Soy and Frequent Dairy Consumption with Subsequent Equol Production Reveals Decreased Gut Health in a Cohort of Healthy Puerto Rican Women
Abstract
1. Introduction
2. Materials and Methods
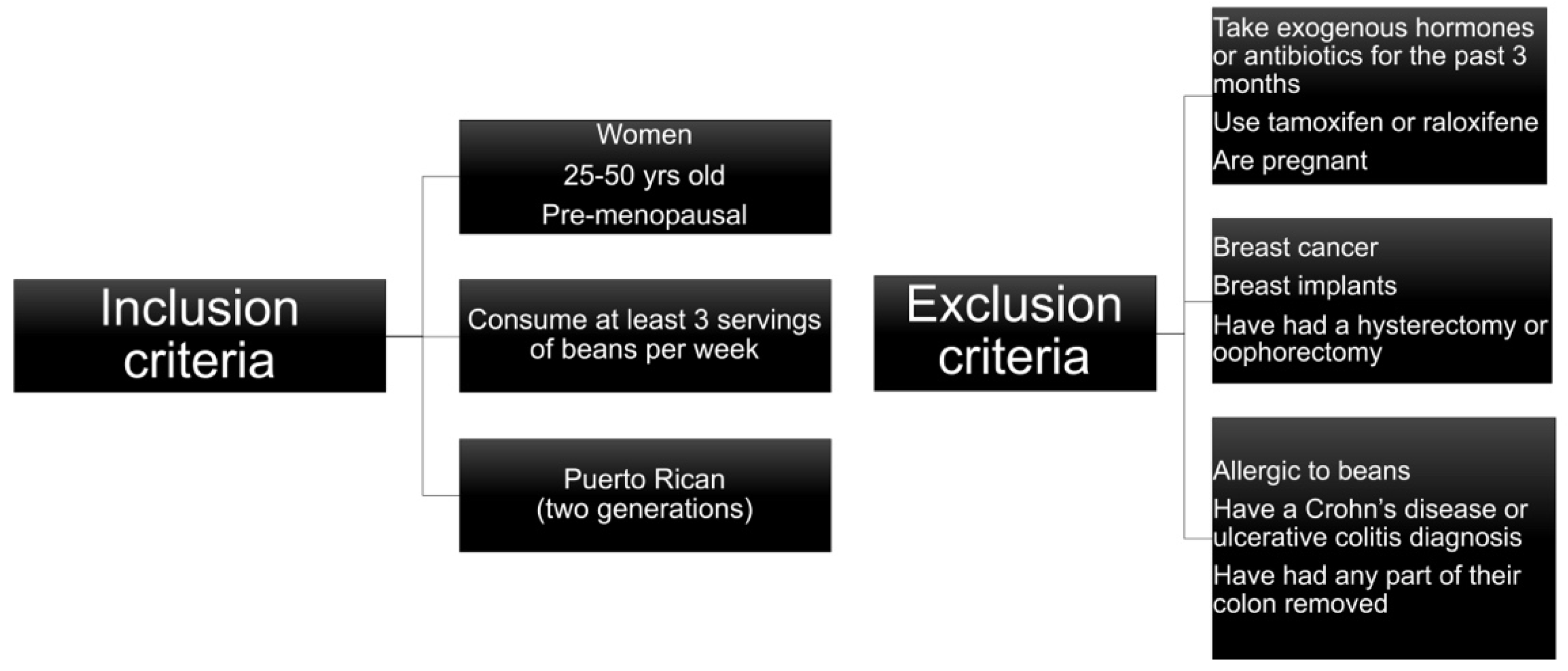
2.1. Study Subjects
2.2. Lifestyle Questionnaire
2.3. Urine Concentration of Metabolites
2.4. Gut Microbiota Data Production and Analysis by Next Generation Sequencing (NGS)
2.4.1. Genomic DNA Extractions from Fecal Samples
