Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae
Abstract
:1. Introduction
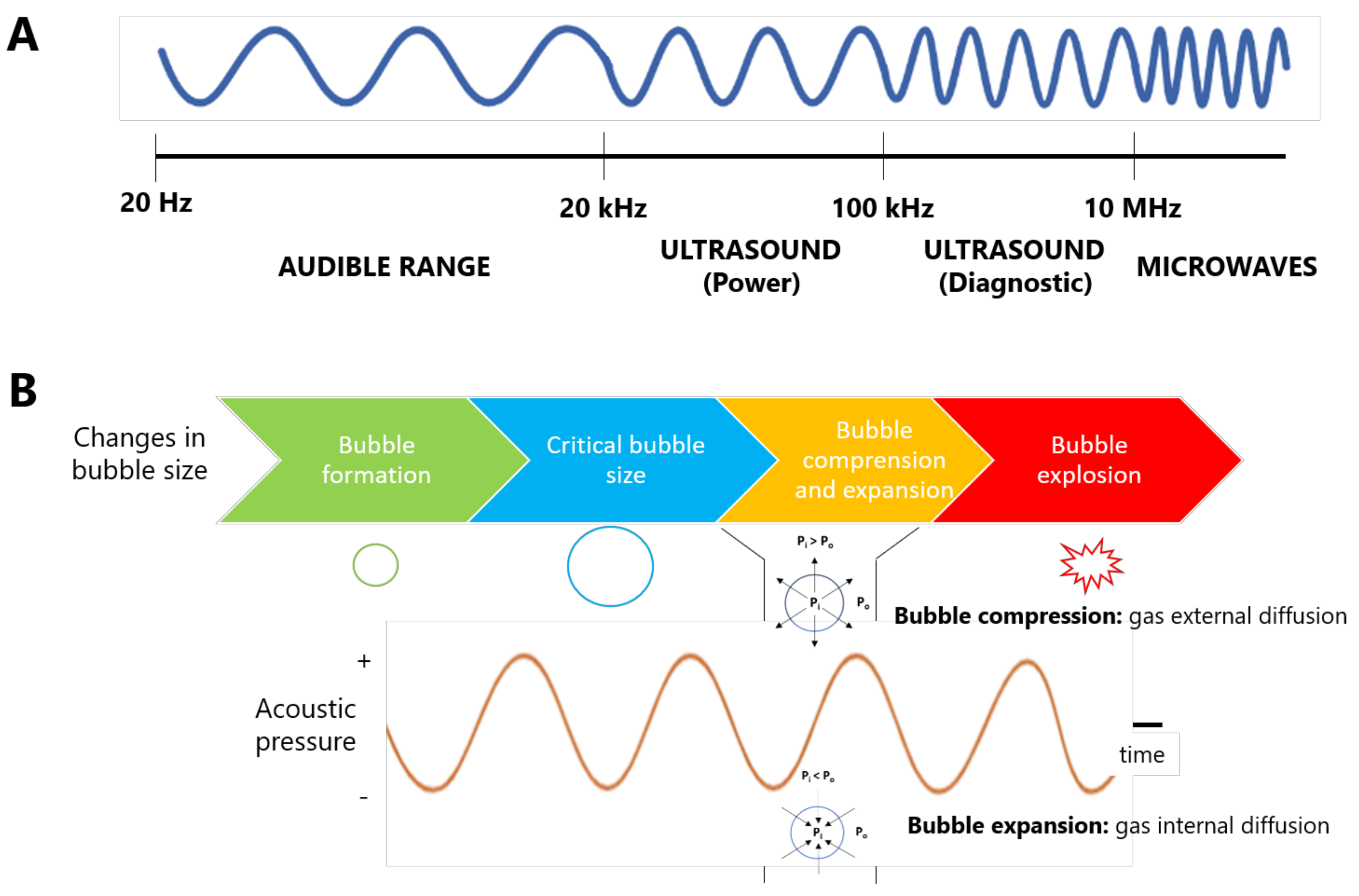
2. Ultrasound-Assisted Extraction (UAE)
2.1. Physicochemical Effects of UAE
2.2. UAE-Associated Mechanisms
- Fragmentation: It consists in the reduction of matrix particle size guided by the ultrasonic action. This mechanism is produced from the collision between particles and shockwaves which are created by the collapse of the bubbles in solution. Consequently, a reduction on solid particle size, increases the solid surface area to develop mass transfer, driving to better extraction yields [62].
- Erosion: It is based on the release of solid structures from the matrix into the extractive solvent, caused by the collapse of cavitation bubbles [62].
- Sonocapillary: The ultrasonic capillary effect involves an enhanced penetration of solvent into the canals and pores of the matrix [63], thus improving the extraction rate, as proved by Pingret et al. (2012), who observed that water holding capacity during the first 10 min of UAE was 70% higher than that of maceration [64].
- Detexturation: It involves the solid matrix destruction caused by ultrasounds [62].
- Sonoporation: This mechanism causes an increase in cell membranes permeability to help the release of intracellular products into the extractive medium, by forming membrane pores [65].
- Local shear stress: The application of ultrasonic waves to liquid media drives to the generation of shear forces onto the matrix surface, causing the later rupture of its structures and the extraction of inner compounds in the solvent [62].
