Making Biodegradable Seedling Pots from Textile and Paper Waste—Part B: Development and Evaluation of Seedling Pots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bio-Composite Sheets
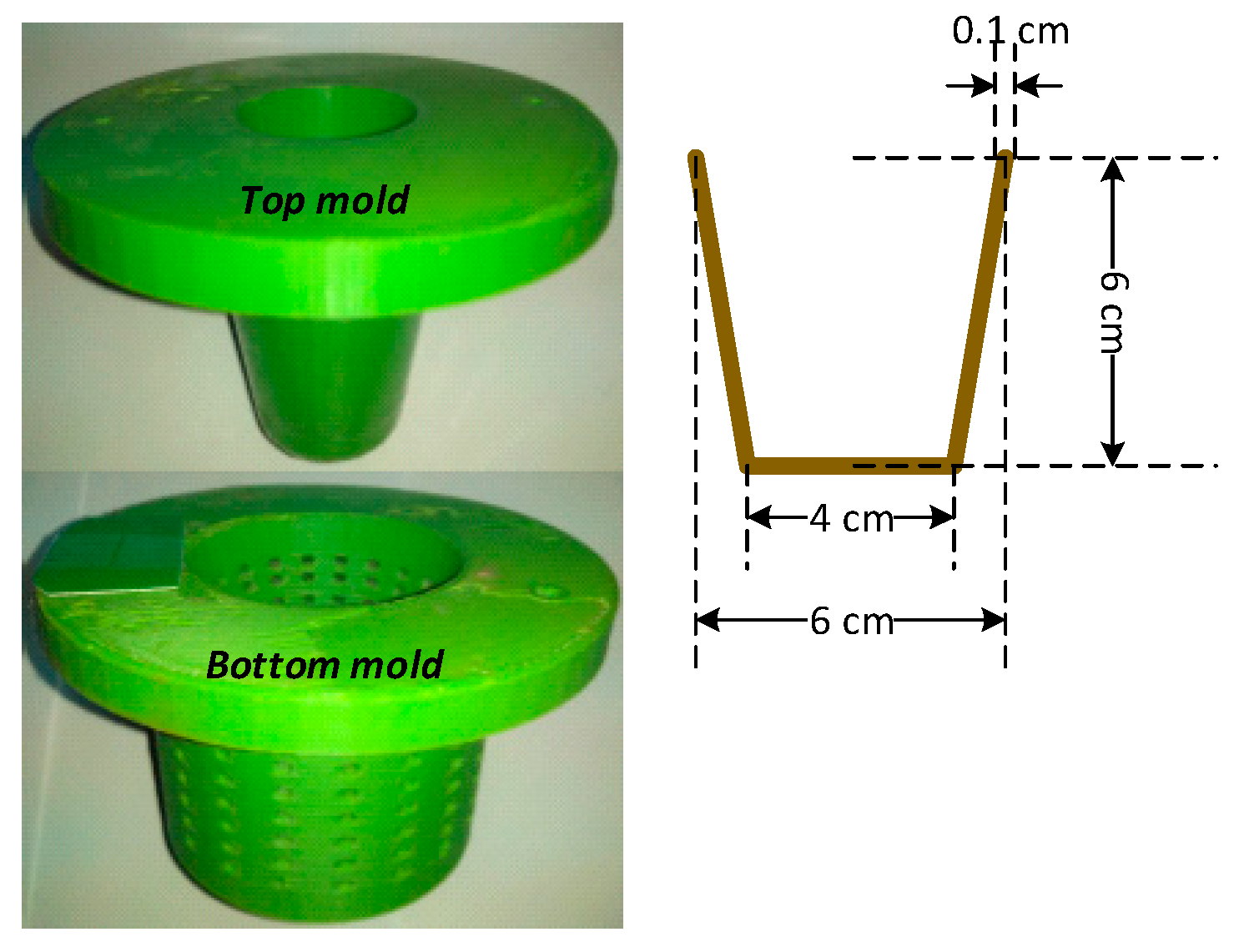
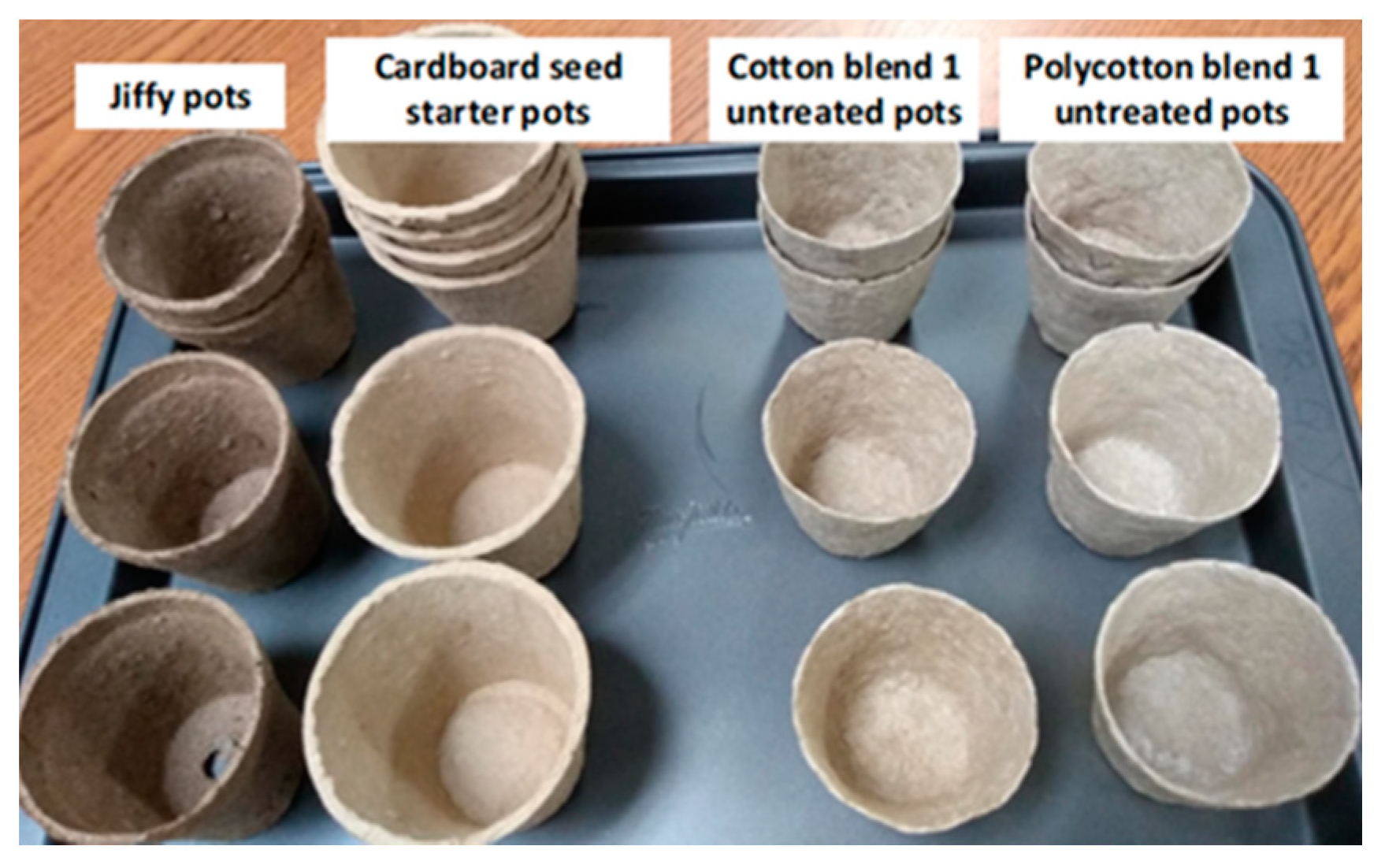
