Bias
4. Discussion
4.1. Overview of Hypothesis Validation Findings
4.2. Summary of Risk of Bias from Included Studies
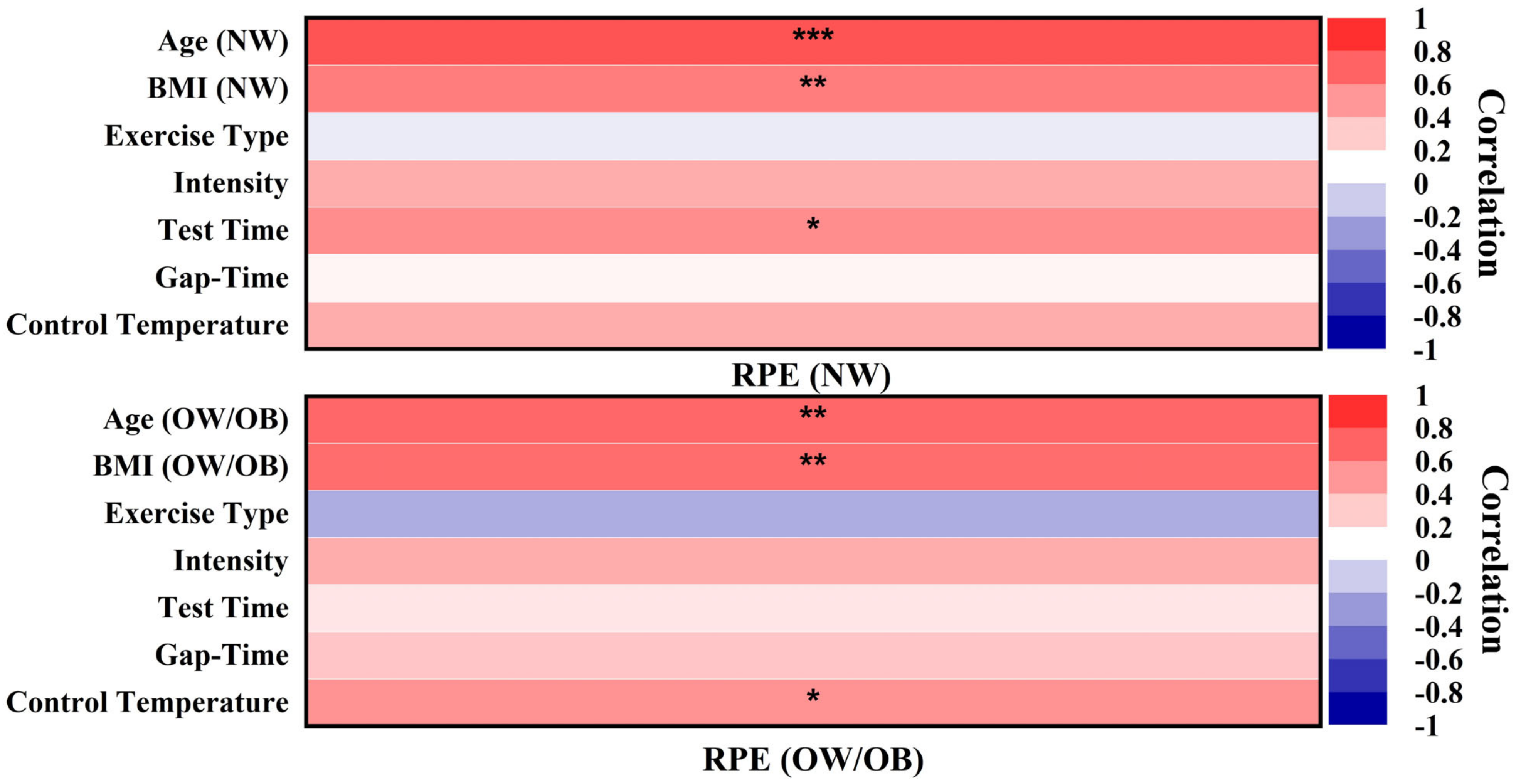
4.3. Summary of Heterogeneity from Included Studies
4.4. Practical Implications and Directions for Future Research
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brisbon, N.; Plumb, J. The asthma and obesity epidemics: The role played by the built environment—A public health perspective. J. Allergy Clin. Immunol. 2005, 115, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, S.B.; Winters, K.P. Overweight and obesity: Prevalence, consequences, and causes of a growing public health problem. Am. J. Med. Sci. 2006, 331, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Children: New Threats to Health. 2020. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/children-new-threats-to-health (accessed on 9 November 2020).
