Study on the Potential Application of Impatiens balsamina L. Flowers Extract as a Natural Colouring Ingredient in a Pastry Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Samples
2.2. Evaluation of Colour Parameters
2.3. Nutritional Composition
2.4. Chemical Composition
2.4.1. Sugars
2.4.2. Fatty Acids
2.4.3. Organic Acids
2.5. Phenolic Composition and Bioactive Potential of Impatiens Flower Extracts
2.5.1. Extract Preparation
2.5.2. Identification and Quantification of Phenolic Compounds
2.5.3. Bioactivities Evaluation
2.6. Incorporation of Natural Colorant in “Bombocas”
2.6.1. Formulation of the “Bombocas”
2.6.2. Evaluation of Colour Parameters, Nutritional Composition, Sugar and Fatty Acid Content and Antioxidant Activity of “Bombocas” during Storage Time
2.7. Statistical Analysis
3. Results
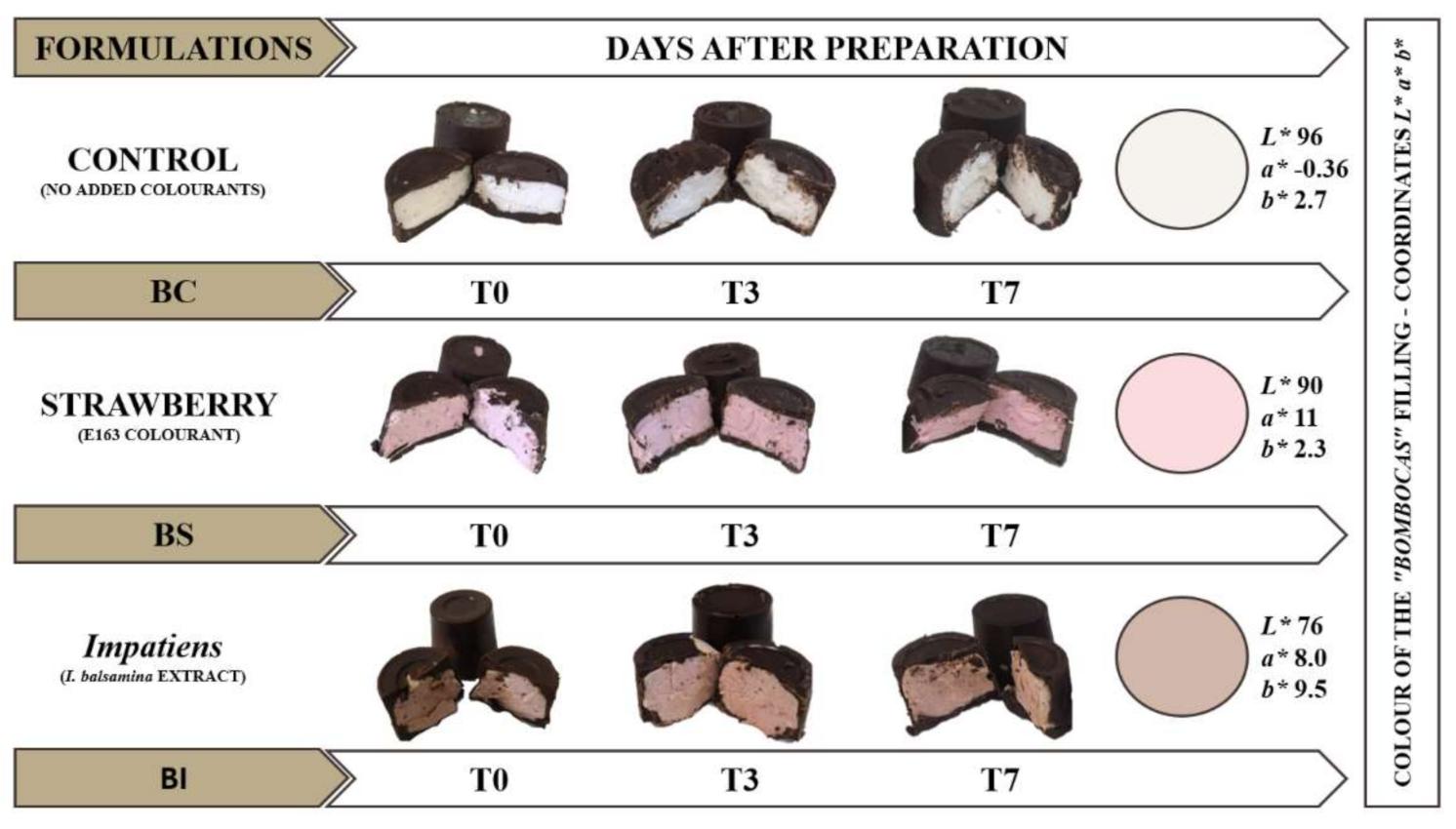
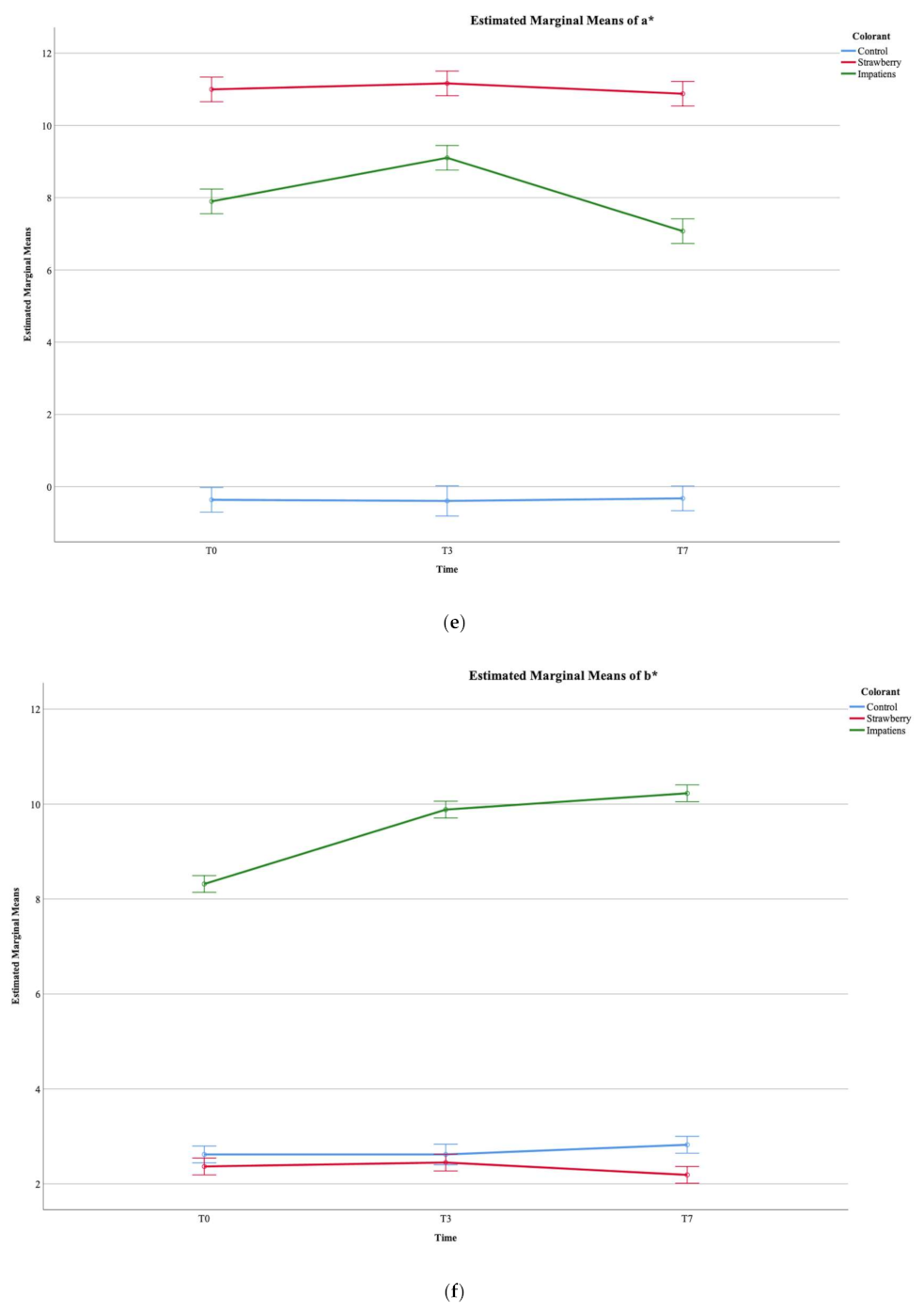
3.1. Evaluation of Colour Parameters
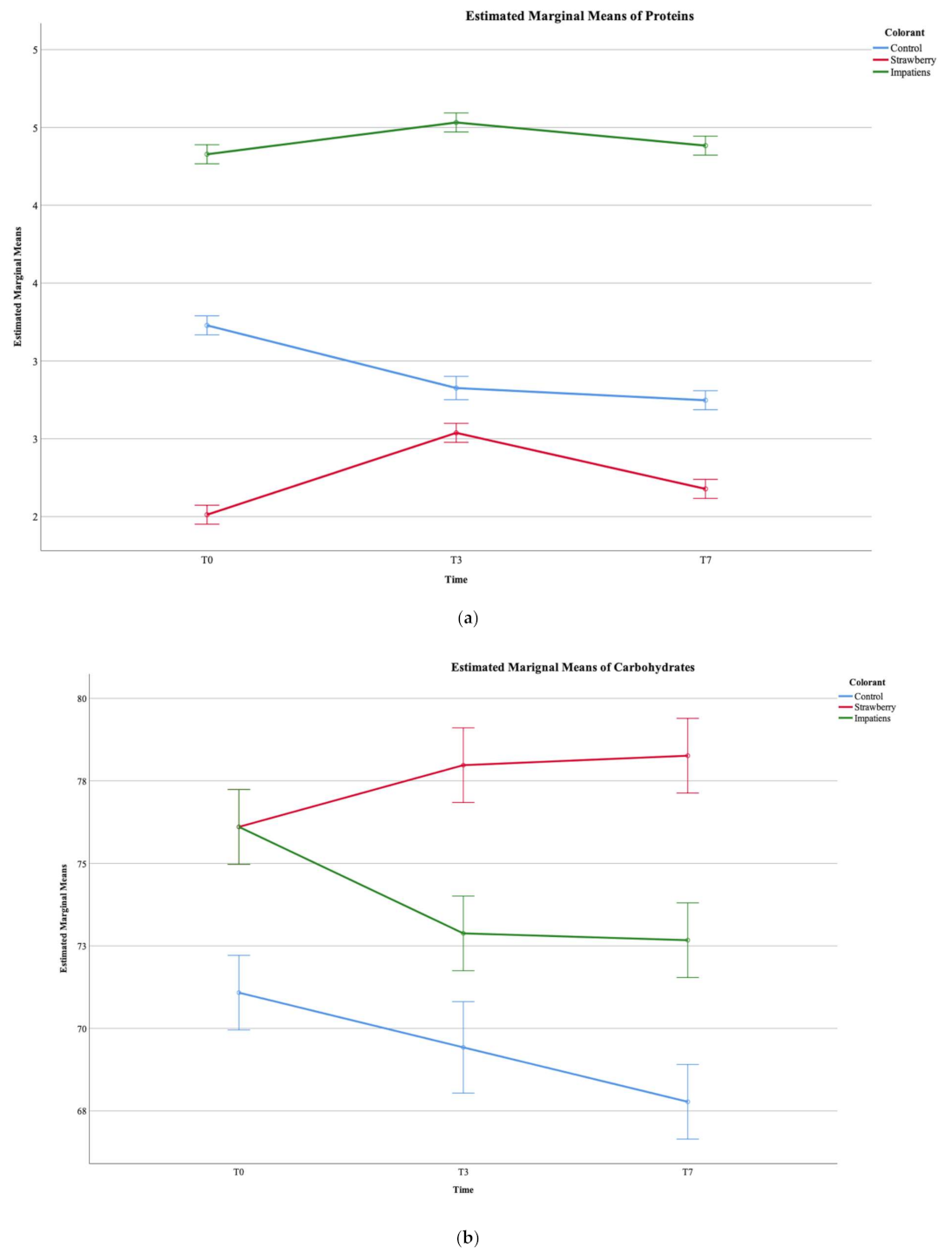
3.2. Nutritional Composition
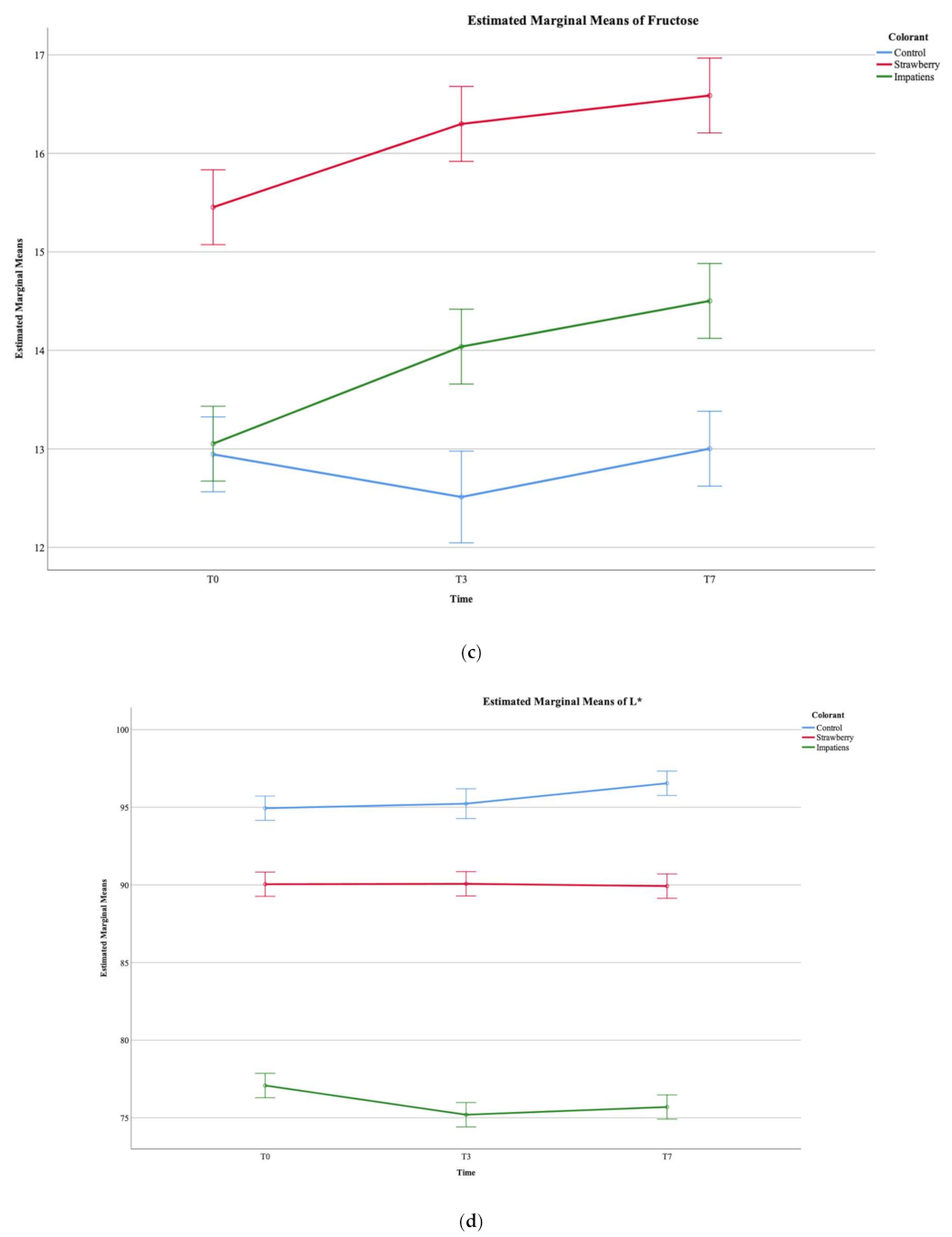
3.3. Chemical Composition
3.4. Identification and Quantification of Phenolic Compounds
3.5. Bioactivities Evaluation
3.6. Incorporation of Natural Colorant in “Bombocas”
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Olas, B.; Urbańska, K.; Bryś, M. Selected food colourants with antiplatelet activity as promising compounds for the prophylaxis and treatment of thrombosis. Food Chem. Toxicol. 2020, 141, 111437. [Google Scholar] [CrossRef] [PubMed]
- Ntrallou, K.; Gika, H.; Tsochatzis, E. Analytical and sample preparation techniques for the determination of food colorants in food matrices. Foods 2020, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, B.; Sperl, R.; Carle, R.; Müller-Maatsch, J. Assessing the sustainability of natural and artificial food colorants. J. Clean. Prod. 2020, 260, 120884. [Google Scholar] [CrossRef]
- Lin, W.-S.; He, P.H.; Chau, C.-F.; Liou, B.-K.; Li, S.; Pan, M.-H. The feasibility study of natural pigments as food colorants and seasonings pigments safety on dried tofu coloring. Food Sci. Hum. Wellness 2018, 7, 220–228. [Google Scholar] [CrossRef]
- Cerreti, M.; Liburdi, K.; Del Franco, F.; Esti, M. Heat and light stability of natural yellow colourants in model beverage systems. Food Addit. Contam. Part. A 2020, 37, 905–915. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Kobylewski, S.; Jacobson, M.F. Toxicology of food dyes. Int. J. Occup. Environ. Health 2012, 18, 220–246. [Google Scholar] [CrossRef]
- Neves, M.I.L.; Silva, E.K.; Meireles, M.A.A. Trends and challenges in the industrialization of natural colorants. Food Public Health 2019, 9, 33–44. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; De Mejia, E.G. Natural pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 2016, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Różyło, R. Recent trends in methods used to obtain natural food colorants by freeze-drying. Trends Food Sci. Technol. 2020, 102, 39–50. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Could fruits be a reliable source of food colorants? Pros and cons of these natural additives. Crit. Rev. Food Sci. Nutr. 2020, 61, 805–835. [Google Scholar] [CrossRef]