2.2. Preparation of Bio-Composite Pots
2.3. Mechanical Tests
2.3.1. For Bio-Composite Sheet
2.3.2. For Seedling Pots
2.4. Degradability Test
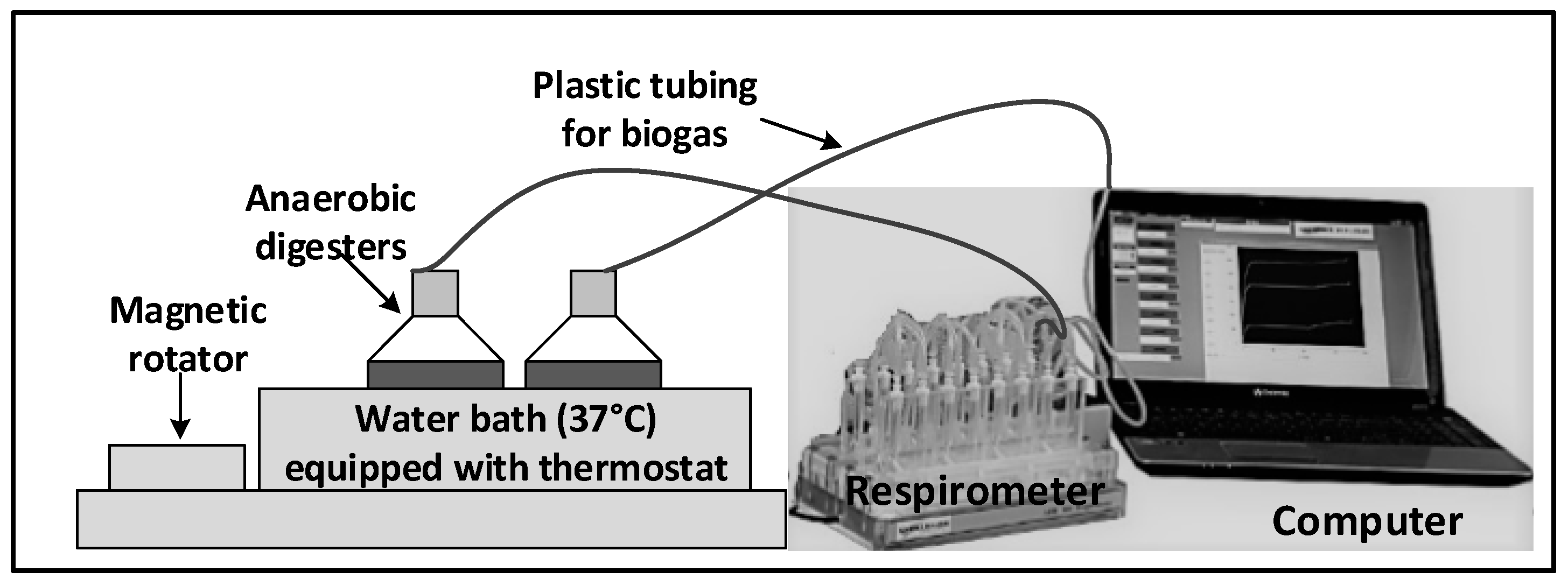
2.4.1. Anaerobic Degradability Test
2.4.2. Soil Burial Test
2.5. Germination Test