- Stronger Focus on Nutrition within Health Services Could Save 3.7 Million Lives by 2025. Available online: https://www.who.int/news/item/04-09-2019-stronger-focus-on-nutrition-within-health-services-could-save-3.7-million-lives-by-20252019 (accessed on 4 September 2019).
- Booth, F.W.; Roberts, C.K. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143. [Google Scholar]
- Janssen, I.; LeBlanc, A.G. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 40. [Google Scholar] [CrossRef]
- Fisher, G.; Brown, A.W. High intensity interval-vs moderate intensity-training for improving cardiometabolic health in overweight or obese males: A randomized controlled trial. PLoS ONE 2015, 10, e0138853. [Google Scholar] [CrossRef] [PubMed]
- Lubans, D.R.; Morgan, P.J. Fundamental movement skills in children and adolescents. Sports Med. 2010, 40, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Cattuzzo, M.T.; dos Santos Henrique, R. Motor competence and health related physical fitness in youth: A systematic review. J. Sports Sci. Med. 2016, 19, 123–129. [Google Scholar] [CrossRef]
- Utesch, T.; Bardid, F. The relationship between motor competence and physical fitness from early childhood to early adulthood: A meta-analysis. Sports Med. 2019, 49, 541–551. [Google Scholar] [CrossRef]
- D’Hondt, E.; Deforche, B. A longitudinal analysis of gross motor coordination in overweight and obese children versus normal-weight peers. Int. J. Obes. 2013, 37, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Paluska, S.A.; Schwenk, T.L. Physical activity and mental health. Sports Med. 2000, 29, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.; Larkin, D. Does motor competence affect self-perceptions differently for adolescent males and females? SAGE Open. 2015, 5, 2158244015615922. [Google Scholar] [CrossRef]
- Viholainen, H.; Aro, T. Adolescents’ school-related self-concept mediates motor skills and psychosocial well-being. Br. J. Educ. Psychol. 2014, 84, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Dinour, L.M.; Bergen, D. The food insecurity–obesity paradox: A review of the literature and the role food stamps may play. J. Am. Diet Assoc. 2007, 107, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Hills, A.P.; Andersen, L.B. Physical activity and obesity in children. Br. J. Sports Med. 2011, 45, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Gebel, K. Built environment, physical activity, and obesity: What have we learned from reviewing the literature? Health Place 2012, 18, 100–105. [Google Scholar] [CrossRef]
- Barnett, L.M.; Lai, S.K. Correlates of gross motor competence in children and adolescents: A systematic review and meta-analysis. Sports Med. 2016, 46, 1663–1688. [Google Scholar] [CrossRef] [PubMed]
- Norton, K.; Norton, L. Position statement on physical activity and exercise intensity terminology. J. Sci. Med. Sport 2010, 13, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Hasani, F.; Haines, T.P. Efficacy of different load intensity and time-under-tension calf loading protocols for Achilles tendinopathy (the LOADIT trial): Protocol for a randomised pilot study. Pilot Feasibility Stud. 2020, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Costigan, S.A.; Eather, N. High-intensity interval training for improving health-related fitness in adolescents: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Kujach, S.; Byun, K. A transferable high-intensity intermittent exercise improves executive performance in association with dorsolateral prefrontal activation in young