2.3. Relevant Parameters Associated with UAE
2.3.1. Physical Parameters
2.3.2. Medium Parameters
2.3.3. Matrix Parameters
3. Marine Algae for the Recovery of Target BCs Using UAE
3.1. Proteins
3.2. Carbohydrates
3.3. Lipids
3.4. Pigments
3.5. Phenolic Compounds
3.6. Micronutrients
4. Combinatorial Approaches of UAE
4.1. UAE Combined with Conventional Techniques
4.2. UAE Combined with New Extraction Techniques
5. Comparison of Extraction Techniques
6. Evaluation of Ultrasound-Assisted Extraction (UAE) Application
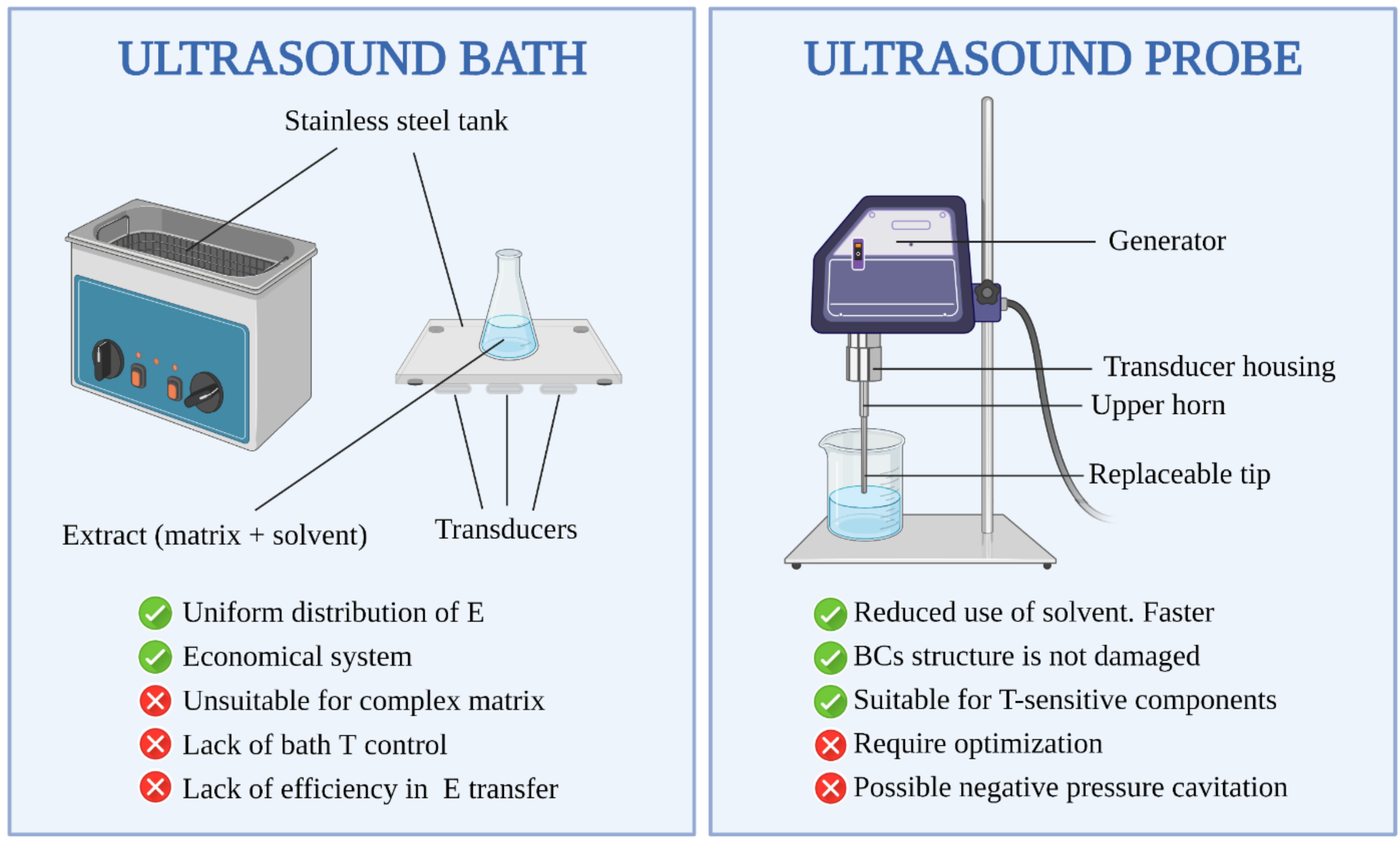
6.1. Benefits and Drawbacks of UAE Equipment
6.2. Environmental Impact of UAE
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| General Terms | |
| BCs | Bioactive compounds |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DW | Dry weight |
| GAE | Gallic acid equivalents |
| LCA | Life-cycle assessment |
| R-PC | R-phycocyanin |
| R-PE | R-phycoerythrin |
| T | Temperature |
| Ultrasound extraction related terms | |
| AC | Acoustic cavitation |
| Cp | Constant pressure |
| ET | Extraction time |
| m | Solvent mass |
| PB | Blake threshold pressure |
| R | Bubble radius |
| UAE | Ultrasound-assisted extraction |
| UI | Ultrasound intensity |
| Extraction techniques | |
| EAE | Enzymatic-assisted extraction |
| HAE | Hydrothermal-assisted extraction |
| MAE | Microwave-assisted extraction |
| PEF | Pulsed electric field-assisted extraction |
| PLE | Pressurized liquid extraction |
| SCFE | Supercritical fluid extraction |
| SLE | Solid-liquid extraction |
| UMAE | Ultrasounds-microwave-assisted extraction |
References
- Ozcan Cetin, E.H.; Cetin, M.S.; Özbay, M.B.; Yaman, N.M.; Könte, H.C.; Ekizler, F.A.; Tak, B.T.; Kara, M.; Temizhan, A.; Özcan, F.; et al. The other side of the medallion in heart failure: Reverse metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2041–2050. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and mediterranean diet: A review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [Green Version]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Adsorption of gallic acid, propyl gallate and polyphenols from Bryophyllum extracts on activated carbon. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Armenta, S.; Garrigues, S.; Esteve-Turrillas, F.A.; de la Guardia, M. Green extraction techniques in green analytical chemistry. TrAC Trends Anal. Chem. 2019, 116, 248–253. [Google Scholar] [CrossRef]
- Ummat, V.; Sivagnanam, S.P.; Rajauria, G.; O’Donnell, C.; Tiwari, B.K. Advances in pre-treatment techniques and green extraction technologies for bioactives from seaweeds. Trends Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a source of valuable antimicrobial compounds: Extraction and applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. The use of invasive algae species as a source of secondary metabolites and biological activities: Spain as case-study. Mar. Drugs 2021, 19, 178. [Google Scholar] [CrossRef]
- Pereira, L. Characterization of bioactive components in edible algae. Mar. Drugs 2020, 18, 65. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gutierrez, C.M.; Guerra-Rivas, G.; Soria-Mercado, I.E.; Ayala-Sánchez, N.E. Marine edible algae as disease preventers. Adv. Food Nutr. Res. 2011, 64, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef] [Green Version]
- Plaza, M.; Santoyo, S.; Jaime, L.; Avalo, B.; Cifuentes, A.; Reglero, G.; García-Blairsy Reina, G.; Señoráns, F.J.; Ibáñez, E. Comprehensive characterization of the functional activities of pressurized liquid and ultrasound-assisted extracts from Chlorella vulgaris. LWT Food Sci. Technol. 2012, 46, 245–253. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M.; Harun, M.R.; Omar, R.; Siajam, S.I. Subcritical water technology for extraction of phenolic compounds from Chlorella sp. microalgae and assessment on its antioxidant activity. Molecules 2017, 22, 1105. [Google Scholar] [CrossRef] [Green Version]
- Berneira, L.M.; de Santi, I.I.; da Silva, C.C.; Venzke, D.; Colepicolo, P.; de Vaucher, R.A.; dos Santos, M.A.Z.; de Pereira, C.M.P. Bioactivity and composition of lipophilic metabolites extracted from Antarctic macroalgae. Braz. J. Microbiol. 2021. [Google Scholar] [CrossRef]
- Khairy, H.M.; El-Sheikh, M.A. Antioxidant activity and mineral composition of three Mediterranean common seaweeds from Abu-Qir Bay, Egypt. Saudi J. Biol. Sci. 2015, 22, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Kolsi, R.B.A.; Fakhfakh, J.; Sassi, S.; Elleuch, M.; Gargouri, L. Physico-chemical characterization and beneficial effects of seaweed sulfated polysaccharide against oxydative and cellular damages caused by alloxan in diabetic rats. Int. J. Biol. Macromol. 2018, 117, 407–417. [Google Scholar] [CrossRef]
- Surayot, U.; You, S.G. Structural effects of sulfated polysaccharides from Codium fragile on NK cell activation and cytotoxicity. Int. J. Biol. Macromol. 2017, 98, 117–124. [Google Scholar] [CrossRef]
- Choi, J.I.; Kim, H.J. Preparation of low molecular weight fucoidan by gamma-irradiation and its anticancer activity. Carbohydr. Polym. 2013, 97, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.R.; Osborne, J.W. Bioactive compounds from Caulerpa racemosa as a potent larvicidal and antibacterial agent. Front. Biol. Beijing 2014, 9, 300–305. [Google Scholar] [CrossRef]
- Guzm, F.; Wong, G.; Rom, T.; Constanza, C. Identification of Antimicrobial Peptides from the Microalgae Tetraselmis suecica (Kylin) Butcher and. Mar. Drugs 2019, 17, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini-Filho, J.; Novoa, A.V.; González, A.E.B.; de Andrade-Wartha, E.R.S.; Portari Mancini, D.A. Free phenolic acids from the seaweed Halimeda monile with antioxidant effect protecting against liver injury. Zeitschrift Naturforschung C J. Biosci. 2009, 64, 657–663. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Patil, S.; Bhutia, S.K.; Jena, M. Enteromorpha compressa extract induces anticancer activity through apoptosis and autophagy in oral cancer. Mol. Biol. Rep. 2020, 47, 9567–9578. [Google Scholar] [CrossRef]