3. Results and Discussion
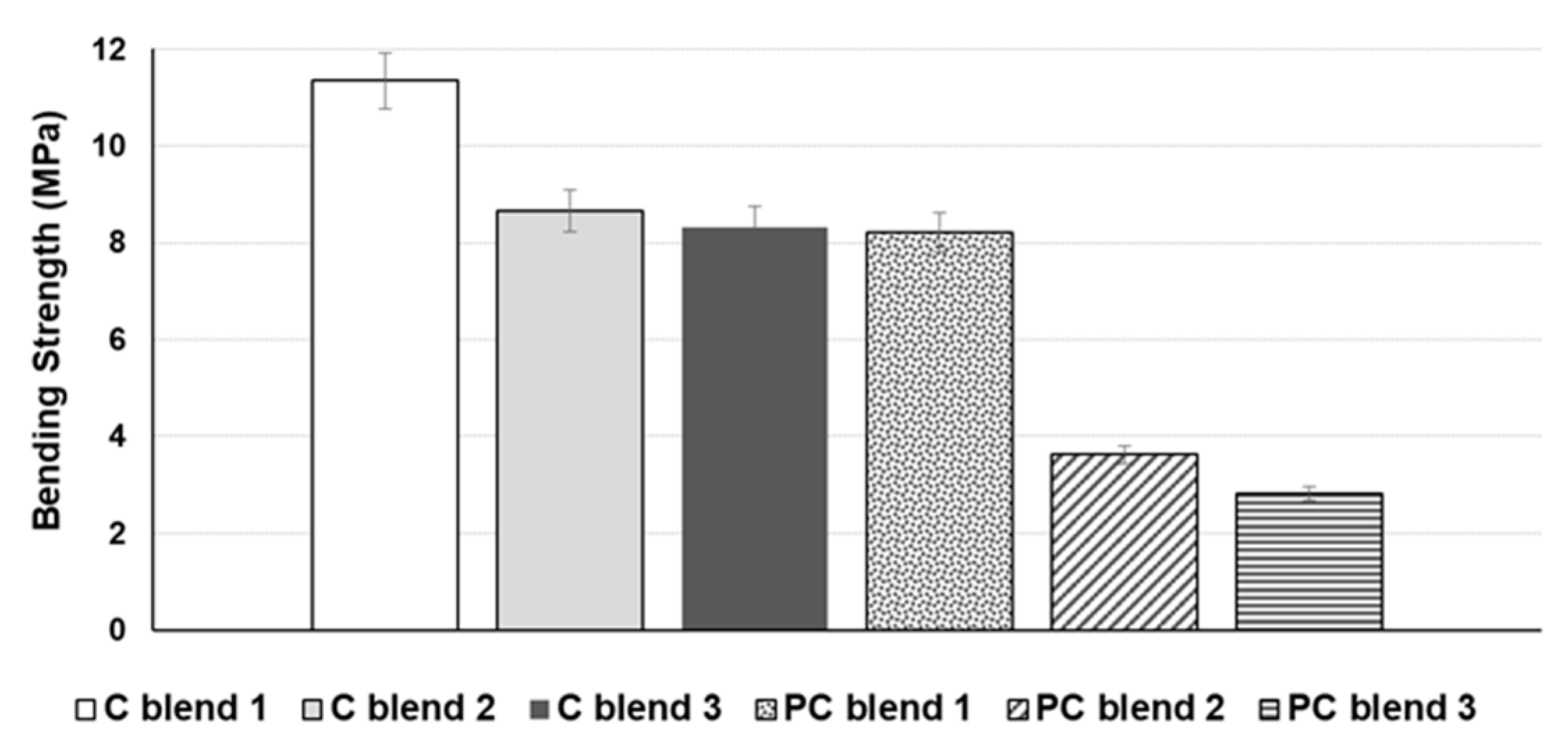
3.1. Tensile Strength and Bending Strength of Bio-Composite Sheet