adults. Neuroimage 2018, 169, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.R.R.; Tessaro, V.H. Influence of acute high-intensity aerobic interval exercise about on selective attention and short-term memory tasks. Percept. Mot. Ski. 2014, 118, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Pinet, B.M.; Prud’homme, D. Exercise intensity prescription in obese individuals. J. Obes. 2008, 16, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Perri, M.G.; Anton, S.D. Adherence to exercise prescriptions: Effects of prescribing moderate versus higher levels of intensity and frequency. J. Health Psychol. 2002, 21, 452. [Google Scholar] [CrossRef]
- Scherr, J.; Wolfarth, B. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur. J. Appl. Physiol. 2013, 113, 147–155. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Groslambert, A.; Mahon, A.D. Perceived exertion. Sports Med. 2006, 36, 911–928. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Fan, X.; Moe, T. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: A meta-analysis. J. Sports Sci. 2002, 20, 873–899. [Google Scholar] [CrossRef]
- Pfeiffer, K.A.; Pivarnik, J.M.; Womack, C.J.; Reeves, M.J. Reliability and validity of the Borg and OMNI rating of perceived exertion scales in adolescent girls. Med. Sci. Sports Exerc. 2002, 34, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Eston, R.G.; Lamb, K.L.; Bain, A. Validity of a perceived exertion scale for children: A pilot study. Percept Mot. Ski. 1994, 78, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Barkley, J.E.; Roemmich, J.N. Validity of the CALER and OMNI-bike ratings of perceived exertion. Med. Sci. Sports Exerc. 2008, 40, 760–766. [Google Scholar] [CrossRef]
- Ritchie, C. Rating of perceived exertion (RPE). J. Physiother. 2012, 58, 62. [Google Scholar] [CrossRef]
- Estevan, I.; Barnett, L.M. Considerations related to the definition, measurement and analysis of perceived motor competence. Sports Med. 2018, 48, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Stodden, D.F.; Goodway, J.D. A developmental perspective on the role of motor skill competence in physical activity: An emergent relationship. Quest 2008, 60, 290–306. [Google Scholar] [CrossRef]
- Harter, S. Developmental and Individual Difference Perspectives on Self-Esteem, 1st ed.; Lawrence Erlbaum: Mahwah, NJ, USA, 2006; pp. 311–334. [Google Scholar]
- Vitor, L.; Lisa, B. Is there an association among actual motor competence, perceived motor competence, physical activity, and sedentary behavior in preschool children? J. Mot. Learn. Dev. 2016, 4, 129–141. [Google Scholar]
- Bronagh, M.; Danielle, P. Investigation into the relationship between adolescents’ perceived and actual fundamental movement skills and physical activity. J. Mot. Learn. Dev. 2018, 6, S424–S439. [Google Scholar]
- Ruri, F.; Emi, T. Relationships among perceived and actual motor skill competence and physical activity in indonesian preschoolers. J. Mot. Learn. Dev. 2018, 6, S403–S423. [Google Scholar]
- Caterina, P.; Ilaria, M. When children’s perceived and actual motor competence mismatch: Sport participation and gender differences. J. Mot. Learn. Dev. 2018, 6, S440–S460. [Google Scholar]
- Vaimanino, R.; Lisa, M.B. The relationship between fundamental movement skills and physical self-perception among adolescent girls. J. Mot. Learn. Dev. 2018, 6, S378–S390. [Google Scholar]