- Boyles, C.; Sobeck, S.J.S. Photostability of organic red food dyes. Food Chem. 2020, 315, 126249. [Google Scholar] [CrossRef]
- De Mejia, E.G.; Zhang, Q.; Penta, K.; Eroğlu, A.; Lila, M.A. The colors of health: Chemistry, bioactivity, and market demand for colorful foods and natural food sources of colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. An Overview on the market of edible flowers. Food Rev. Int. 2019, 36, 258–275. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2019, 129, 108868. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, B.; Huang, W.; Amrouche, A.T.; Maurizio, B.; Simal-Gandara, J.; Tundis, R.; Xiao, J.; Zou, L.; Lu, B. Edible flowers as functional raw materials: A review on anti-aging properties. Trends Food Sci. Technol. 2020, 106, 30–47. [Google Scholar] [CrossRef]
- Pires, E.O.; Caleja, C.; Garcia, C.C.; Ferreira, I.C.; Barros, L. Current status of genus impatiens: Bioactive compounds and natural pigments with health benefits. Trends Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Szewczyk, K. Phytochemistry of the genus impatiens (Balsaminaceae): A review. Biochem. Syst. Ecol. 2018, 80, 94–121. [Google Scholar] [CrossRef]
- Pereira, E.; Antonio, A.; Barreira, J.; Barros, L.; Bento, A.A.; Ferreira, I. Gamma irradiation as a practical alternative to preserve the chemical and bioactive wholesomeness of widely used aromatic plants. Food Res. Int. 2015, 67, 338–348. [Google Scholar] [CrossRef]
- AOAC International. AOAC Official Methods of Analysis, 20th ed.; AOAC International: Washington, DC, USA, 2016. [Google Scholar]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized analysis of organic acids in edible mushrooms from portugal by ultra fast liquid chromatography and photodiode array detection. Food Anal. Methods 2012, 6, 309–316. [Google Scholar] [CrossRef]
- Pereira, E.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chem. 2012, 130, 394–403. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Bessada, S.M.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant ac-tivity of Coleostephus myconis (L.) Rchb. f.: An underexploited and highly disseminated species. Ind. Crop. Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Gonçalves, G.A.; Soares, A.A.; Correa, R.C.G.; Barros, L.; Haminiuk, C.W.I.; Peralta, R.M.; Bracht, A. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced ar-thritis. J. Funct. Foods 2017, 33, 408–418. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crop. Prod. 2018, 128, 496–503. [Google Scholar] [CrossRef]
- Jabeur, I.; Tobaldini, F.; Martins, N.; Barros, L.; Martins, I.; Calhelha, R.C.; Henriques, M.; Silva, S.; Achour, L.; Santos-Buelga, C.; et al. Bioactive properties and functional constituents of Hypericum androsaemum L.: A focus on the phenolic profile. Food Res. Int. 2016, 89, 422–431. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an In Vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; van Griensven, L.J.L.D. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant. Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- Pires, E.; Pereira, E.; Pereira, C.; Dias, M.; Calhelha, R.; Ćirić, A.; Soković, M.; Hassemer, G.; Garcia, C.; Caleja, C.; et al. Chemical composition and bioactive characterisation of Impatiens walleriana. Molecules 2021, 26, 1347. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Szewczyk, K.; Heise, E.M.; Piwowarski, J.P. Preliminary characterization and bioactivities of some Impatiens L. water-soluble polysaccharides. Molecules 2018, 23, 631. [Google Scholar] [CrossRef]