- Alves, C.; Pinteus, S.; Simões, T.; Horta, A.; Silva, J.; Tecelão, C.; Pedrosa, R. Bifurcaria bifurcata: A key macro-alga as a source of bioactive compounds and functional ingredients. Int. J. Food Sci. Technol. 2016, 51, 1638–1646. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A green approach for alginate extraction from Sargassum muticum brown seaweed using ultrasound-assisted technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef]
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksii using response surface methodology. J. Appl. Phycol. 2017, 29, 3161–3173. [Google Scholar] [CrossRef]
- Sivagnanam, S.P.; Yin, S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar. Drugs 2015, 13, 3422–3442. [Google Scholar] [CrossRef]
- Buedenbender, L.; Astone, F.A.; Tasdemir, D. Bioactive Molecular Networking for Mapping the Antimicrobial Constituents of the Baltic Brown Alga Fucus vesiculosus. Mar. Drugs 2020, 18, 311. [Google Scholar] [CrossRef] [PubMed]
- Cernadas, H.; Flórez-fernández, N.; González-mu, M.J.; Torres, M.D. Food and Bioproducts Processing Retrieving of high-value biomolecules from edible Himanthalia elongata brown seaweed using hydrothermal processing. Food Bioprod. Process. 2019, 7, 275–286. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Int. 2017, 99, 995–1001. [Google Scholar] [CrossRef]
- Raguraman, V.; Mubarakali, D.; Narendrakumar, G.; Thirugnanasambandam, R.; Kirubagaran, R.; Thajuddin, N. Unraveling rapid extraction of fucoxanthin from Padina tetrastromatica: Purification, characterization and biomedical application. Process Biochem. 2018, 73, 211–219. [Google Scholar] [CrossRef]
- Haslin, C.; Lahaye, M.; Pellegrini, M.; Chermann, J.C. In Vitro Anti-HIV Activity of Sulfated Cell-Wall Polysaccharides from Gametic, Carposporic and Tetrasporic Stages of the Mediterraean Red Alga Asparagopsis armata. Planta Med. 2001, 67, 301–305. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, Y.; Liu, K.; Hua, H.; Zhu, H.; Pei, Y. Gracilarioside and gracilamides from the Red alga Gracilaria asiatica. J. Nat. Prod. 2006, 69, 1488–1491. [Google Scholar] [CrossRef]
- Barceló-villalobos, M.; Figueroa, F.L.; Korbee, N. Production of Mycosporine-Like Amino Acids from Gracilaria vermiculophylla (Rhodophyta) Cultured Through One Year in an Integrated Multi-trophic Aquaculture (IMTA) System. Mar. Biotechnol. 2017, 19, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Topuz, O.K.; Gokoglu, N.; Yerlikaya, P.; Ucak, I.; Gumus, B. Optimization of Antioxidant Activity and Phenolic Compound Extraction Conditions from Red Seaweed (Laurencia obtuse). J. Aquat. Food Prod. Technol. 2015, 25, 414–422. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Kee, M.B.O.; Fitzgerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Harnedy, P.A.; FitzGerald, R.J. In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. J. Appl. Phycol. 2013, 25, 1793–1803. [Google Scholar] [CrossRef]
- Huang, C.; Chen, W.; Gao, Y.; Chen, G.; Lin, H.V. Enzyme-Assisted Method for Phycobiliproteins Extraction from Porphyra and Evaluation of Their Bioactivity. Processes 2021, 9, 560. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Identification of Bioactive Peptides with α_Amylase Inhibitory Potential from Enzymatic Protein Hydrolysates of Red Seaweed (Porphyra spp.). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Dey, S.; Rathod, V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yin, X.; Zeng, Q.; Zhu, L.; Chen, J. Isolation, structure, and surfactant properties of polysaccharides from Ulva lactuca L. from South China Sea. Int. J. Biol. Macromol. 2015, 79, 577–582. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring ultrasound, microwave and ultrasound-microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Vaquero, M.; O’Doherty, J.V.; Tiwari, B.K.; Sweeney, T.; Rajauria, G. Enhancing the extraction of polysaccharides and antioxidants from macroalgae using sequential hydrothermal-assisted extraction followed by ultrasound and thermal technologies. Mar. Drugs 2019, 17, 457. [Google Scholar] [CrossRef] [Green Version]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soulé, F.; Bhaw-Luximon, A.; Meilhac, O.; D’Hellencourt, C.L.; Jhurry, D.; Couprie, J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef]
- Rahimi, F.; Tabarsa, M.; Rezaei, M. Ulvan from green algae Ulva intestinalis: Optimization of ultrasound-assisted extraction and antioxidant activity. J. Appl. Phycol. 2016, 28, 2979–2990. [Google Scholar] [CrossRef]
- Vázquez-Rodríguez, B.; Gutiérrez-Uribe, J.A.; Antunes-Ricardo, M.; Santos-Zea, L.; Cruz-Suárez, L.E. Ultrasound-assisted extraction of phlorotannins and polysaccharides from Silvetia compressa (Phaeophyceae). J. Appl. Phycol. 2020, 32, 1441–1453. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Saien, J.; Daneshamoz, S. Experimental studies on the effect of ultrasonic waves on single drop liquid–liquid extraction. Ultrason. Sonochem. 2018, 40, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.; Ashokkumar, M. The physical and chemical effects of ultrasound. In Ultrasound Technologies for Food and Bioprocessing; Springer: New York, NY, USA, 2011; pp. 1–12. ISBN 9781441974716. [Google Scholar]
- Leong, T.; Ashokkumar, M.; Kentish, S. The growth of bubbles in an acoustic field by rectified diffusion. In Handbook of Ultrasonics and Sonochemistry; Springer: Singapore, 2016; pp. 69–98. ISBN 9789812872784. [Google Scholar]
- Young, F.R. Cavitation. McGraw-Hil.: London, UK, 1989. [Google Scholar]
- Leighton, T.G. The Acoustic Bubble; Academ: San Diego, CA, USA, 1994. [Google Scholar]
- Leong, T.; Ashokkumar, M.; Sandra, K. The fundamentals of power ultrasound—A review. Acoust. Aust. 2011, 39, 54–63. [Google Scholar]
- Lee, J.; Kentish, S.E.; Ashokkumar, M. The effect of surface-active solutes on bubble coalescence in the presence of ultrasound. J. Phys. Chem. B 2005, 109, 5095–5099. [Google Scholar] [CrossRef] [PubMed]
- Crum, L.A. Measurements of the growth of air bubbles by rectified diffusion. J. Acoust. Soc. Am. 1980, 68, 203–211. [Google Scholar] [CrossRef]