- Toftegaard-Stoeckel, J.; Groenfeldt, V. Children’s self-perceived bodily competencies and associations with motor skills, body mass index, teachers’ evaluations, and parents’ concerns. J. Sports Sci. 2010, 28, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- LeGear, M.; Greyling, L. A window of opportunity? Motor skills and perceptions of competence of children in Kindergarten. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Breslin, G.; Murphy, M. The effect of teachers trained in a fundamental movement skills programme on children’s self-perceptions and motor competence. Eur. Phys. Educ. Rev. 2012, 18, 114–126. [Google Scholar] [CrossRef]
- Maike, T.; Dennis, D. Pictorial scale of physical self-concept for younger children (P-PSC-C): A feasibility study. J. Mot. Learn. Dev. 2018, 6, S391–S402. [Google Scholar]
- Borg, D.N.; Stewart, I.B. The impact of environmental temperature deception on perceived exertion during fixed-intensity exercise in the heat in trained-cyclists. Physiol. Behav. 2018, 194, 333–340. [Google Scholar] [CrossRef]
- Hobbs, M.; Griffiths, C. Examining longitudinal associations between the recreational physical activity environment, change in body mass index, and obesity by age in 8864 Yorkshire Health Study participants. Soc. Sci. Med. 2019, 227, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.; Bull, F. Development of the world health organization global physical activity questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Hidding, L.M.; Chinapaw, M.J. An updated systematic review of childhood physical activity questionnaires. Sports Med. 2018, 48, 2797–2842. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaf, J.; Altman, D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, comparison, outcomes and study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Sackett, D.L.; Rosenberg, W.M. Evidence based medicine: What it is and what it isn’t. Br. Med. J. 1996, 312, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Mokkink, L.B.; Boers, M. COSMIN risk of bias tool to assess the quality of studies on reliability or measurement error of outcome measurement instruments: A Delphi study. BMC Med. Res. Methodol. 2020, 20, 293. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J. Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019; Volume 8, pp. 187–241. [Google Scholar]
- Tompkins, C.L.; Flanagan, T.; Lavoie, J.; Brock, D.W. Heart rate and perceived exertion in healthy weight and obese children during a self-selected physical activity program. J. Phys. Act. Health 2015, 12, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Ekkekakis, P.; Lind, E. Exercise does not feel the same when you are overweight: The impact of self-selected and imposed intensity on affect and exertion. Int. J. Obes. 2005, 30, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Elsangedy, H.M.; Krinski, K.; Costa, E.C.; Haile, L.; Fonteles, A.I.; da Silva Timossi, L.; da Silva, S.G. The rating of perceived exertion is not different at the ventilatory threshold in sedentary women with different body mass indices. J. Exerc. Sci. Fit. 2013, 11, 102–106. [Google Scholar] [CrossRef]
- Elsangedy, H.M.; Nascimento, P.H.; Machado, D.G. Poorer positive affect in response to self-paced exercise among the obese. Physiol. Behav. 2018, 189, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Coquart, J.B.; Lemaire, C.; Dubart, A.E.; Douillard, C.; Luttenbacher, D.P.; Wibaux, F.; Garcin, M. Prediction of peak oxygen uptake from sub-maximal ratings of perceived exertion elicited during a graded exercise test in obese women. Psychophysiology 2009, 46, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Wallman, K.E.; Campbell, L. Test–retest reliability of the Aerobic Power Index submaximal exercise test in an obese population. J. Sci. Med. Sport 2006, 10, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Hulens, M.; Vansant, G.; Claessens, A.; Lysens, R.; Muls, E. Predictors of 6-minute walk test results in lean, obese and morbidly obese women. Scand. J. Med. Sci. Sports 2003, 13, 98–105. [Google Scholar] [CrossRef]