- Tran, T.-D.; Nguyen, T.-T.; Do, T.-H.; Huynh, T.-N.; Tran, C.-D.; Thai, K.-M. Synthesis and antibacterial activity of some heterocyclic chalcone analogues alone and in combination with antibiotics. Molecules 2012, 17, 6684–6696. [Google Scholar] [CrossRef]
- Szewczyk, K.; Bonikowski, R.; Maciąg-Krajewska, A.; Abramek, J.; Bogucka-Kocka, A. Lipophilic components and evaluation of the cytotoxic and antioxidant activities of Impatiens glandulifera Royle and Impatiens noli-tangere L. (Balsaminaceae). Grasas Y Aceites 2018, 69, 270. [Google Scholar] [CrossRef]
- Chua, L.S. Untargeted MS-based small metabolite identification from the plant leaves and stems of Impatiens balsamina. Plant. Physiol. Biochem. 2016, 106, 16–22. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyżowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2020, 61, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, R.; González-Miquel, M.; González, E.J.; Díaz, I.; Rodríguez, M. Overview of neoteric solvents as extractants in food industry: A focus on phenolic compounds separation from liquid streams. Food Res. Int. 2020, 136, 109558. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Kuhnert, N. Identification and characterization of the phenolic glycosides of Lagenaria siceraria stand. (Bottle Gourd) fruit by liquid chromatography—Tandem mass spectrometry. J. Agric. Food Chem. 2014, 62, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Mandim, F.; Petropoulos, S.A.; Fernandes, Â.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Chemical composition of Cynara cardunculus L. var. altilis Heads: The impact of harvesting time. Agronomy 2020, 10, 1088. [Google Scholar] [CrossRef]
- Anjos, O.; Fernandes, R.; Cardoso, S.M.; Delgado, T.; Farinha, N.; Paula, V.; Estevinho, M.L.M.F.; Carpes, S.T. Bee pollen as a natural antioxidant source to prevent lipid oxidation in black pudding. LWT 2019, 111, 869–875. [Google Scholar] [CrossRef]
- Sut, S.; Zengin, G.; Dall’Acqua, S.; Gazdová, M.; Šmejkal, K.; Bulut, G.; Dogan, A.; Haznedaroglu, M.Z.; Aumeeruddy, M.Z.; Maggi, F.; et al. Paeonia arietina and Paeonia kesrounansis bioactive constituents: NMR, LC-DAD-MS fingerprinting and in vitro assays. J. Pharm. Biomed. Anal. 2018, 165, 1–11. [Google Scholar] [CrossRef]
- Ning, Z.-W.; Zhai, L.-X.; Huang, T.; Peng, J.; Hu, D.; Xiao, H.-T.; Wen, B.; Lin, C.-Y.; Zhao, L.; Bian, Z.-X. Identification of α-glucosidase inhibitors from cyclocarya paliurus tea leaves using UF-UPLC-Q/TOF-MS/MS and molecular docking. Food Funct. 2019, 10, 1893–1902. [Google Scholar] [CrossRef]