- Piyasena, P.; Mohareb, E.; McKellar, R.C. Inactivation of microbes using ultrasound: A review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef]
- Laborde, J.L.; Bouyer, C.; Caltagirone, J.P.; Gérard, A. Acoustic cavitation field prediction at low and high frequency ultrasounds. Ultrasonics 1998, 36, 581–587. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Mason, T.J. Sonochemistry. Encycl. Chem. Technol. 2007, 247, 1439–1445. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Malykh, V.N.; Petrov, G.S. On sonocapillary effect. In Proceedings of the 5th World Congress on Ultrasonics (WCU), Paris, France, 7–10 September 2003; pp. 435–438. [Google Scholar]
- Pingret, D.; Fabiano-Tixier, A.S.; Bourvellec, C.L.; Renard, C.M.G.C.; Chemat, F. Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. J. Food Eng. 2012, 111, 73–81. [Google Scholar] [CrossRef]
- Meullemiestre, A.; Breil, C.; Abert-Vian, M.; Chemat, F. Microwave, ultrasound, thermal treatments, and bead milling as intensification techniques for extraction of lipids from oleaginous Yarrowia lipolytica yeast for a biojetfuel application. Bioresour. Technol. 2016, 211, 190–199. [Google Scholar] [CrossRef]
- Toma, M.; Fukutomi, S.; Asakura, Y.; Koda, S. A calorimetric study of energy conversion efficiency of a sonochemical reactor at 500 kHz for organic solvents. Ultrason. Sonochem. 2011, 18, 197–208. [Google Scholar] [CrossRef]
- Mason, T.J.; Peters, D. An introduction to the uses of power ultrasound in chemistry. In Practical Sonochemistry; Sonochemistry; Oxford University Press: New York, NY, USA, 1999; pp. 1–48. [Google Scholar]
- Santos, H.M.; Lodeiro, C.; Capelo-Martinez, J.L. The Power of Ultrasound. In Ultrasound in Chemistry: Analytical Applications; Capelo-Martínez, J.-L., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 1–16. [Google Scholar]
- Sun, Y.; Liu, D.; Chen, J.; Ye, X.; Yu, D. Effects of different factors of ultrasound treatment on the extraction yield of the all-trans-β-carotene from citrus peels. Ultrason. Sonochem. 2011, 18, 243–249. [Google Scholar] [CrossRef]
- Esclapez, M.D.; Sáez, V.; Milán-Yáñez, D.; Tudela, I.; Louisnard, O.; González-García, J. Sonoelectrochemical treatment of water polluted with trichloroacetic acid: From sonovoltammetry to pre-pilot plant scale. Ultrason. Sonochem. 2010, 17, 1010–1020. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, X.; Wang, L.; Zhou, S.; Feng, H.; Chen, W.N.; Lau, R. Ultrasound assisted extraction of carbohydrates from microalgae as feedstock for yeast fermentation. Bioresour. Technol. 2013, 128, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Mahali, M.; Sibi, G. Extraction Methods and Functional Properties of Protein from Arthospira platensis for Bioavailability of Algal Proteins. Int. J. Pharm. Chem. 2019, 5, 20. [Google Scholar] [CrossRef]
- Orr, V.C.A.; Plechkova, N.V.; Seddon, K.R.; Rehmann, L. Disruption and Wet Extraction of the Microalgae Chlorella vulgaris Using Room-Temperature Ionic Liquids. ACS Sustain. Chem. Eng. 2016, 4, 591–600. [Google Scholar] [CrossRef]
- Lu, W.; Alam, M.A.; Pan, Y.; Wu, J.; Wang, Z.; Yuan, Z. A new approach of microalgal biomass pretreatment using deep eutectic solvents for enhanced lipid recovery for biodiesel production. Bioresour. Technol. 2016, 218, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Sališová, M.; Toma, Š.; Mason, T.J. Comparison of conventional and ultrasonically assisted extractions of pharmaceutically active compounds from Salvia officinalis. Ultrason. Sonochem. 1997, 4, 131–134. [Google Scholar] [CrossRef]
- García-Pérez, P.; Lozano-Milo, E.; Landín, M.; Gallego, P.P. Combining medicinal plant in vitro culture with machine learning technologies for maximizing the production of phenolic compounds. Antioxidants 2020, 9, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K. Effect of drying and extraction conditions on the recovery of bioactive compounds from Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 2947–2960. [Google Scholar] [CrossRef]
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Altena, I.A.V.; Scarlett, C.J. The Effects of Drying on Physico-Chemical Properties and Antioxidant Capacity of the Brown Alga (Hormosira banksii (Turner) Decaisne). J. Food Process. Preserv. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Menshova, R.V.; Anastyuk, S.D.; Ermakova, S.P.; Shevchenko, N.M.; Isakov, V.I.; Zvyagintseva, T.N. Structure and anticancer activity in vitro of sulfated galactofucan from brown alga Alaria angusta. Carbohydr. Polym. 2015, 132, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.A.; Da Silveira, J.M.; Vidal, É.M.; Ribeiro, N.T.; Veiga Burkert, C.A. Cell Disruption of Chaetoceros calcitrans by Microwave and Ultrasound in Lipid Extraction. Int. J. Chem. Eng. 2018. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.; Liu, N.; Zhang, J.; Yang, Q.; Hua, S.; Song, H.; Xia, C. Optimization of ultrasound-assisted extraction parameters of chlorophyll from Chlorella vulgaris residue after lipid separation using response surface methodology. J. Food Sci. Technol. 2014, 51, 2006–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debiagi, P.E.A.; Trinchera, M.; Frassoldati, A.; Faravelli, T.; Vinu, R.; Ranzi, E. Algae characterization and multistep pyrolysis mechanism. J. Anal. Appl. Pyrolysis 2017, 128, 423–436. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Perković, Z.P. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Parial, D.; Khatoon, N.; Chaudhuri, A.; Senroy, S. Effect of Formulated Algal Diet on growth performance of Labeo rohita Hamilton. J. Algal Biomass Util. 2011, 2, 1–9. [Google Scholar]
- Chakraborty, S.; Santra, S.C. Biochemical composition of eight benthic algae collected from Sunderban. Indian J. Mar. Sci. 2008, 37, 329–332. [Google Scholar]
- Jayshree, A.; Jayashree, S.; Thangaraju, N. Chlorella vulgaris and Chlamydomonas reinhardtii: Effective antioxidant, antibacterial and anticancer mediators. Indian J. Pharm. Sci. 2016, 78, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Darwish, R.; Gedi, M.A.; Akepach, P.; Assaye, H.; Zaky, A.S.; Gray, D.A. Chlamydomonas reinhardtii is a potential food supplement with the capacity to outperform Chlorella and Spirulina. Appl. Sci. 2020, 10, 6736. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Mustapa Kamal, S.M.; Harun, M.R.; Omar, R.; Siajam, S.I. Extraction of phenolic compounds from Chlorella sp. microalgae using pressurized hot water: Kinetics study. Biomass Convers. Biorefinery 2020, 1–9. [Google Scholar] [CrossRef]