- Marinov, B.; Kostianev, S.; Turnovska, T. Ventilatory efficiency and rate of perceived exertion in obese and non-obese children performing standardized exercise. Clin. Physiol. Funct. 2002, 22, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.M.; Green, J.M.; Davis, J.K.; Geary, C.L.; Voth, N.R. Differentiated ratings of perceived exertion between overweight and non-overweight children during submaximal cycling. Int. J. Health Sci. 2019, 13, 3. [Google Scholar]
- McGuigan, M.R.; Al Dayel, A.; Tod, D.; Foster, C.; Newton, R.U.; Pettigrew, S. Use of session rating of perceived exertion for monitoring resistance exercise in children who are overweight or obese. Pediatr. Exerc. Sci. 2008, 20, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, M.; Fink, P.W.; Page, W.H.; Shultz, S.P. Stationary exercise in overweight and normal weight children. Pediatr. Exerc. Sci. 2019, 31, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Santos, M.A.; Barreto-Filho, J.A. Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch Gerontol. Geriatr. 2016, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.R.; Fisher, G. Exercise training and energy expenditure following weight loss. Med. Sci. Sports Exerc. 2015, 47, 1950. [Google Scholar] [CrossRef]
- Byrne, N.M.; Hills, A.P. Relationships between HR and VO2 in the obese. Med. Sci. Sports Exerc. 2002, 34, 1419–1427. [Google Scholar] [CrossRef]
- Gibson, E.J.; Pick, A.D. An Ecological Approach to Perceptual Learning and Development, 1st ed.; Oxford University Press: Oxford, UK, 2000; pp. 14–24. [Google Scholar]
- Noordstar, J.J.; van der Net, J. The change in perceived motor competence and motor task values during elementary school: A longitudinal cohort study. Br. J. Dev. Psychol. 2016, 34, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Harter, S.; Pike, R. The pictorial scale of perceived competence and social acceptance for young children. Child Dev. 1984, 1969–1982. [Google Scholar] [CrossRef]
- Goodway, J.D.; Rudisill, M.E. Perceived physical competence and actual motor skill competence of African American preschool children. Adapt. Phys. Act. Q. 1997, 14, 314–326. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body mass index: Obesity, BMI, and health: A critical review. Nutr. Today 2015, 50, 117. [Google Scholar] [CrossRef]
- Uchida, M.C.; Teixeira, L.F. Does the timing of measurement alter session-RPE in boxers? J. Sports Sci. Med. 2014, 13, 59. [Google Scholar]
- Justine, M.; Ishak, A. Effects of sub-maximal exercise on energy expenditure and heart rate recovery in individuals with normal weight, overweight and obesity. J. Phys. Educ. Sport 2018, 18, 1632–1639. [Google Scholar]
- Skinner, J.; Hutsler, R. Perception of effort during different types of exercise and under different environmental conditions. Med. Sci. Sports 1973, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Crewe, H.; Tucker, R. The rate of increase in rating of perceived exertion predicts the duration of exercise to fatigue at a fixed power output in different environmental conditions. Eur. J. Appl. Physiol. 2008, 103, 569–577. [Google Scholar] [CrossRef]