2.4.2. 16S rDNA Amplifications and Illumina Sequencing
2.4.3. Read QC and Bioinformatic Analyses
2.5. Statistical Analysis
2.6. Ethics Statement
3. Results
3.1. Demographic, Anthropometric, Lifestyle and Dietary Factors
3.2. Urinary Metabolites
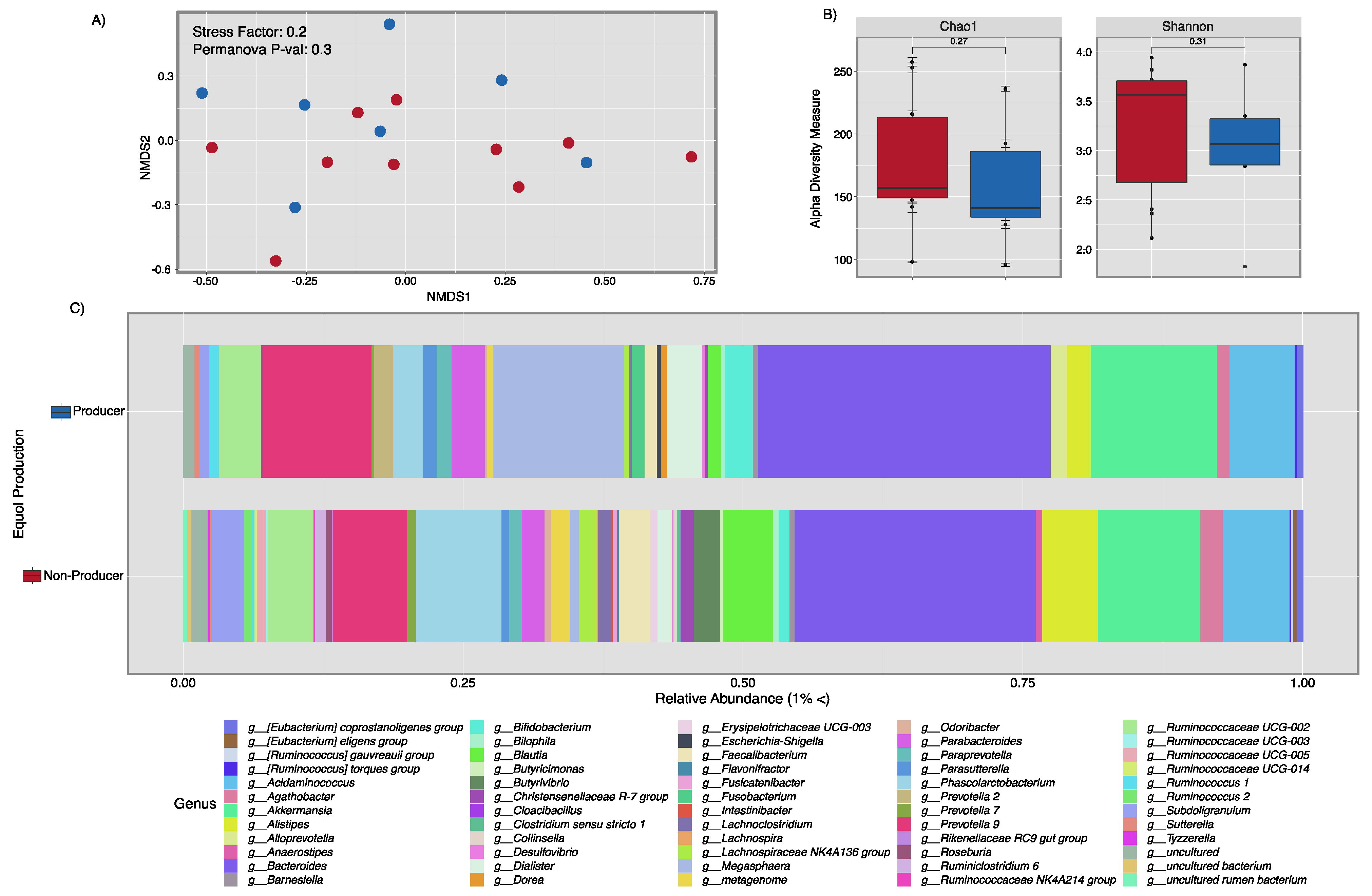
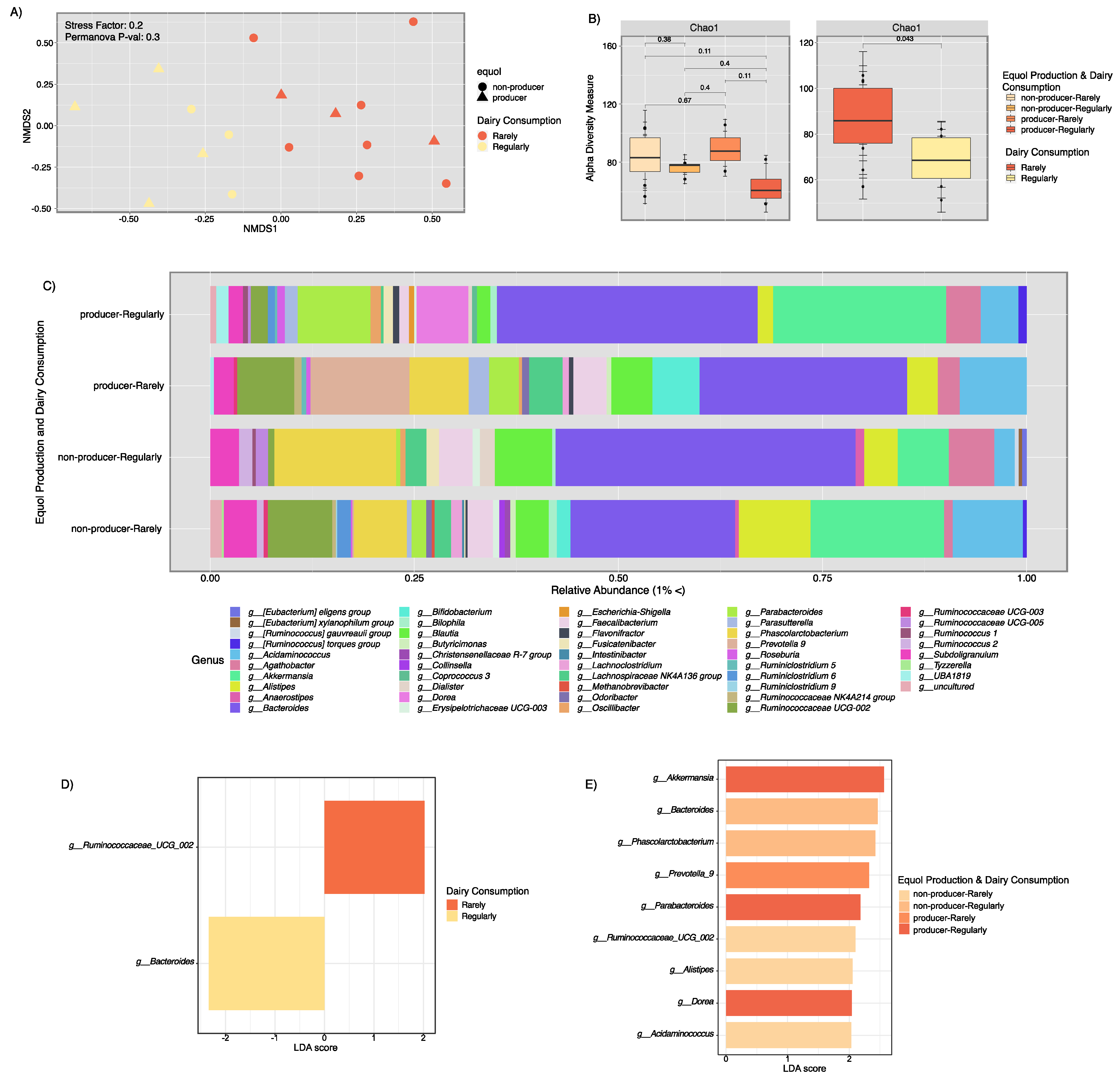
3.3. Microbial Composition Analysis and Metabolic Pathway Inference Results from Fecal Samples of Healthy Puerto Rican Women Correlated with Equol Production and Soy Consumption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nwosu, O.K.; Ubaoji, K.I. Nutraceuticals: History, Classification and Market Demand. In Functional Foods and Nutraceuticals; Egbuna, C., Dable-Tupas, G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; p. 642. [Google Scholar]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans; U.S. Department of Health and Human Services and U.S. Department of Agriculture: Washington, DC, USA, 2015.