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, G.-S.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2016, 214, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.; Dias, M.I.; Calhelha, R.C.; Alves, M.J.; Santos-Buelga, C.; Ferreira, I.C.; Barros, L. Development of new bilberry (Vaccinium myrtillus L.) based snacks: Nutritional, chemical and bioactive features. Food Chem. 2020, 334, 127511. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, Z.; Zhu, H.; Hu, C.; Liu, R.; Young, J.C.; Tsao, R. Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Res. Int. 2012, 46, 250–259. [Google Scholar] [CrossRef]
- Hosokawa, K.; Fukunaga, Y.; Fukushi, E.; Kawabata, J. Five acylated pelargonidin glucosides in the red flowers of Hyacinthus orientalis. Phytochemistry 1995, 40, 567–571. [Google Scholar] [CrossRef]
- Oliveira, J.; Alhinho da Silva, M.; Teixeira, N.; De Freitas, V.; Salas, E. Screening of anthocyanins and anthocya-nin-derived pigments in red wine grape pomace using LC-DAD/MS and MALDI-TOF techniques. J. Agric. Food Chem. 2015, 63, 7636–7644. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, K.; Henning, S.; Soendergaard, M. Antioxidant activity and phenolic content of chinese balsam (Impatiens chinensis). FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Moroeanu, V.; Albu, C.; Ursu, T.-M.; Zanfirescu, A.; Negres, S.; Chirita, C.; Radu, G.L. Anti-inflammatory and antioxidant activities of the Impatiens noli-tangere and stachys officinalis polyphenolic-rich extracts. Rev. Bras. Farm. 2018, 28, 57–64. [Google Scholar] [CrossRef]
- Ding, Z.-S.; Jiang, F.-S.; Chen, N.-P.; Lv, G.-Y.; Zhu, C.-G. Isolation and identification of an anti-tumor component from leaves of impatiens balsamina. Molecules 2008, 13, 220–229. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lin, Y.-H. Anti-gastric adenocarcinoma activity of 2-Methoxy-1,4-naphthoquinone, an anti-Helicobacter pylori compound from Impatiens balsamina L. Fitoterapia 2012, 83, 1336–1344. [Google Scholar] [CrossRef]
- Kim, C.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Son, M.W.; Kim, K.H.; Lee, K.R. Two new phenolic compounds from the white flower of Impatiens balsamina. Phytochem. Lett. 2015, 14, 215–220. [Google Scholar] [CrossRef]
- Yang, X.; Summerhurst, D.K.; Koval, S.F.; Ficker, C.; Smith, M.L.; Bernards, M.A. Isolation of an antimicrobial compound from Impatiens balsamina L. using bioassay-guided fractionation. Phytother. Res. 2001, 15, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Yeganehzad, S.; Ptichkina, N.; Kodatsky, Y.; Kliukina, O.; Nepovinnykh, N.; Naji-Tabasi, S. Study on foaming, rheological and thermal properties of gelatin-free marshmallow. Food Hydrocoll. 2019, 93, 335–341. [Google Scholar] [CrossRef]
- Magalhães, A.L.T.D. Effect of Sucrose Substitutes on the Rheological and Sensory Characteristics of Aerated Sweet “Marshmallow” Type, Formulated with Guava Juice (Psidium guajava L.). Ph.D. Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2019. Available online: http://repositorio.unicamp.br/handle/REPOSIP/255074 (accessed on 5 February 2021).
- Periche, A.; Heredia, A.; Escriche, I.; Andrés, A.M.; Castelló, M.L. Potential use of isomaltulose to produce healthier marshmallows. LWT 2015, 62, 605–612. [Google Scholar] [CrossRef]