- Tikuisis, P.; Mclellan, T.M. Perceptual versus physiological heat strain during exercise-heat stress. Med. Sci. Sports Exerc. 2002, 34, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Smith, P. Effects of caffeine ingestion on rating of perceived exertion during and after exercise: A meta-analysis. Scand J. Med. Sci. Sports 2005, 15, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Rhim, H.C.; Kim, S.J. Effect of citrulline on post-exercise rating of perceived exertion, muscle soreness, and blood lactate levels: A systematic review and meta-analysis. J. Sport Health Sci. 2020, 9, 553–561. [Google Scholar] [CrossRef]
- Halford, G.S.; Cowan, N. Separating cognitive capacity from knowledge: A new hypothesis. Trends Cogn. Sci. 2007, 11, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A. Brain Maturation and Cognitive Development: Comparative and Cross-Cultural Perspectives, 2nd ed.; Routledge: New York, NY, USA, 2017; pp. 225–253. [Google Scholar]
- Whitley, E.; Ball, J. Statistics review 4: Sample size calculations. J. Crit. Care. 2002, 6, 1–7. [Google Scholar]
- Singh, A.S.; Masuku, M.B. Sampling techniques & determination of sample size in applied statistics research: An overview. IJECM 2014, 2, 1–22. [Google Scholar]
- Bearden, W.O.; Sharma, S. Sample size effects on chi square and other statistics used in evaluating causal models. J. Mark. Res. 1982, 19, 425–430. [Google Scholar] [CrossRef]
- Bonett, D.G.; Wright, T.A. Sample size requirements for estimating Pearson, Kendall and Spearman correlations. Psychometrika 2000, 65, 23–28. [Google Scholar] [CrossRef]
- Park, D.C.; Bischof, G.N. The aging mind: Neuroplasticity in response to cognitive training. Dialogues Clin. Neurosci. 2013, 15, 109. [Google Scholar] [PubMed]
- Parsons, B.; Magill, T. Enhancing cognitive function using perceptual-cognitive training. Clin. EEG Neurosci. 2016, 47, 37–47. [Google Scholar] [CrossRef]
- Dishman, R.K. Prescribing exercise intensity for healthy adults using perceived exertion. Med. Sci. Sports Exerc. 1994, 26. [Google Scholar] [CrossRef]
- Reed, J.L.; Pipe, A.L. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can. J. Cardiol. 2016, 32, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.; Bar-Or, O. Use of the Borg scale in exercise prescription for overweight youth. Can. J. Sport Sci. 1990, 15, 120–125. [Google Scholar] [PubMed]
- Aamot, I.L.; Forbord, S.H. Does rating of perceived exertion result in target exercise intensity during interval training in cardiac rehabilitation? A study of the Borg scale versus a heart rate monitor. J. Sports Sci. Med. 2014, 17, 541–545. [Google Scholar] [CrossRef]

| Reference | Country | Sample Size (M1/F2) | Mean Age (Years) | Mean BMI | Exercise Modality | Exercise Protocol | Physiological Criterion | PE 9 Scale |
|---|---|---|---|---|---|---|---|---|
| Connie et al. 2015 [55] | USA | 52 (34/18) | NW 3: 8.6 SD: 0.9 OB: 8.7 SD: 0.7 | NW: 16 SD: 1.2 OB: 23 SD: 1.3 | 45 min school PA | Vigorous Continuous | HR | Borg scale (6–20) Test time: end of exercise |
| Ekkekakis et al. 2005 [56] | USA | 25 (25/0) | NW: 43.67 SD: 4.24 OW: 43 SD: 5.4 | NW: 22.34 SD: 1.82 OW: 31.06 SD: 4.91 | 20 min treadmill exercise | Low Intermittent | VO2peak and HR | Borg scale (6–20) Test time: each 5 min |
| Hassan et al. 2013 [57] | Brazil | 66 (66/0) | NW: 30.8 SD: 9.3 OB: 33.5 SD: 8.5 OW: 34.8 SD: 8.6 | NW: 22 SD: 1.6 OB: 34.9. SD: 4.1 OW: 26.4 SD: 1.3 | Maximal graded treadmill | Low Continuous Standard temperature | HRmax, VEmax, VO2VT, %VO2 26, VTmax, %HRmax 25, VT, HRVT, VO2max, and RERmax | Borg scale (6–20) Test time: the last 15 s |