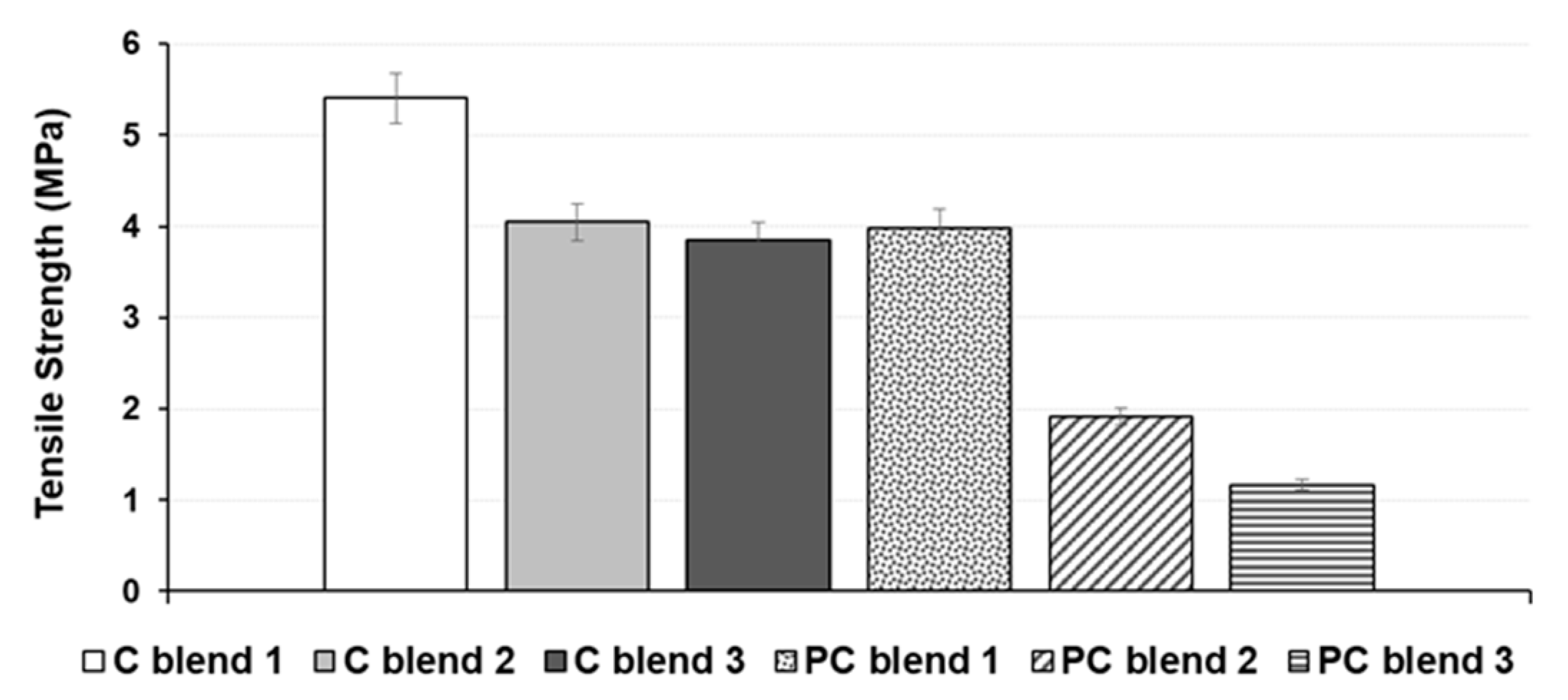
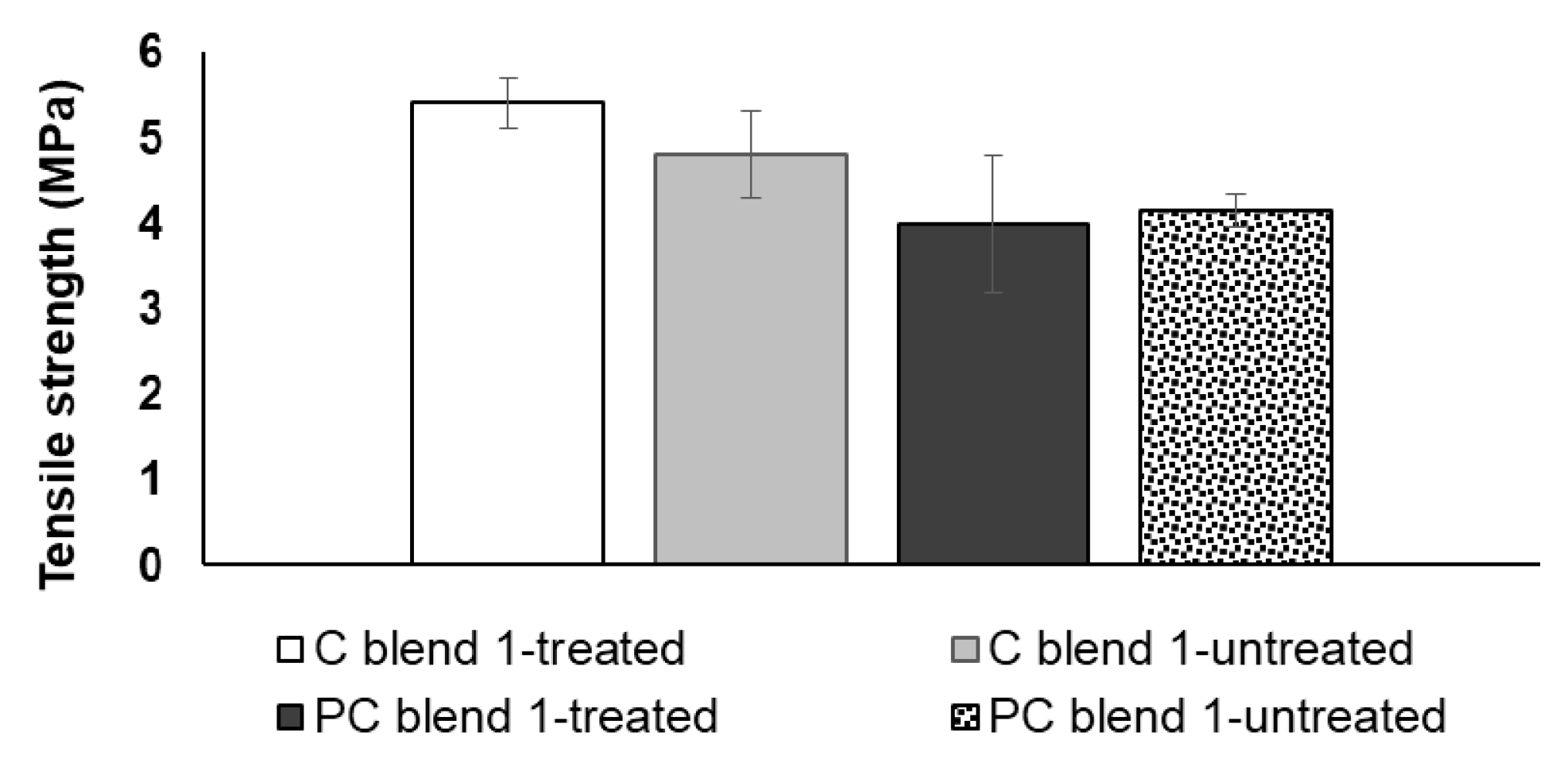
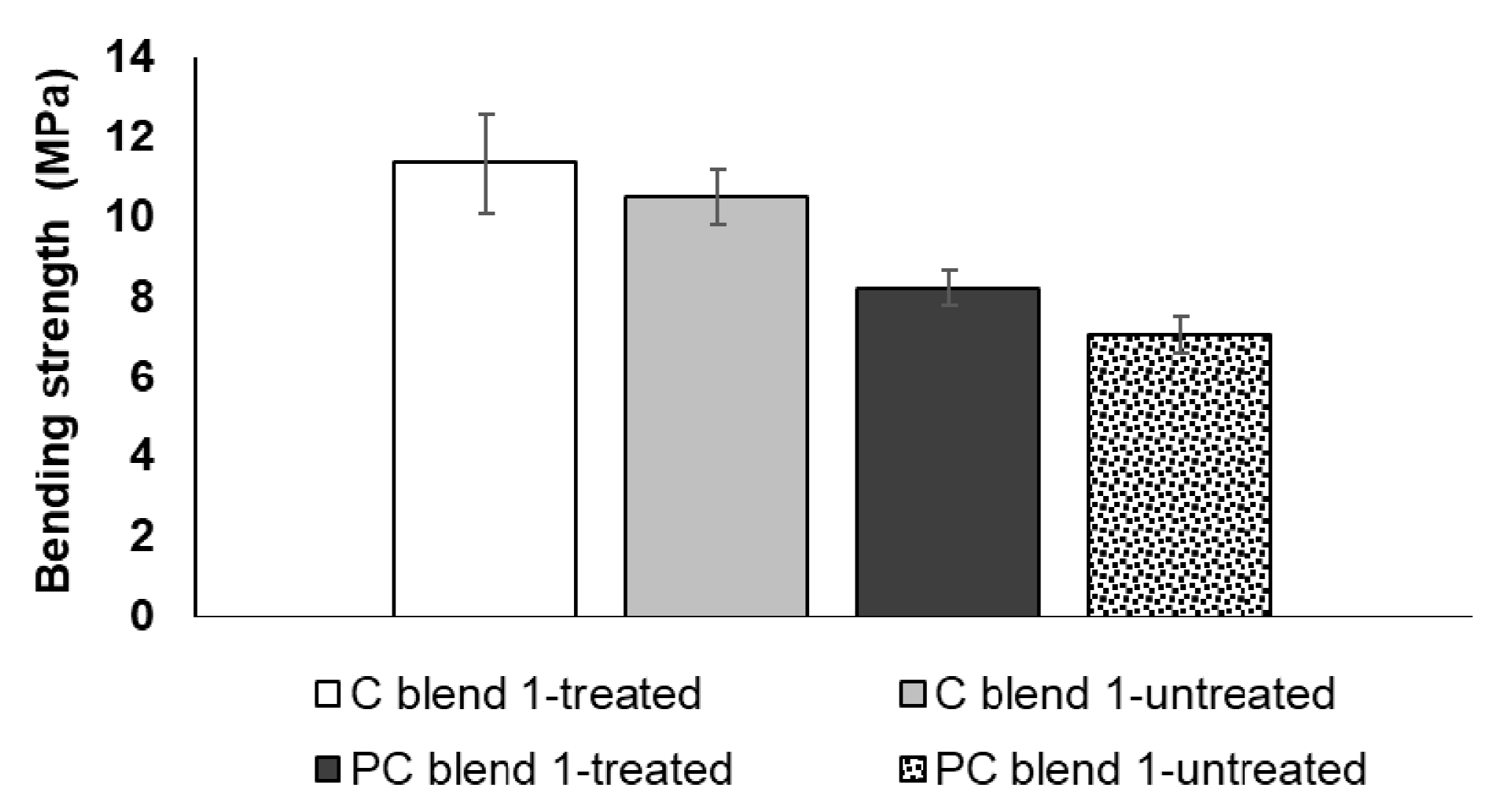