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef]
- Godoy-Vitorino, F. Human microbial ecology and the rising new medicine. Ann. Transl. Med. 2019, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Lee, Y.K. Microflora of the Gastrointestinal Tract: A Review. Methods Mol. Biol. 2004, 268, 491–502. [Google Scholar] [CrossRef]
- Adlercreutz, H. Phytoestrogens: Epidemiology and a possible role in cancer protection. Environ. Health Perspect. 1995, 103 (Suppl. S7), 103–112. [Google Scholar] [PubMed]
- Rafii, F. The Role of Colonic Bacteria in the Metabolism of the Natural Isoflavone Daidzin to Equol. Metabolites 2015, 5, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montemayor, M.M.; Otero-Franqui, E.; Martinez, J.; De La Mota-Peynado, A.; Cubano, L.A.; Dharmawardhane, S. Individual and combined soy isoflavones exert differential effects on metastatic cancer progression. Clin. Exp. Metastasis 2010, 27, 465–480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De la Parra, C.; Otero-Franqui, E.; Martinez-Montemayor, M.; Dharmawardhane, S. The soy isoflavone equol may increase cancer malignancy via up-regulation of eukaryotic protein synthesis initiation factor eIF4G. J. Biol. Chem. 2012, 287, 41640–41650. [Google Scholar] [CrossRef]
- Patisaul, H.B. Endocrine disruption by dietary phyto-oestrogens: Impact on dimorphic sexual systems and behaviours. Proc. Nutr. Soc. 2016, 76, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, S.; Halaby, R. Dietary Isoflavones and Breast Cancer Risk. Medicines 2017, 4, 18. [Google Scholar] [CrossRef]
- Ahmad, A.; Ramasamy, K.; Majeed, A.B.; Mani, V. Enhancement of beta-secretase inhibition and antioxidant activities of tempeh, a fermented soybean cake through enrichment of bioactive aglycones. Pharm. Biol. 2015, 53, 758–766. [Google Scholar] [CrossRef]
- Koo, J.; Cabarcas-Petroski, S.; Petrie, J.L.; Diette, N.; White, R.J.; Schramm, L. Induction of proto-oncogene BRF2 in breast cancer cells by the dietary soybean isoflavone daidzein. BMC Cancer 2015, 15, 905. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Ko, B.S.; Ryuk, J.; Liu, M.; Zhang, W.; Park, S. Equol Decreases Hot Flashes in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food 2019, 22, 127–139. [Google Scholar] [CrossRef]
- Yoshikata, R.; Myint, K.Z.Y.; Ohta, H. Effects of Equol Supplement on Bone and Cardiovascular Parameters in Middle-Aged Japanese Women: A Prospective Observational Study. J. Altern. Complement. Med. 2018, 24, 701–708. [Google Scholar] [CrossRef]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Ihara, M.; Lopez, O.; Kakuta, C.; Lopresti, B.; Higashiyama, A.; Aizenstein, H.; Chang, Y.F.; Mathis, C.; Miyamoto, Y.; et al. Effect of S-equol and Soy Isoflavones on Heart and Brain. Curr. Cardiol. Rev. 2019, 15, 114–135. [Google Scholar] [CrossRef]
- Dixon, R.A.; Pasinetti, G. Flavonoids and Isoflavonoids: From Plant Biology to Agriculture and Neuroscience. Plant. Physiol. 2010, 154, 453–457. [Google Scholar] [CrossRef]