| Hassan et al. 2018 [58] | Brazil | 66 (66/0) | NW: 30.8 SD: 9.3 OB: 33.5 SD: 8.5 OW: 34.8 SD: 8.6 | NW: 22 SD: 1.6 OB: 34.9. SD: 4.1 OW: 26.4 SD: 1.3 | GXT | Low Intermittent | HR andVO2 | Borg scale (6–20) Test time: the last 15 s |
| Jeremy et al. 2009 [59] | USA | 43 (43/0) | OB: 50.5 SD: 8.1 | OB: 39.6 SD: 6.1 | GXT | Moderate Continuous Standard temperature | VO2max 27 | Borg scale (6–20) Test time: the last 15 s |
| Karen et al. 2006 [60] | Australia | 22 (19/3) | OB: 45.7 SD: 9.8 | OB: 33.6 SD: 2.6 | Submaximal cycle test | Low Intermittent aerobic, and power index Standard temperature | VO2 | Borg scale (6–20) Test time: recorded 55 s |
| Hulens et al. 2003 [61] | Belgium | 300 (300/0) | NW 3: 39 SD 7: 11.8 OB 5: 39.5 SD: 11.4 OB morbidity: 38.9 SD: 12.4 | NW: 22.1 SD: 2.1 OB: 32.3 SD: 1.9 OB morbidity: 40.7 SD: 4.4 | GXT 6 | Low Continuous | VO2peak 17 and HR 10 | Borg scale (6–20) Test time: the last 15 s |
| Marinov et al. 2002 [62] | Bulgaria | 60 (30/30) | NW: 11.0 SD: 3.1 OB: 10.9 SD: 3.1 | NW: 18.8 SD: 2.7 OB: 27.4 SD: 4.5 | Cardiopulmonary exercise test | Moderate Continuous | VT14, BF 15, VE 12, VE⁄ MVV 16, VO2peak, VO2 11, VO2⁄FFM 18, VO2⁄BSA 19, VO2⁄HR 20, HR, RER 21, AT 24, VE/VO2 23, VE ⁄ VCO2 22, and VCO2/VO2 13 | Borg scale (0–10) Test time: end of exercise |
| Matthew et al. 2019 [63] | USA | 21 (12/9) | NW: 9.4 SD: 0.5 OW 4: 10.2 SD: 0.4 NW: 10.3 SD: 0.6 OW: 10.5 SD: 0.7 | NW: 16.3 SD: 1.7 OW: 23.1 SD: 2.2 NW: 16 SD: 2 OW: 23.5 SD: 1.9 | Self-selected: 20 min submaximal cycling | Low Continuous | HR VO2/VO2peak 70%peak HR | OMNI scale Test time: each 5 min |
| Michael et al. 2008 [64] | Australia | 61 (28/33) | OB: 9.7 SD: 1.4 | OB: 25.6 SD: 3.4 | Resistance exercise | Low Intermittent | NA 8 | OMNI scale Test time: end of exercise |
| Mostafa et al. 2019 [65] | New Zealand | 25 (13/12) | NW: 12.23 SD: 1.23 OW: 12.1 SD: 1.22 | NW: 17.35 SD: 2.64 OW: 28.7 SD: 7.05 | Butt kick Stationary running Frontal kick | Low Intermittent Standard temperature | HR | Borg scale (6–20) Test time: end of exercise |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Sun, C.; Sun, B.; Chen, X.; Tan, Z. Systematic Review and Meta-Analysis of the Relationship between Actual Exercise Intensity and Rating of Perceived Exertion in the Overweight and Obese Population. Int. J. Environ. Res. Public Health 2021, 18, 12912. https://doi.org/10.3390/ijerph182412912
Yu H, Sun C, Sun B, Chen X, Tan Z. Systematic Review and Meta-Analysis of the Relationship between Actual Exercise Intensity and Rating of Perceived Exertion in the Overweight and Obese Population. International Journal of Environmental Research and Public Health. 2021; 18(24):12912. https://doi.org/10.3390/ijerph182412912
Chicago/Turabian StyleYu, Hongli, Chen Sun, Bo Sun, Xiaohui Chen, and Zhijun Tan. 2021. "Systematic Review and Meta-Analysis of the Relationship between Actual Exercise Intensity and Rating of Perceived Exertion in the Overweight and Obese Population" International Journal of Environmental Research and Public Health 18, no. 24: 12912. https://doi.org/10.3390/ijerph182412912
APA StyleYu, H., Sun, C., Sun, B., Chen, X., & Tan, Z. (2021). Systematic Review and Meta-Analysis of the Relationship between Actual Exercise Intensity and Rating of Perceived Exertion in the Overweight and Obese Population. International Journal of Environmental Research and Public Health, 18(24), 12912. https://doi.org/10.3390/ijerph182412912