3.2. Tensile Strength and Bending Strength of Treated and Untreated Bio-Composite Sheets
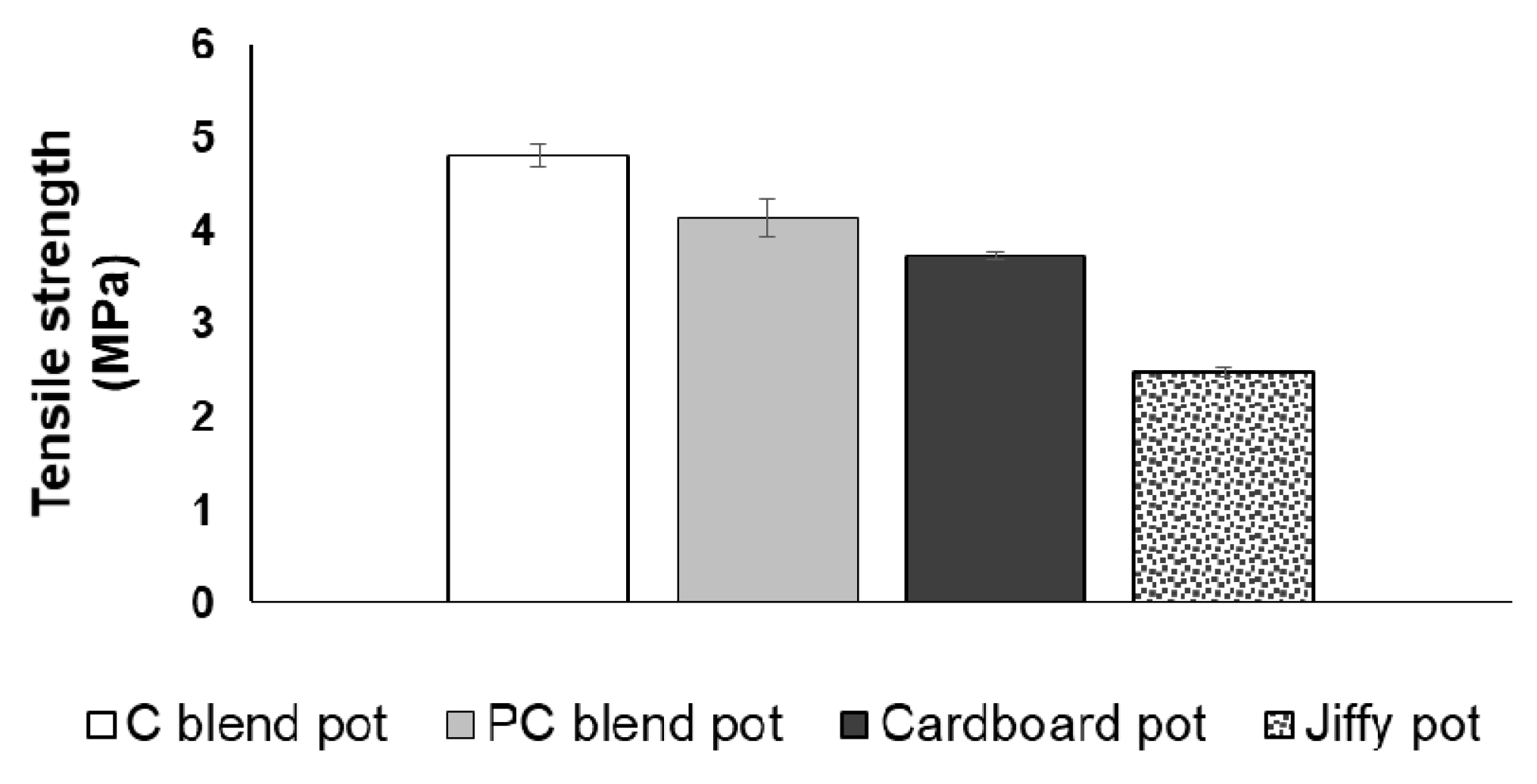