- Kim, E.; Woo, M.S.; Qin, L.; Ma, T.; Beltran, C.D.; Bao, Y.; Bailey, J.A.; Corbett, D.; Ratan, R.R.; Lahiri, D.K.; et al. Daidzein Augments Cholesterol Homeostasis via ApoE to Promote Functional Recovery in Chronic Stroke. J. Neurosci. 2015, 35, 15113–15126. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Fu, G.; Shen, J.; Shen, K.; Xu, Z.; Wang, Y.; Jin, B.; Pan, H. Ameliorative Effect of Daidzein on Cisplatin-Induced Nephrotoxicity in Mice via Modulation of Inflammation, Oxidative Stress, and Cell Death. Oxidative Med. Cell. Longev. 2017, 2017, 3140680. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D. The history and basic science development of soy isoflavones. Menopause 2017, 24, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Gaya, P.; Medina, M.; Sanchez-Jimenez, A.; Landete, J.M. Phytoestrogen Metabolism by Adult Human Gut Microbiota. Molecules 2016, 21, 1034. [Google Scholar] [CrossRef]
- Marrian, G.F.; Haslewood, G.A.D. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares’ urine. Biochem. J. 1932, 26, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Axelson, M.; Kirk, D.N.; Farrant, R.D.; Cooley, G.; Lawson, A.M.; Setchell, K.D. The identification of the weak oestrogen equol [7-hydroxy-3-(4′-hydroxyphenyl)chroman] in human urine. Biochem. J. 1982, 201, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Common, R.; Ainsworth, L. Identification of equol in the urine of the domestic fowl. Biochim. Biophys. Acta (BBA) Bioenerg. 1961, 53, 403–404. [Google Scholar] [CrossRef]
- Macrae, H.F.; Dale, D.G.; Common, R.H. Formation in vivo of 16-epiestriol and 16-ketoestradiol-17 beta from estriol by the laving hen and occurrence of equol in hen’s urine and feces. Can. J. Biochem. Physiol. 1960, 38, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355s–1362s. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Wähälä, K.; Adlercreutz, H. Identification of isoflavone metabolites dihydrodaidzein, dihydrogenistein, 6′-OH-O-dma, and cis-4-OH-equol in human urine by gas chromatography-mass spectroscopy using authentic reference compounds. Anal. Biochem. 1999, 274, 211–219. [Google Scholar] [CrossRef]
- Bannwart, C.; Adlercreutz, H.; Fotsis, T.; Wähälä, K.; Hase, T.; Brunlow, G. Identification of O-desmethylangolensin, a metabolite of daidzein, and of matairesinol, one likely plant precursor of the animal lignan enterolactone, in human urine. Finn. Chem. Lett. 1984, 1984, 120–125. [Google Scholar]
- Bannwart, C.; Adlercreutz, H.; Wähälä, K.; Kotiaho, T.; Hesso, A.; Brunow, G.; Hase, T. Identification of the phyto-oestrogen 3′,7-dihydroxyisoflavan, an isomer of equol, in human urine and cow’s milk. Biomed. Environ. Mass Spectrom. 1988, 17, 1–6. [Google Scholar] [CrossRef]
- Kuhnle, G.G.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen Content of Foods of Animal Origin: Dairy Products, Eggs, Meat, Fish, and Seafood. J. Agric. Food Chem. 2008, 56, 10099–10104. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-Equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Newton, K.M.; Bowles, E.J.A.; Yong, M.; Lampe, J.W. Demographic, anthropometric, and lifestyle factors and dietary intakes in relation to daidzein-metabolizing phenotypes among premenopausal women in the United States. Am. J. Clin. Nutr. 2008, 87, 679–687. [Google Scholar] [CrossRef]
- Iino, C.; Shimoyama, T.; Iino, K.; Yokoyama, Y.; Chinda, D.; Sakuraba, H.; Fukuda, S.; Nakaji, S. Daidzein Intake Is Associated with Equol Producing Status through an Increase in the Intestinal Bacteria Responsible for Equol Production. Nutrients 2019, 11, 433. [Google Scholar] [CrossRef]