- Velichkova, K.; Sirakov, I. Growth parameters, protein and photosynthetic pigment content of Chlorella vulgaris cultivated under photoautotrophic and mixotrophic conditions. Bulg. J. Agric. Sci. 2018, 24, 150–155. [Google Scholar]
- Shakya, R.; Adhikari, S.; Mahadevan, R.; Shanmugam, S.R.; Nam, H.; Hassan, E.B.; Dempster, T.A. Influence of biochemical composition during hydrothermal liquefaction of algae on product yields and fuel properties. Bioresour. Technol. 2017, 243, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Safafar, H.; Nørregaard, P.U.; Ljubic, A.; Møller, P.; Holdt, S.L.; Jacobsen, C. Enhancement of protein and pigment content in two Chlorella species cultivated on industrial process water. J. Mar. Sci. Eng. 2016, 4, 84. [Google Scholar] [CrossRef] [Green Version]
- Akköz, C.; Arslan, D.; Ünver, A.; Özcan, M.M.; Yilmaz, B. Chemical composition, total phenolic and mineral contents of Enteromorpha intestinalis (L.) kütz. and Cladophora glomerata (L.) kütz. seaweeds. J. Food Biochem. 2011, 35, 513–523. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological activities of two macroalgae from Adriatic coast of Montenegro. Saudi J. Biol. Sci. 2015, 22, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical constituents and biological activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef] [Green Version]
- Cofrades, S.; López-Lopez, I.; Bravo, L.; Ruiz-Capillas, C.; Bastida, S.; Larrea, M.T.; Jiménez-Colmenero, F. Nutritional and antioxidant properties of different brown and red Spanish edible seaweeds. Food Sci. Technol. Int. 2010, 16, 361–370. [Google Scholar] [CrossRef]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; El Majdoub, Y.O.; Kounnoun, A.; Miceli, N.; Taviano, M.F.; Mondello, L.; et al. The contribution of carotenoids, phenolic compounds, and flavonoids to the antioxidative properties of marine microalgae isolated from mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef] [Green Version]
- D’armas, H.; Jaramillo, C.; D’armas, M.; Echavarría, A.; Valverde, P. Proximate Composition of Several Macroalgae from the Coast of Salinas Bay, Ecuador. Rev. Biol. Trop. 2019, 67, 61–68. [Google Scholar]
- Warsidah, W.; Mega Sari Juane Sofiana, I.S.; Syarif Irwan Nurdiansyah, D. Phytochemical Screening, Total Phenolic Compounds and Antioxidant Activity of Tropical Brown Algae Padina pavonica L. from Kabung Island, West Kalimantan. Infobic 2021, 17. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bhattacharjee, P.; Satpati, G.G.; Pal, R. Spray dried extract of Phormidium valderianum as a promising source of natural antioxidant. Int. J. Food Sci. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardouin, K.; Bedoux, G.; Burlot, A.S.; Nyvall-Collén, P.; Bourgougnon, N. Enzymatic recovery of metabolites from seaweeds: Potential applications. In Advances in Botanical Research; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 71, pp. 279–320. [Google Scholar]
- Kepekçi, R.A.; Saygideger, S.D. Enhancement of phenolic compound production in Spirulina platensis by two-step batch mode cultivation. J. Appl. Phycol. 2012, 24, 897–905. [Google Scholar] [CrossRef]
- Satpati, G.G.; Pal, R. Biochemical composition and lipid characterization of marine green alga Ulva rigida-a nutritional approach. J. Algal Biomass Util. 2011, 2, 10–13. [Google Scholar]
- Vernès, L.; Abert-Vian, M.; El Maâtaoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of ultrasound for green extraction of proteins from spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar] [CrossRef]
- Sankaran, R.; Manickam, S.; Yap, Y.J.; Ling, T.C.; Chang, J.S.; Show, P.L. Extraction of proteins from microalgae using integrated method of sugaring-out assisted liquid biphasic flotation (LBF) and ultrasound. Ultrason. Sonochem. 2018, 48, 231–239. [Google Scholar] [CrossRef]
- Yucetepe, A.; Saroglu, O.; Daskaya-Dikmen, C.; Bildik, F.; Ozcelik, B. Optimisation of Ultrasound-Assisted Extraction of Protein from Spirulina platensis Using RSM. Food Technol. Econ. Eng. Phys. Prop. Czech J. Food Sci 2018, 36, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Zurano, A.; Morillas-España, A.; González-López, C.V.; Lafarga, T. Optimisation of Protein Recovery from Arthrospira platensis by Ultrasound-Assisted Isoelectric Solubilisation/Precipitation. Processes 2020, 8, 1586. [Google Scholar] [CrossRef]
- Cesário, M.T.; da Fonseca, M.M.R.; Marques, M.M.; de Almeida, M.C.M.D. Marine algal carbohydrates as carbon sources for the production of biochemicals and biomaterials. Biotechnol. Adv. 2018, 36, 798–817. [Google Scholar] [CrossRef] [PubMed]
- Priscilla de Souza, M.; Sanchez-Barrios, A.; Medianeira Rizzetti, T.; Brittes Benitez, L.; Hoeltz, M.; de Cassia de Souza Schneider, R.; de Farias Neves, F. Concepts and Trends for Extraction and Application of Microalgae Carbohydrates. In Microalgae-From Physiology to Application; IntechOpen: London, UK, 2020. [Google Scholar]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Lupatini, A.L.; de Oliveira Bispo, L.; Colla, L.M.; Costa, J.A.V.; Canan, C.; Colla, E. Protein and carbohydrate extraction from S. platensis biomass by ultrasound and mechanical agitation. Food Res. Int. 2017, 99, 1028–1035. [Google Scholar] [CrossRef]
- Jeon, B.H.; Choi, J.A.; Kim, H.C.; Hwang, J.H.; Abou-Shanab, R.A.I.; Dempsey, B.A.; Regan, J.M.; Kim, J.R. Ultrasonic disintegration of microalgal biomass and consequent improvement of bioaccessibility/bioavailability in microbial fermentation. Biotechnol. Biofuels 2013, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Eldalatony, M.M.; Kabra, A.N.; Hwang, J.H.; Govindwar, S.P.; Kim, K.H.; Kim, H.; Jeon, B.H. Pretreatment of microalgal biomass for enhanced recovery/extraction of reducing sugars and proteins. Bioprocess Biosyst. Eng. 2016, 39, 95–103. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 87–134. ISBN 9780857095121. [Google Scholar]