- Yudhistira, B.; Affandi, D.R.; Nusantari, P.N. Effect of green spinach (Amaranthus tricolor L.) and tomato (Solanum lycopersicum) addition in physical, chemical, and sensory properties of marshmallow as an alternative prevention of iron deficiency anemia. IOP Conf. Series Earth Environ. Sci. 2018, 102, 012007. [Google Scholar] [CrossRef]
- De Oliveira Melo, F.; Nascimento, R.S.; dos Santos, T.G.; de França Andrade, S.; Leite, K.S.; Neto, J.H.; Constant, P.B.L. Desenvolvimento de balas de gelatina de morango enriquecida com extrato de hibisco (Hibiscus Sabdarifa L.). Braz. J. Dev. 2020, 6, 47561–47571. [Google Scholar] [CrossRef]
- Ali, E.; Al-Askalany, S.; Ghandor, H. Evaluation of sensory, physicochemical changes of Marshmallow (Children Candy) by Addition Natural colors. Bull. Natl. Nutr. Inst. Arab. Repub. Egypt 2017, 50, 219–243. [Google Scholar] [CrossRef][Green Version]
- Artamonova, M.; Piliugina, I.; Samokhvalova, O.; Murlykina, N.; Kravchenko, O.; Fomina, I.; Grigorenko, A. Study of the properties of marshmallow with the sudanese rose and black chokeberry dyes upon storage. Eureka: Life Sci. 2017, 3, 15–23. [Google Scholar] [CrossRef][Green Version]
| BO | BP | p-Value | |
|---|---|---|---|
| Colour Parameters | |||
| L* | 46 ± 2 | 27 ± 1 | <0.001 |
| a* | 46 ± 2 | 45 ± 3 | 0.314 |
| b* | 53 ± 3 | 14 ± 1 | <0.001 |
| Nutritional Composition | |||
| Ash (g/100 g fw) | 0.26 ± 0.02 | 0.26 ± 0.01 | 0.376 |
| Protein (g/100 g fw) | 0.33 ± 0.01 | 0.315 ± 0.001 | 0.001 |
| Fat (g/100 g fw) | 0.13 ± 0.01 | 0.10 ± 0.01 | <0.001 |
| Carbohydrates (g/100 g fw) | 4.2 ± 0.1 | 4.76 ± 0.02 | <0.001 |
| Energy (kcal/100 g fw) | 19.2 ± 0.4 | 21.145 ± 0.003 | <0.001 |
| Energy (kJ/100 g fw) | 80 ± 2 | 88.53 ± 0.01 | <0.001 |
| Sugars | |||
| Fructose (g/100 g fw) | 0.866 ± 0.003 | 0.933 ± 0.001 | <0.001 |
| Glucose (g/100 g fw) | 1.23 ± 0.02 | 1.34 ± 0.01 | <0.001 |
| Total sugars (g/100 g fw) | 1.2 ± 0.02 | 1.34 ± 0.01 | <0.001 |
| Fatty Acids (%) | |||
| Caprylic Acid (C8:0) | 0.25 ± 0.01 | 0.11 ± 0.01 | <0.001 |
| Capric Acid (C10:0) | 0.65 ± 0.02 | 0.26 ± 0.01 | <0.001 |
| Undecylic Acid (C11:0) | 2.87 ± 0.04 | 0.90 ± 0.03 | <0.001 |
| Lauric acid (C12:0) | 0.77 ± 0.03 | 0.38 ± 0.02 | <0.001 |
| Tridecyl acid (C13:0) | 0.061 ± 0.002 | 0.048 ± 0.002 | <0.001 |
| Myristic acid (14:0) | 2.29 ± 0.06 | 1.28 ± 0.06 | <0.001 |
| Myristoleic acid (C14:1) | 0.97 ± 0.04 | 0.59 ± 0.03 | <0.001 |
| Pentadecenoic acid (C15:1) | 9.8 ± 0.1 | 9.2 ± 0.3 | <0.001 |
| Palmitic acid (C16:0) | 1.66 ± 0.07 | 1.04 ± 0.02 | <0.001 |
| Palmitoleic acid (C16:1) | 0.51 ± 0.01 | 0.43 ± 0.02 | <0.001 |
| cis-10-Heptadecenoic acid (C17:1) | 2.64 ± 0.06 | 2.4 ± 0.1 | <0.001 |
| Stearic acid (C18:0) | 31.65 ± 0.09 | 24.2 ± 0.2 | <0.001 |
| Linoleic acid (C18:2n6) | 20.8 ± 0.6 | 26.1 ± 0.3 | <0.001 |
| γ-linoleic acid (C18:3n6) | 14.4 ± 0.1 | 21.4 ± 0.4 | <0.001 |
| Linolenic acid (C18:3n3) | 0.56 ± 0.01 | 0.62 ± 0.01 | <0.001 |
| Arachidic acid (C20:0) | 0.35 ± 0.02 | 0.37 ± 0.01 | <0.001 |
| Eicosenoic acid (20:1) | 0.169 ± 0.002 | 0.15 ± 0.01 | <0.001 |
| Eicosadienoic acid (C20:2) | 0.31 ± 0.01 | 0.217 ± 0.008 | <0.001 |
| Eicosatrienoic acid (C20:3n3) | 3.9 ± 0.1 | 3.6 ± 0.2 | <0.001 |
| Dihomo-γ-linolenic acid (C20:3n6) | 0.073 ± 0.001 | 0.202 ± 0.003 | <0.001 |