- Rowland, I.R.; Wiseman, H.; Sanders, T.A.; Adlercreutz, H.; Bowey, E.A. Interindividual Variation in Metabolism of Soy Isoflavones and Lignans: Influence of Habitual Diet on Equol Production by the Gut Microflora. Nutr. Cancer 2000, 36, 27–32. [Google Scholar] [CrossRef]
- Atkinson, C.; Frankenfeld, C.; Lampe, J.W. Gut Bacterial Metabolism of the Soy Isoflavone Daidzein: Exploring the Relevance to Human Health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.-H. The antioxidant activity of daidzein metabolites, O-desmethylangolensin and equol, in HepG2 cells. Mol. Med. Rep. 2013, 9, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.L.; Jackson, E.A.; Churchill, L.; Lampe, J.W.; Leung, K.; Ockene, J.K. Impact of dose, frequency of administration, and equol production on efficacy of isoflavones for menopausal hot flashes: A pilot randomized trial. Menopause 2013, 20, 936–945. [Google Scholar] [CrossRef] [PubMed]
- De la Parra, C.; Borrero-Garcia, L.D.; Cruz-Collazo, A.; Schneider, R.J.; Dharmawardhane, S. Equol, an isoflavone metabolite, regulates cancer cell viability and protein synthesis initiation via c-Myc and eIF4G. J. Biol. Chem. 2015, 290, 6047–6057. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Belosay, A.; Hartman, J.A.; Song, H.; Zhang, Y.; Wang, W.; Doerge, D.R.; Helferich, W.G. Dietary soy isoflavones increase metastasis to lungs in an experimental model of breast cancer with bone micro-tumors. Clin. Exp. Metastasis 2015, 32, 323–333. [Google Scholar] [CrossRef]
- Rowland, I.; Wiseman, H.; Sanders, T.; Adlercreutz, H.; Bowey, E. Metabolism of oestrogens and phytoestrogens: Role of the gut microflora. Biochem. Soc. Trans. 1999, 27, 304–308. [Google Scholar] [CrossRef]
- Frankenfeld, C.L. O-desmethylangolensin: The importance of equol’s lesser known cousin to human health. Adv. Nutr. 2011, 2, 317–324. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.-H. O-desmethylangolensin inhibits the proliferation of human breast cancer MCF-7 cells by inducing apoptosis and promoting cell cycle arrest. Oncol. Lett. 2013, 6, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L.; Atkinson, C.; Wahala, K.; Lampe, J.W. Obesity prevalence in relation to gut microbial environments capable of producing equol or O-desmethylangolensin from the isoflavone daidzein. Eur. J. Clin. Nutr. 2014, 68, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.M.; Lampe, J.W.; Newton, K.M.; Gundersen, G.; Fuller, S.; Reed, S.D.; Frankenfeld, C.L. Being overweight or obese is associated with harboring a gut microbial community not capable of metabolizing the soy isoflavone daidzein to O- desmethylangolensin in peri- and post-menopausal women. Maturitas 2017, 99, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Skor, H.E.; Fitzgibbons, E.D.; Scholes, D.; Chen, C.; Wähälä, K.; Schwartz, S.M.; Lampe, J.W. Overnight urinary isoflavone excretion in a population of women living in the United States, and its relationship to isoflavone intake. Cancer Epidemiol. Biomark. Prev. 2002, 11, 253–260. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Godoy-Vitorino, F.; Romaguera, J.; Zhao, C.; Vargas-Robles, D.; Ortiz-Morales, G.; Vazquez-Sanchez, F.; Sanchez-Vazquez, M.; de la Garza-Casillas, M.; Martinez-Ferrer, M.; White, J.R.; et al. Cervicovaginal Fungi and Bacteria Associated with Cervical Intraepithelial Neoplasia and High-Risk Human Papillomavirus Infections in a Hispanic Population. Front. Microbiol. 2018, 9, 2533. [Google Scholar] [CrossRef]