- Chen, Z.; Wang, L.; Qiu, S.; Ge, S. Determination of Microalgal Lipid Content and Fatty Acid for Biofuel Production. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Araujo, G.S.; Matos, L.J.B.L.; Fernandes, J.O.; Cartaxo, S.J.M.; Gonçalves, L.R.B.; Fernandes, F.A.N.; Farias, W.R.L. Extraction of lipids from microalgae by ultrasound application: Prospection of the optimal extraction method. Ultrason. Sonochem. 2013, 20, 95–98. [Google Scholar] [CrossRef]
- Natarajan, R.; Chen, X.; Lau, R. Ultrasound Applications in Lipid Extractions from Microalgae. Biomass Convers. Biorefinery 2020, 1–9. [Google Scholar] [CrossRef]
- Adam, F.; Abert-Vian, M.; Peltier, G.; Chemat, F. “Solvent-free” ultrasound-assisted extraction of lipids from fresh microalgae cells: A green, clean and scalable process. Bioresour. Technol. 2012, 114, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments content (Chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Tandeau De Marsac, N. Phycobiliproteins and phycobilisomes: The early observations. Photosynth. Res. 2003, 76, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, D.P.; Rech, R.; Marczak, L.D.F.; Mercali, G.D. Ultrasound as an alternative technology to extract carotenoids and lipids from Heterochlorella luteoviridis. Bioresour. Technol. 2017, 224, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Deenu, A.; Naruenartwongsakul, S.; Kim, S.M. Optimization and economic evaluation of ultrasound extraction of lutein from Chlorella vulgaris. Biotechnol. Bioprocess Eng. 2013, 18, 1151–1162. [Google Scholar] [CrossRef]
- Pereira, A.G.; Jimenez-Lopez, C.; Fraga, M.; Lourenço-Lopes, C.; García-Oliveira, P.; Lorenzo, J.M.; Perez-Lamela, C.; Prieto, M.A.; Simal-Gandara, J. Extraction, Properties, and Applications of Bioactive Compounds Obtained from Microalgae. Curr. Pharm. Des. 2020, 26, 1929–1950. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.T.; Jacobsen, C.; Holdt, S.L. Emerging technologies for the extraction of marine phenolics: Opportunities and challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of ultrasound frequency, extraction time and solvent for the recovery of polyphenols, phlorotannins and associated antioxidant activity from brown seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, M.C.; Moon, S.H.; Jeon, B.T.; Jeon, Y.J. Potential use of ultrasound in antioxidant extraction from Ecklonia cava. Algae 2013, 28, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Martínez–Hernández, G.B.; Castillejo, N.; del Carrión–Monteagudo, M.M.; Artés, F.; Artés-Hernández, F. Nutritional and bioactive compounds of commercialized algae powders used as food supplements. Food Sci. Technol. Int. 2018, 24, 172–182. [Google Scholar] [CrossRef]
- Dhargalkar, V.K.; Verlecar, X.N. Southern Ocean seaweeds: A resource for exploration in food and drugs. Aquaculture 2009, 287, 229–242. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, S.K. Nutritional and digestive health benefits of seaweed. In Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 64, pp. 17–28. ISBN 9780123876690. [Google Scholar]
- Kumar, M.; Gupta, V.; Kumari, P.; Reddy, C.R.K.; Jha, B. Assessment of nutrient composition and antioxidant potential of Caulerpaceae seaweeds. J. Food Compos. Anal. 2011, 24, 270–278. [Google Scholar] [CrossRef]
- Zheng, L.; Wen, G.; Yuan, M.; Gao, F. Ultrasound-Assisted Extraction of Total Flavonoids from Corn Silk and Their Antioxidant Activity. J. Chem. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Kadam, S.U.; Donnell, C.P.O.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Plaza, M.; Šnóblová, M.; Lojková, L. Development of new efficient method for isolation of phenolics from sea algae prior to their rapid resolution liquid chromatographic–tandem mass spectrometric determination. J. Pharm. Biomed. Anal. 2017, 135, 87–96. [Google Scholar] [CrossRef]
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štěrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef] [PubMed]
- Tzima, K.; Brunton, N.P.; Lyng, J.G.; Frontuto, D.; Rai, D.K. The effect of Pulsed Electric Field as a pre-treatment step in Ultrasound Assisted Extraction of phenolic compounds from fresh rosemary and thyme by-products. Innov. Food Sci. Emerg. Technol. 2021, 69, 102644. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Zeng, X.A.; Rahaman, A.; Siddeeg, A.; Aadil, R.M.; Ahmed, Z.; Li, J.; Niu, D. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. J. Food Sci. Technol. 2019, 56, 2355–2364. [Google Scholar] [CrossRef]
- Wiyarno, B.; Yunus, R.M.; Mel, M. Extraction of algae oil from Nannocloropsis sp.: A study of Soxhlet and Ultrasonic-Assisted Extractions. J. Appl. Sci. 2011, 11, 3607–3612. [Google Scholar] [CrossRef]
- Pereira, T.; Barroso, S.; Mendes, S.; Amaral, R.A.; Dias, J.R.; Baptista, T.; Saraiva, J.A.; Alves, N.M.; Gil, M.M. Optimization of phycobiliprotein pigments extraction from red algae Gracilaria gracilis for substitution of synthetic food colorants. Food Chem. 2020, 321, 126688. [Google Scholar] [CrossRef]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3. [Google Scholar] [CrossRef]
- Nascentes, C.C.; Korn, M.; Arruda, M.A.Z. A fast ultrasound-assisted extraction of Ca, Mg, Mn and Zn from vegetables. Microchem. J. 2001, 69, 37–43. [Google Scholar] [CrossRef]
- Nascentes, C.C.; Korn, M.; Sousa, C.S.; Arruda, M.A.Z. Use of Ultrasonic Baths for Analytical Applications: A New Approach for Optimisation Conditions. J. Braz. Chem. Soc. 2001, 12, 57–63. [Google Scholar] [CrossRef]
- Güney, M.; Elik, A. Comparison of Probe with Bath Ultrasonic Leaching Procedures for Preparation to Heavy Metal Analysis of Bio-Collectors Prior to Atomic Absorption Spectrometry. Commun. Soil Sci. Plant Anal. 2017, 48, 1741–1752. [Google Scholar] [CrossRef]