| Behenic acid (C22:0) | 0.396 ± 0.004 | 2.18 ± 0.01 | <0.001 |
| Tricosanoic acid (C23:0) | 3.5 ± 0.2 | 3.3 ± 0.1 | <0.001 |
| Tetracosanoic acid (C24:0) | 0.491 ± 0.005 | 0.31 ± 0.01 | <0.001 |
| Tetracosenoic acid (C24:1) | 0.16 ± 0.01 | 0.18 ± 0.01 | <0.001 |
| SFA | 44.9 ± 0.5 | 34.37 ± 0.06 | <0.001 |
| MUFA | 14.13 ± 0.08 | 12.9 ± 0.5 | <0.001 |
| PUFA | 40.9 ± 0.6 | 52.7 ± 0.5 | <0.001 |
| Organic Acids (g/100 g fw) | |||
| Oxalic acid | 8.1 ± 0.7 | 6.13 ± 0.03 | <0.001 |
| Quinic acid | 13.4 ± 0.2 | 11.6 ± 0.2 | <0.001 |
| Malic acid | 16.7 ± 0.1 | 15.1 ± 0.3 | 0.336 |
| Succinic acid | 43.9 ± 0.9 | 59.8 ± 0.9 | <0.001 |
| Ascorbic acid | 40.4 ± 0.3 | 41.3 ± 0.5 | 0.002 |
| Total | 123 ± 2 | 134.0 ± 0.4 | <0.001 |
| Non-Antdocyanin Phenolic Compounds | |||||||
| Peak | Rt (min) | λmax (nm) | [M-H]− | Main Fragment ESI- MS2 [Intensity (Relative %)] | Tentative Identification | Quantification (mg/g) | |
| BO | BP | ||||||
| 1 | 7.99 | 309 | 325 | 307(5),265(76), 235(100),205(5),163(5) | O-p-Coumaroyl-α-hexoside | 0.52 ± 0.02 | 1.32 ± 0.05 * |
| 2 | 9.13 | 308 | 325 | 307(5),265(82), 235(100),205(5),163(5) | O-p-Coumaroyl-β-hexoside | 0.43 ± 0.02 | 1.04 ± 0.01 * |
| 3 | 10.15 | 324 | 517 | 311(15),269(100) | Apigenin-O-malonyl-hexoside | 1.175 ± 0.005 | 0.98 ± 0.02 * |
| 4 | 16.07 | 346 | 609 | 447(21),285(100) | Kaempherol-O-hexoside-O-hexoside | 1.92 ± 0.04 | 3.48 ± 0.02 * |
| 5 | 17.13 | 348 | 667 | 625(100),463(10),301(20) | Quercetin-acetyl-O-hexoside-O-hexoside | 0.9997 ± 0.0002 | 1.105 ± 0.003 * |
| 6 | 17.93 | 342 | 595 | 301(100) | Quercetin-O-hexosyl-pentoside | 1.27 ± 0.01 | 2.02 ± 0.01* |
| 7 | 20.3 | 346 | 651 | 609(100),447(8),285(53) | Kaempherol-O-acetylhexoside-O-hexoside | 2.23 ± 0.03 | 4.014 ± 0.004 * |
| 8 | 21.94 | 451 | 447 | 285(110) | Kaempherol-3-O-glucoside | 1.325 ± 0.005 | 1.804 ± 0.002 * |
| 9 | 23.82 | 342 | 543 | 431(28),285(100) | Kaempherol-O-hexoside-O-deoxyhexoside | 1.484 ± 0.002 | 1.43 ± 0.02 * |
| TPA | 0.95 ± 0.04 | 2.36 ± 0.04 * | |||||
| Tflav | 10.4 ± 0.07 | 14.843 ± 0.005 * | |||||
| TNAC | 11.4 ± 0.1 | 17.19 ± 0.04 * | |||||
| Antdocyanin Phenolic Compounds | |||||||
| Peak | Rt (min) | λmax (nm) | [H]+ | Main Fragment ESI- MS2 [Intensity (Relative %)] | Tentative Identification | Quantification (mg/g) | |
| BO | BP | ||||||
| 10 | 14.62 | 500 | 595 | 271(100) | Pelargonidin-O-dihexoside | 7.4 ± 0.5 | 1.2 ± 0.1 * |
| 11 | 21.01 | 501 | 637 | 475(30),271(100) | Pelargonidin-O-hexoside-O-acetylhexoside | 0.82 ± 0.01 | 0.29 ± 0.02 * |
| 12 | 34.04 | 506 | 741 | 579(100),271(12) | Pelargonidin-O-hexoside-O-deoxyhexosyl-hexoside | 0.38 ± 0.02 | 0.62 ± 0.03 * |
| 13 | 36.62 | 510 | 801 | 639(25),331(100) | Malvidin-O-coumaroylhexoside-O-hexoside isomer I | 0.25 ± 0.01 | 1 ± 0.1 * |
| 14 | 37.56 | 511 | 801 | 639(25),331(100) | Malvidin-O-coumaroylhexoside-O-hexoside isomer II | 0.26 ± 0.03 | 0.73 ± 0.09 * |
| 15 | 38.48 | 504 | 741 | 579(100),271(15) | Pelargonidin-O-hexoside-O-deoxyhexosyl-hexoside | 0.8 ± 0.1 | 0.0001 ± 0.00003 * |
| 16 | 39.73 | 510 | 801 | 331(100) | Malvidin-O-coumaroylhexoside-O-hexoside isomer III | 0.57 ± 0.02 | 2.7 ± 0.3 * |