- Gonzalez, A.; Navas-Molina, J.A.; Kosciolek, T.; McDonald, D.; Vázquez-Baeza, Y.; Ackermann, G.; DeReus, J.; Janssen, S.; Swafford, A.D.; Orchanian, S.B.; et al. Qiita: Rapid, web-enabled microbiome meta-analysis. Nat. Methods 2018, 15, 796–798. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1963. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Guadamuro, L.; Dohrmann, A.B.; Tebbe, C.C.; Mayo, B.; Delgado, S. Bacterial communities and metabolic activity of faecal cultures from equol producer and non-producer menopausal women under treatment with soy isoflavones. BMC Microbiol. 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mäkelä, T.; Hase, T.; Adlercreutz, H.; Kurzer, M.S. Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes. J. Steroid Biochem. Mol. Biol. 1994, 50, 205–212. [Google Scholar] [CrossRef]
- Wang, L.-Q. Mammalian phytoestrogens: Enterodiol and enterolactone. J. Chromatogr. B 2002, 777, 289–309. [Google Scholar] [CrossRef]
- Frankenfeld, C.L. Dairy consumption is a significant correlate of urinary equol concentration in a representative sample of US adults. Am. J. Clin. Nutr. 2011, 93, 1109–1116. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J. Microbiome and Gut Dysbiosis. Exp. Suppl. 2018, 109, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Cady, N.; Peterson, S.R.; Freedman, S.N.; Mangalam, A.K. Beyond Metabolism: The Complex Interplay Between Dietary Phytoestrogens, Gut Bacteria, and Cells of Nervous and Immune Systems. Front. Neurol. 2020, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, L.; Flórez, A.B.; Verbruggen, S.; Redruello, B.; Verhoeven, J.; Venema, K.; Mayo, B. Modulation of equol production via different dietary regimens in an artificial model of the human colon. J. Funct. Foods 2020, 66, 103819. [Google Scholar] [CrossRef]
- Guadamuro, L.; Azcárate-Peril, M.A.; Tojo, R.; Mayo, B.; Delgado, S. Use of high throughput amplicon sequencing and ethidium monoazide dye to track microbiota changes in an equol-producing menopausal woman receiving a long-term isoflavones treatment. AIMS Microbiol. 2019, 5, 102–116. [Google Scholar] [CrossRef]
- Hughes, R.L.; Kable, M.E.; Marco, M.; Keim, N.L. The Role of the Gut Microbiome in Predicting Response to Diet and the Development of Precision Nutrition Models. Part II: Results. Adv. Nutr. 2019, 10, 979–998. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-C.; Lee, C.-H.; Wang, H. Exploring the Association of Autism Spectrum Disorders and Constipation through Analysis of the Gut Microbiome. Int. J. Environ. Res. Public Health 2021, 18, 667. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Saier, M.H. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Brown, K.; DeCoffe, D.; Molcan, E.; Gibson, D.L. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 2012, 4, 1095–1119. [Google Scholar] [CrossRef]


| Demographics and Anthropometrics | Mean ± SD |
|---|---|
| Age (years) | 38.9 ± 7.8 |
| Menarche (years) | 12.3 ± 1.9 |
| BMI | 29.7 ± 8.1 |
| Education (highest degree earned) | % |
| Elementary School | 1.3 |
| Intermediate School | 16.3 |
| High School | 10 |
| University | 72.5 |
| Income (per year) | |
| <$10,000 | 37.5 |
| $20,000–$30,000 | 40 |
| $30,000–$40,000 | 10 |
| >$50,000 | 7.5 |