- Bimakr, M.; Ganjloo, A.; Zarringhalami, S.; Ansarian, E. Ultrasound-assisted extraction of bioactive compounds from Malva sylvestris leaves and its comparison with agitated bed extraction technique. Food Sci. Biotechnol. 2017, 26, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Lavilla, I.; Bendicho, C. Fundamentals of Ultrasound-Assisted Extraction. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–316. ISBN 9780128096154. [Google Scholar]
- Panda, D.; Manickam, S. Cavitation technology-the future of greener extraction method: A review on the extraction of natural products and process intensification mechanism and perspectives. Appl. Sci. 2019, 9, 766. [Google Scholar] [CrossRef] [Green Version]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Dieu, V.; Vauchel, P.; Pradal, D.; Dimitrov, K. Reduction of environmental impacts of caffeine extraction from guarana by using ultrasound assistance. Food Bioprod. Process. 2021, 127, 266–275. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gomez-Barrio, L.P.; Zhao, M.; Tiwari, B.; Knutsen, S.H.; Ballance, S.; Zobel, H.K.; Nilsson, A.E.; Krewer, C.; Östergren, K.; et al. Alternative protocols for the production of more sustainable agar-based extracts from Gelidium sesquipedale. Algal Res. 2021, 55, 102254. [Google Scholar] [CrossRef]
- Berbel, J.; Posadillo, A. Review and Analysis of Alternatives for the Valorisation of Agro-Industrial Olive Oil By-Products. Sustainability 2018, 10, 237. [Google Scholar] [CrossRef] [Green Version]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-products of agri-food industry as tannin-rich sources: A review of tannins’ biological activities and their potential for valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef]
- Miranda, I.; Simões, R.; Medeiros, B.; Nampoothiri, K.M.; Sukumaran, R.K.; Rajan, D.; Pereira, H.; Ferreira-Dias, S. Valorization of lignocellulosic residues from the olive oil industry by production of lignin, glucose and functional sugars. Bioresour. Technol. 2019, 292. [Google Scholar] [CrossRef]
- Barbulova, A.; Colucci, G.; Apone, F. New trends in cosmetics: By-products of plant origin and their potential use as cosmetic active ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Del Mar Contreras, M.; Romero, I.; Moya, M.; Castro, E. Olive-derived biomass as a renewable source of value-added products. Process Biochem. 2020, 97, 43–56. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart advanced solvents for bioactive compounds recovery from agri-food by-products: A review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.; Alves, R.C.; Oliveira, M.B.P.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Phyllum | Algae | Main BCs | Biological Activity | Ref. |
|---|---|---|---|---|
| Chlorophyta | Chlorella vulgaris | Carotenoids, fatty acids, polyphenols | Antioxidant, antimicrobial | [15,16] |
| Ulva intestinalis | Lipophilic compounds | Antioxidant, antimicrobial | [17] | |
| Ulva lactuca | Carotenoids, polyphenols | Antioxidant | [18] | |
| Codium fragile | Polysaccharides, pigments | Antioxidant, anticancer | [19,20,21] | |
| Caulerpa racemosa | Fatty acid methyl esters, | Antibacterial, larvicidal | [22] | |
| Tetraselmis suecica | Peptides | Antimicrobial | [23] | |
| Halimeda monile | Polyphenols | Antioxidant, liver-protective | [24] | |
| Enteromorpha compressa | Polyphenols | Antioxidant | [25] | |
| Ochrophyta | Bifurcaria bifurcata | Polyphenols, diterpenes | Antioxidant, antimicrobial, anticancer | [26] |
| Sargassum muticum | Polysaccharides | Anticancer | [27] | |
| Hormosira banksii | Polyphenols | Antioxidant | [28] | |
| Saccharina japonica, Sargassum horneri | Fatty acids, polyphenols, pigments | Antioxidant, antimicrobial, antihypertension | [29] | |
| Fucus vesiculosus | Galactolipids, polyphenols | Antimicrobial | [30] | |
| Himanthalia elongata | Polysaccharides, polyphenols | Antitumoral, antioxidant | [31,32] | |
| Padina tetrastromatica | Pigments | Antioxidant, cytoprotective effects | [33] | |
| Rhodophyta | Jania rubens, Pterocladia capillacea | Carotenoids, polyphenols | Antioxidant | [18] |
| Asparagopsis armata | Polysaccharides | Antiviral | [34] | |
| Gracialaria vermiculophylla | Lipids, proteins | Anticancer, antioxidant | [35,36] | |
| Laurencia obtusa | Polyphenols | Antioxidant | [37] | |
| Curdiea racovitzae | Lipophilic compounds | Antimicrobial | [17] | |
| Palmaria palmata | Proteins | Antioxidant, cardioprotective, anti-diabetic | [38,39] | |
| Porphyra sp. | Proteins, pigments | Antioxidant, anti-diabetic, anticancer, anti-hypertensive | [40,41] |
| Algae | Proteins | Carbohydrates | Lipids | Pigments | Phenolic Compounds | Minerals | Ref. |
|---|---|---|---|---|---|---|---|
| Arthrospira platensis | 65.2% | 5.9% | 10.1% | - | - | 3.2% | [83] |
| Bifurcaria birfurcata | 8.6% | - | 5.8% | - | 9.6 mg PGE/g | - | [26,84] |
| Catenella repens | 9.3% | 32.2% | 9.5% | 0.18% | - | - | [85] |
| Catenella repens | 8.4% | 29.0% | 5.3% | 5.6 mg/g | - | - | [86] |
| Chaetomorpha ligustica | 40.9% | 22.3% | 4.1% | 6.1 mg/g | - | - | [86] |
| Chlamydomonas reinhardtii | 46% | 22% | 24% | 35.5 mg/g | 150 mg GAE/g | 4% | [87,88] |
| Chlorella spp. | 17.9% | 48.1% | 16.3% | 10.8 g/L | 58.2 mg GAE/g | 2.7% | [83,89,90] |
| Chlorella spp. | 14.6% | 49.7% | 30.3% | 39.5 mg/g | 58.2 mg GAE/g | 3.0% | [89,91,92] |
| Dictyota ceylinica | 3.3% | 18.5% | 2.6% | 5.4 mg/g | 0.08% | - | [84,86] |
| Enteromorpha intestinalis | 13.1% | 52.3% | 4.6% | 0.06% | 0.03 mg GAE/g | 1.92% | [85,93] |
| Enteromorpha intestinalis | 6.2% | 30.6% | 7.1% | - | 0.41 mg PE/mg | 1.92% | [86,93,94] |
| Fucus spp. | 1–17% | 66–26% | 0.4–5% | - | 28.2–204.2 mg PGE/g | - | [84,95] |
| Himanthalia elongata | 5% | - | 1.5% | - | 151.3 mg GAE/g | - | [84,96] |
| Nannochloropsis spp. | 39.3% | 6.5% | 15.4% | - | 33.2 mg GAE/g | 5.4% | [83,97] |
| Nannochloropsis spp. | 18.2% | 16.0% | 49.3% | - | 33.2 mg GAE/g | 7.4% | [91,97] |
| Padina pavonica | 5.6% | 43.4% | 0.4% | - | 20.3 mg GAE/g | 24.9% | [98,99] |
| Phormidium valderianum | 25.6% | 3.2% | 3.2% | 0.15% | 0.97 mg GAE/g | - | [85,100] |
| Polysiphonia mollis | 16.6% | 25.8% | 5.8% | 2.6 mg/g | - | - | [86] |
| Porphyridium spp. | 38.8% | 13.0% | 12.0% | - | - | 5.3% | [83] |