| 17 | 40.28 | 509 | 783 | 579(100),475(34),271(25) | Pelargonidin-O-p-coumaroylhexoside-O-acetyl-hesoxide | 0.89 ± 0.09 | 1.3 ± 0.2 * |
| 18 | 41.11 | 511 | 813 | 609(100),301(14) | Peonidin-O-acetylhexoside-O-p-coumaroylhexoside | 0.43 ± 0.09c | 0.9 ± 0.2 * |
| 19 | 41.71 | 504 | 783 | 579(100),475(34),271(35) | Pelargonidin-O-p-coumaroylhexoside-O-acetyl-hexoside | 2.5 ± 0.2 | 1.5 ± 0.1 * |
| 20 | 42.7 | 511 | 843 | 639(100),331(34) | Malvidin-O-acetylhexoside-O-coumaroylhexoside | n.d. | 2.3 ± 0.1 * |
| 21 | 43.12 | 511 | 813 | 609(100),301(17) | Peonidin-O-acetylhexoside-O-coumaroylhexoside | 0.7 ± 0.1 | n.d. * |
| 22 | 43.35 | 511 | 639 | 331(100) | Malvidin-O-coumaroylhexoside | 0.8 ± 0.2 | n.d. * |
| 23 | 44.14 | 515 | 843 | 639(61),331(23) | Malvidin-O-acetylhexoside-O-coumaroylhexoside | n.d. | 6.4 ± 0.5 * |
| TAC | 15.7 ± 0.7 | 19 ± 1 * | |||||
| BO | BP | Positive Control | |||||
|---|---|---|---|---|---|---|---|
| Antioxidant activity (Ec50 values; µg/mL) | Trolox | ||||||
| Oxidative hemolysis inhibition assay (OxHLIA) | 42 ± 2 | 29 ± 2 * | 85.2 ± 2 | ||||
| Anti-inflammatory (GI50 values; µg/mL) | Dexamethasone | ||||||
| RAW264.7 | 281 ± 12 | 164 ± 7 * | 6.30 ± 0.4 | ||||
| Tumour cell lines (GI50 values; µg/mL) | Ellipticine | ||||||
| HeLa | 121 ± 3 | 90 ± 6 * | 1.03 ± 0.09 | ||||
| HepG2 | 201 ± 6 | 135 ± 9 * | 1.10 ± 0.09 | ||||
| MCF-7 | 253 ± 9 | 155 ± 15 * | 1.02 ± 0.02 | ||||
| NCI-H460 | 293 ± 12 | 167 ± 13 * | 1.01 ± 0.01 | ||||
| Non-tumour cell lines (GI50 values; µg/mL) | Ellipticine | ||||||
| PLP2 | >400 | >400 | 1.40 ± 0.1 | ||||
| Antibacterial activity | B.c. | S.a. | L.m. | E.c. | P.a. | S.t. | |
| BO | MIC | 0.10 | 0.20 | 0.20 | 0.05 | 0.10 | 0.20 |
| MBC | 0.20 | 0.40 | 0.40 | 0.10 | 0.20 | 0.40 | |
| BP | MIC | 0.05 | 0.20 | 0.20 | 0.075 | 0.20 | 0.20 |
| MBC | 0.10 | 0.40 | 0.40 | 0.10 | 0.40 | 0.40 | |
| Antifungal activity | A.fun. | A.v. | A.n. | P.f. | P.o | P.v.c | |
| BO | MIC | 0.012 | 0.025 | 0.012 | 0.012 | 0.006 | 0.025 |
| MFC | 0.025 | 0.05 | 0.025 | 0.025 | 0.012 | 0.05 | |
| BP | MIC | 0.025 | 0.025 | 0.025 | 0.025 | 0.012 | 0.025 |
| MFC | 0.05 | 0.05 | 0.05 | 0.05 | 0.025 | 0.05 | |
| Humidity (g/100 g) | Ash (g/100 g) | Protein (g/100 g) | Fat (g/100 g) | Carbohydrates (g/100 g) | Energy (Kcal) | Energy (Kj) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colourant Type (CT) | Control | 27 ± 2 | 0.174 ± 0.004 | 2.9 ± 0.2 | 0.066 ± 0.004 | 69 ± 2 | 290 ± 7 | 1214 ± 30 | ||||
| Strawberry | 20 ± 1 | 0.191 ± 0.002 | 2.2 ± 0.2 | 0.067 ± 0.003 | 77 ± 2 | 319 ± 5 | 1337 ± 23 | |||||
| Impatiens | 24 ± 3 | 0.182 ± 0.006 | 4.4 ± 0.1 | 0.06 ± 0.04 | 74 ± 2 | 311 ± 5 | 1301 ± 19 | |||||
| p-value (n = 27) | Tukey Test | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Time Interval (TI) | T0 | 25 ± 2 | 0.181 ± 0.006 | 3 ± 1 | 0.062 ± 0.003 | 74 ± 2 | 308 ± 8 | 1289 ± 33 | ||||
| T3 | 22 ± 3 | 0.187 ± 0.008 | 3 ± 1 | 0.069 ± 0.003 | 74 ± 4 | 310 ± 14 | 1296 ± 59 | |||||
| T7 | 25 ± 4 | 0.180 ± 0.001 | 3 ± 1 | 0.066 ± 0.003 | 73 ± 5 | 305 ± 18 | 1276 ± 75 | |||||
| p-value (n = 3) | Tukey Test | <0.001 | <0.001 | <0.001 | <0.001 | 0.009 | 0.177 | 0.178 | ||||