| Smoker | |
| No | 67.9 |
| Yes | 32.1 |
| Constipation | |
| No | 31.6 |
| Yes | 61.4 |
| Exercise | |
| No | 52.5 |
| Yes | 47.5 |
| Medical Insurance | |
| No | 5 |
| Yes | 95 |
| Type of Insurance | |
| Government | 31 |
| Private | 69 |
| Breast Cancer Family History | |
| No | 66.3 |
| Yes | 27.5 |
| Do not know | 6.3 |
| Food | Not Consume (%) | Consume (%) |
|---|---|---|
| Beans | 13.8 | 86.2 |
| Dairy | 2.5 | 96.3 |
| Fruit | 13.8 | 86.2 |
| Meat | 3.8 | 96.2 |
| Soy | 58.8 | 41.2 |
| Vegetables | 15.0 | 85.0 |
| Metabolite | Metabolite Correlated to | r | p Value |
|---|---|---|---|
| ODMA | Enterodiol | 0.287 | 0.0100 |
| Enterolactone | 0.071 | 0.5320 | |
| Daidzein | 0.744 | 0.0001 | |
| DHD | 0.000 | 0.9990 | |
| Equol | 0.487 | 0.0001 | |
| Genistein | 0.885 | 0.0001 | |
| Daidzein | Enterodiol | 0.054 | 0.6370 |
| Enterolactone | −0.019 | 0.8650 | |
| DHD | 0.284 | 0.0001 | |
| Equol | 0.151 | 0.1810 | |
| Genistein | 0.635 | 0.0001 | |
| Equol | Enterodiol | 0.463 | 0.0001 |
| Enterolactone | 0.145 | 0.2010 | |
| DHD | −0.034 | 0.7630 | |
| Genistein | 0.605 | 0.0001 | |
| Genistein | Enterodiol | 0.434 | 0.0100 |
| Enterolactone | 0.102 | 0.3670 | |
| DHD | 0.074 | 0.5150 | |
| Enterodiol | Enterolactone | 0.573 | 0.0001 |
| DHD | 0.000 | 0.9990 | |
| Enterolactone | DHD | −0.058 | 0.6120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacourt-Ventura, M.Y.; Vilanova-Cuevas, B.; Rivera-Rodríguez, D.; Rosario-Acevedo, R.; Miranda, C.; Maldonado-Martínez, G.; Maysonet, J.; Vargas, D.; Ruiz, Y.; Hunter-Mellado, R.; et al. Soy and Frequent Dairy Consumption with Subsequent Equol Production Reveals Decreased Gut Health in a Cohort of Healthy Puerto Rican Women. Int. J. Environ. Res. Public Health 2021, 18, 8254. https://doi.org/10.3390/ijerph18168254
Lacourt-Ventura MY, Vilanova-Cuevas B, Rivera-Rodríguez D, Rosario-Acevedo R, Miranda C, Maldonado-Martínez G, Maysonet J, Vargas D, Ruiz Y, Hunter-Mellado R, et al. Soy and Frequent Dairy Consumption with Subsequent Equol Production Reveals Decreased Gut Health in a Cohort of Healthy Puerto Rican Women. International Journal of Environmental Research and Public Health. 2021; 18(16):8254. https://doi.org/10.3390/ijerph18168254
Chicago/Turabian StyleLacourt-Ventura, Mercedes Y., Brayan Vilanova-Cuevas, Delmarie Rivera-Rodríguez, Raysa Rosario-Acevedo, Christine Miranda, Gerónimo Maldonado-Martínez, Johanna Maysonet, Darlene Vargas, Yelitza Ruiz, Robert Hunter-Mellado, and et al. 2021. "Soy and Frequent Dairy Consumption with Subsequent Equol Production Reveals Decreased Gut Health in a Cohort of Healthy Puerto Rican Women" International Journal of Environmental Research and Public Health 18, no. 16: 8254. https://doi.org/10.3390/ijerph18168254
APA StyleLacourt-Ventura, M. Y., Vilanova-Cuevas, B., Rivera-Rodríguez, D., Rosario-Acevedo, R., Miranda, C., Maldonado-Martínez, G., Maysonet, J., Vargas, D., Ruiz, Y., Hunter-Mellado, R., Cubano, L. A., Dharmawardhane, S., Lampe, J. W., Baerga-Ortiz, A., Godoy-Vitorino, F., & Martínez-Montemayor, M. M. (2021). Soy and Frequent Dairy Consumption with Subsequent Equol Production Reveals Decreased Gut Health in a Cohort of Healthy Puerto Rican Women. International Journal of Environmental Research and Public Health, 18(16), 8254. https://doi.org/10.3390/ijerph18168254