| Rhizoclonium riparium | 21.1% | 15.3% | 3.4% | 4.6 mg/g | - | - | [86] |
| Sargassum spp. | 9–20% | 4–68% | 0.5–3.9% | - | 1.68% | - | [84,101] |
| Spirulina platensis | 61.5% | 5.7% | 2.6% | 0.08% | 2.4–5.0 mg GAE/g | - | [85,102] |
| Spirulina platensis | 52% | 23% | 14% | 9.4 mg/g | - | 10% | [88] |
| Ulva lactuca | 8.5% | 35.3% | 4.4% | 5.0 mg/g | 0.30–0.45% | - | [18,86] |
| Ulva lactuca | 5.5% | 45.5% | 0.3% | - | 0.30–0.45% | 27.0% | [18,88] |
| Ulva rigida | 6.6% | 22.0% | 12.0% | 21% | 23% | - | [103] |
| Source | Compounds | Extraction Approach | Yield | Ref. |
|---|---|---|---|---|
| UAE combined with conventional techniques | ||||
| Ulva lactuca | Polysaccharides (ULP1, ULP2) | SLE (2% NaOH, 90 °C, 5 h) + UAE (1 h) | 17.57% | [44] |
| Kappaphycus alvarezii and Euchema aenticulatum. | Carrageenans | SLE (water: 10 g/L, pH 7) + UAE (90 °C, 150 W, 15 min) | 50–55% | [47] |
| Fucus vesiculosus | Phlorotannins | SLE (50% ethanol) + UAE | 568.9 ± 9.9 mg PGE/g | [49] |
| Sargassum muticum | Alginate | UAE + Sonication (3% alkali and 93% ethanol, 86 °C). | 13.6% | [27] |
| Gelidium pusillum | Phycobiliproteins (R-PE and R-PC) | UAE + Maceration | 77% R-PE and 93% R-PC | [137] |
| UAE combined with new extraction techniques | ||||
| Ascophyllum nodosum | FSPs/Soluble carbohydrates/Phenolics | UMAE (UAE +MAE) | 3.5 g F/100 g DM/10.4 g G eq/100 g DM/2.6 g GA eq/100 g DM | [45] |
| Cystoseira abies-marina, Undaria pinnatifida, Sargassum muticum, Chondrus crispus | Phenolic compounds, (PTC, 3,4-HB, p-HBA, p-CA, VA, p-HB, CA, SY, VN, P-CHA, FA, SA, GA, SIA). | UAE + Ika Ultra-Turrax® | 4.31 µg/g | [138] |
| UAE + PLE | 11.8 µg/g | |||
| Sargassum muticum, Sargassum vulgare, Hypnea spinella, Porphyra sp., Undaria pinnatifida, Chondrus crispus, Halopytis incurvus | 8 Isoflavones (Di, Geni, Ono, Dai, Sis, Gen, For, Bio). | UAE-SCFE | Up to 230 ng/g (Geni) and 100% recovery in C. crispus | [139]. |
| Ascophyllum. nodosum | F | UAE (30 min) + HAE (30 min) | 2.97 g F/100 g DM | [46] |
| Laminaria hyperborea | Gl | UAE (15 min) + HAE (30 min) | 0.9 g Gl/100 g DM | [46] |
| Algae | Compound | Extraction Techniques | Conditions | Recovery | Ref. |
|---|---|---|---|---|---|
| A. platensis | Proteins | UAE | W, RT, 60 min | 84% | [73] |
| Alkali extraction | 1 M NaOH, RT, 15 min | 75% | |||
| EAE | NaH2PO3 buffer, 1% cellulase, 50 °C, 180 min | 81% | |||
| Thermal extraction | 120 °C, 5 min | 64% | |||
| MAE | W, 1000 W, 3 min | 79% | |||
| A. nodosum | FSPs, total soluble carbohydrates | UAE | 0.1 M HCl, RT, 5 min, 20 kHz, 500 W, 50% sonication amplitude | 195.4 mg F/100 g DM/2573 mg G eq./100 g DM | [45] |
| MAE | 0.1 M HCl, 1000 W, 5 min, 2450 MHz | 1699.8 mg F/100 g DM/3317.4 mg G eq./100 g DM | |||
| UMAE | 0.1 M HCl, 100 W, 5 min, 20 kHz, 100% sonication amplitude, 2450 MHz | 3.5 g F/100 g DM | |||
| 0.1 M HCl, 600 W, 5 min, 20 kHz, 100% sonication amplitude, 2450 MHz | 10.4 g G eq/100 g DM | ||||
| L. hyperborea | Laminarin | UAE | 0.1 M HCl, RT, 15 min, 20 kHz | 6.2% | [136] |
| SLE | W, 70 °C, 150 min | 4.3% | |||
| A. nodosum | UAE | 0.1 M HCl, RT, 15 min, 20 kHz | 5.8% | ||
| SLE | W, 70 °C, 150 min | 4.6% | |||
| Nanochloropsis sp. | Free fatty acids | UAE | 98% EtOH, 69.62 °C, 5 min, 20 kHz, 500 W, 50% sonication amplitude | 7% | [142] |
| Soxhlet | 70% EtOH, 200 min | 9.4% | |||
| A. nodosum | Phenolic compounds | UAE | 0.1 M HCl, RT, 5 min, 20 kHz, 500 W, 50% sonication amplitude | 2340.5 mg GA eq./100 g DM | [45] |
| MAE | 0.1 M HCl, 600 W, 5 min, 2450 MHz | 1790.9 mg GA eq./100 g DM | |||
| UMAE | 0.1 M HCl, 100 W, 5 min, 20 kHz, 100% sonication amplitude, 2450 MHz | 2605.89 mg GA eq./100 g DM | |||
| L. hyperborea | Phenolic compounds | UAE | W, RT, 15 min, 20 kHz | 0.4% | [136] |
| SLE | W, 70 °C, 150 min | 0.4% | |||
| A. nodosum | UAE | W, RT, 15 min, 20 kHz | 0.16% | ||
| SLE | W, 70 °C, 150 min | 0.17% | |||
| H. banksii | Phenolic compounds | UAE | 70% EtOH, 30 °C, 60 min, 150 W | 23.1 mg/g DM | [28] |
| SLE | 70% EtOH, 30 °C, 12 h | 16.2 mg/g DM | |||
| E. cava | Phenolic compounds | UAE | 50% MeOH, 30 °C, 12 h, 40 kHZ, 200 W | 6.2 g/100 g DM | [128] |
| SLE | 50% MeOH, 24 h | 6.4 g/100 g DM | |||
| G. pusillum | Phycobiliproteins (R-PE and R-PC) | UAE | 0.1 M PBS, 30 °C, 10 min, 120 µm sonication amplitude | 0.16 mg/g DM/0.11 mg/g DM | [137] |
| Serial extraction | 0.1 M PBS, 4 °C, 60 min (repeated) | 2.0 mg/g DM/1.28 mg/g DM | |||
| SLE + UAE | 0.1 M PBS, RT, 45 min; 10 min, 120 µm sonication amplitude | 1.6 mg/g DM/1.2 mg/g DM | |||
| Homogenization + UAE | 0.1 M PBS, 35 °C, 45 min, 15,000 RPM; 10 min, 120 µm sonication amplitude | 1.4 mg/g DM/0.9 mg/g DM | |||
| Homogenization | 0.1 M PBS, 35 °C, 45 min, 15,000 RPM | 1.3 mg/g DM/0.8 mg/g DM | |||
| SLE | 0.1 M PBS, RT, 45 min | 1.2 mg/g DM/0.8 mg/g DM | |||
| G. gracilis | R-PE | UAE | 0.26 M PBS; 10 min, 45 kHz, 400 W | 1.6 mg/g DM | [143] |
| SLE | 0.1 M PBS, RT, 10 min | 3.6 mg/g DM | |||
| HPAE | 0.1 M PBS, 5 min, 300 MPa | 1.3 mg/g DM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. https://doi.org/10.3390/ijerph18179153
Carreira-Casais A, Otero P, Garcia-Perez P, Garcia-Oliveira P, Pereira AG, Carpena M, Soria-Lopez A, Simal-Gandara J, Prieto MA. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. International Journal of Environmental Research and Public Health. 2021; 18(17):9153. https://doi.org/10.3390/ijerph18179153
Chicago/Turabian StyleCarreira-Casais, Anxo, Paz Otero, Pascual Garcia-Perez, Paula Garcia-Oliveira, Antia G. Pereira, Maria Carpena, Anton Soria-Lopez, Jesus Simal-Gandara, and Miguel A. Prieto. 2021. "Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae" International Journal of Environmental Research and Public Health 18, no. 17: 9153. https://doi.org/10.3390/ijerph18179153
APA StyleCarreira-Casais, A., Otero, P., Garcia-Perez, P., Garcia-Oliveira, P., Pereira, A. G., Carpena, M., Soria-Lopez, A., Simal-Gandara, J., & Prieto, M. A. (2021). Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. International Journal of Environmental Research and Public Health, 18(17), 9153. https://doi.org/10.3390/ijerph18179153