| TC×IT (n = 81) | p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| C16:0 (%) | C18:0 (%) | C18:1 (%) | C18:2 (%) | SFA (%) | MUFA (%) | PUFA (%) | Fructose | Glucose | Sucrose | |||
| Colourant Type (CT) | Control | 55 ± 4 | 16 ± 2 | 18 ± 2 | 10.7 ± 0.5 | 71 ± 2 | 18 ± 2 | 10.7 ± 0.5 | 12.8 ± 0.4 | 13.8 ± 0.3 | 33 ± 1 | |
| Strawberry | 49 ± 2 | 16.3 ± 0.7 | 25 ± 2 | 9 ± 1 | 65 ± 1 | 25 ± 2 | 10 ± 1 | 16.1 ± 0.6 | 16.8 ± 0.6 | 31 ± 1 | ||
| Impatiens | 56 ± 5 | 16 ± 2 | 18 ± 2 | 10 ± 1 | 72 ± 3 | 18 ± 2 | 10 ± 1 | 13.9 ± 0.7 | 15 ± 1 | 35 ± 2 | ||
| p-value (n = 27) | Teste Tukey | <0.001 | 0.008 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Time Interval (TI) | T0 | 56 ± 4 | 14.6 ± 0.9 | 19 ± 3 | 10.1 ± 0.9 | 71 ± 3 | 19 ± 3 | 10.1 ± 0.9 | 14 ± 1 | 14 ± 1 | 32 ± 2 | |
| T3 | 55 ± 4 | 15.9 ± 0.4 | 20 ± 4 | 9.2 ± 0.7 | 65 ± 1 | 20 ± 4 | 9.2 ± 0.7 | 14 ± 2 | 15 ± 1 | 33 ± 3 | ||
| T7 | 48 ± 2 | 18.2 ± 0.9 | 23 ± 3 | 10 ± 1 | 72 ± 3 | 23 ± 3 | 10 ± 1 | 15 ± 2 | 16 ± 1 | 34 ± 1 | ||
| p-value (n = 3) | Tukey Test | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| TC × IT (n = 81) | p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.005 | <0.001 | 0.007 | |
| OxHLIA | Control (BC) | Strawberry (BS) | Impatiens (BI) | Trolox | ||||||||
| (IC50, µg/mL) | T0 | w.a. | 124 ± 8 | 212 ± 29 | 8.8 ± 0.5 | |||||||
| T3 | w.a. | w.a. | 267 ± 222 | - | ||||||||
| T7 | w.a. | w.a. | 486 ± 57 | - | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, E.d.O., Jr.; Pereira, E.; Carocho, M.; Pereira, C.; Dias, M.I.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Garcia, C.C.; Ferreira, I.C.F.R.; et al. Study on the Potential Application of Impatiens balsamina L. Flowers Extract as a Natural Colouring Ingredient in a Pastry Product. Int. J. Environ. Res. Public Health 2021, 18, 9062. https://doi.org/10.3390/ijerph18179062
Pires EdO Jr., Pereira E, Carocho M, Pereira C, Dias MI, Calhelha RC, Ćirić A, Soković M, Garcia CC, Ferreira ICFR, et al. Study on the Potential Application of Impatiens balsamina L. Flowers Extract as a Natural Colouring Ingredient in a Pastry Product. International Journal of Environmental Research and Public Health. 2021; 18(17):9062. https://doi.org/10.3390/ijerph18179062
Chicago/Turabian StylePires, Eleomar de O., Jr., Eliana Pereira, Márcio Carocho, Carla Pereira, Maria Inês Dias, Ricardo C. Calhelha, Ana Ćirić, Marina Soković, Carolina C. Garcia, Isabel C. F. R. Ferreira, and et al. 2021. "Study on the Potential Application of Impatiens balsamina L. Flowers Extract as a Natural Colouring Ingredient in a Pastry Product" International Journal of Environmental Research and Public Health 18, no. 17: 9062. https://doi.org/10.3390/ijerph18179062
APA StylePires, E. d. O., Jr., Pereira, E., Carocho, M., Pereira, C., Dias, M. I., Calhelha, R. C., Ćirić, A., Soković, M., Garcia, C. C., Ferreira, I. C. F. R., Caleja, C., & Barros, L. (2021). Study on the Potential Application of Impatiens balsamina L. Flowers Extract as a Natural Colouring Ingredient in a Pastry Product. International Journal of Environmental Research and Public Health, 18(17), 9062. https://doi.org/10.3390/ijerph18179062