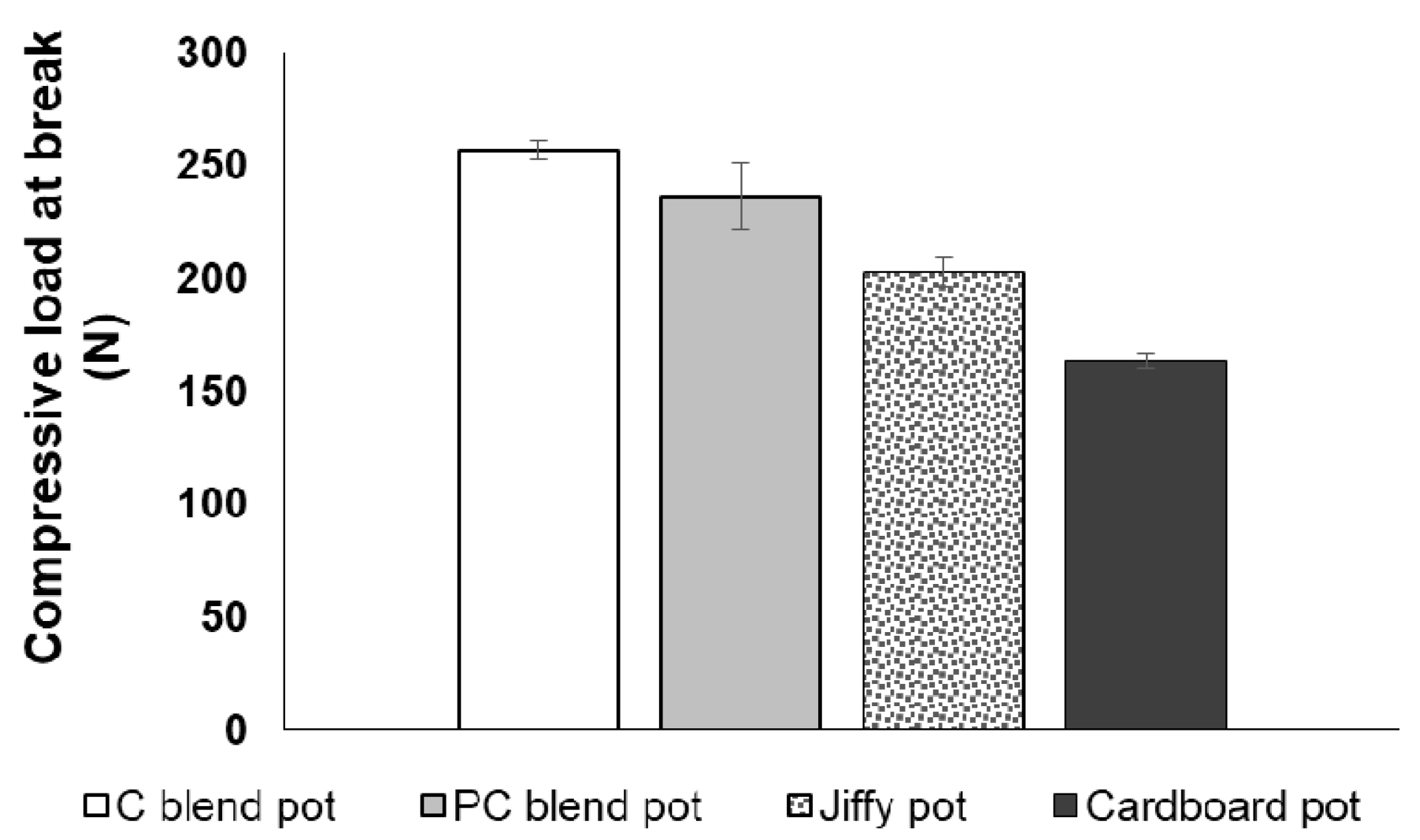
3.3. Tensile Strength and Compressive Strength of Seedling Pots
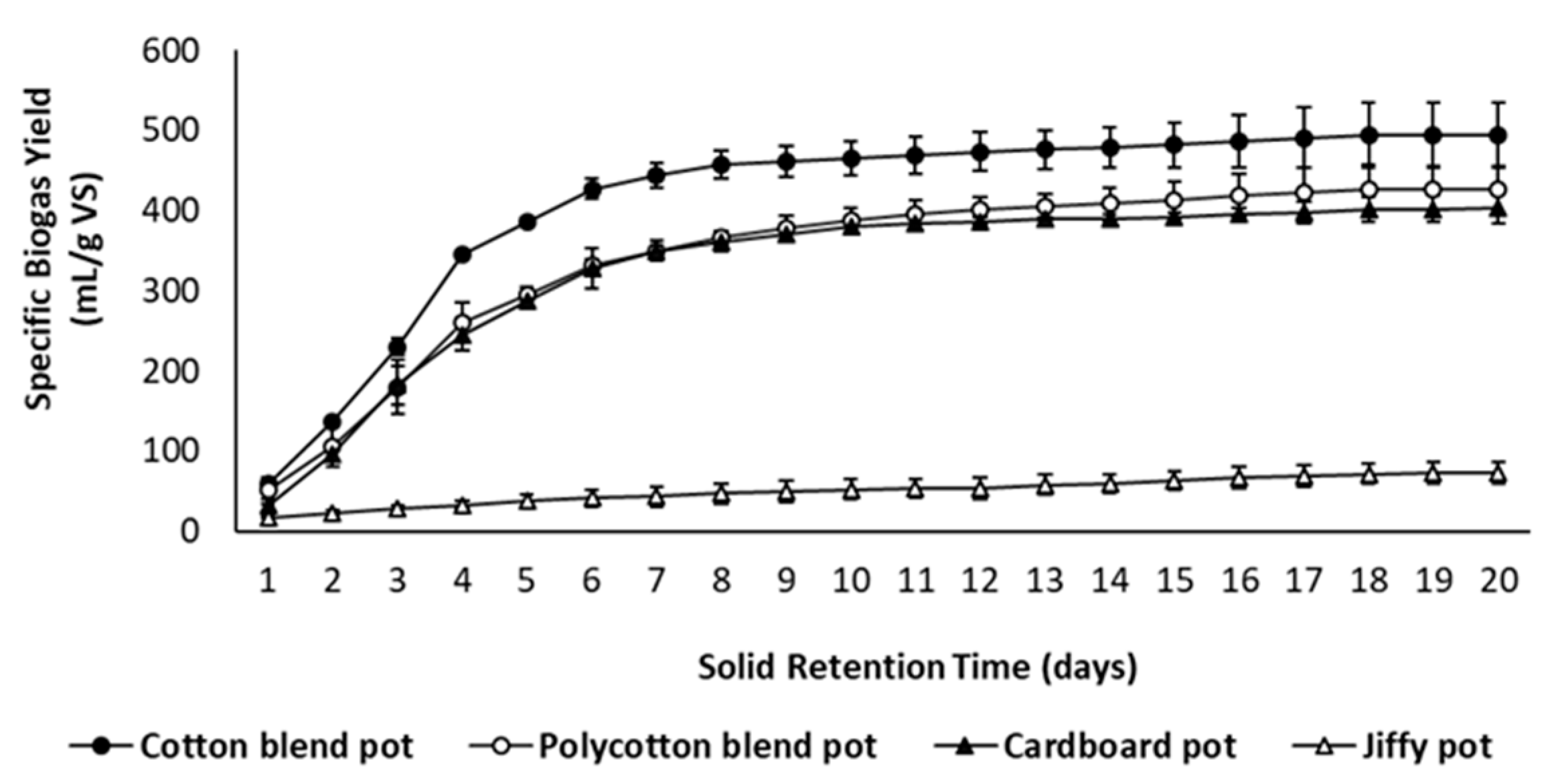
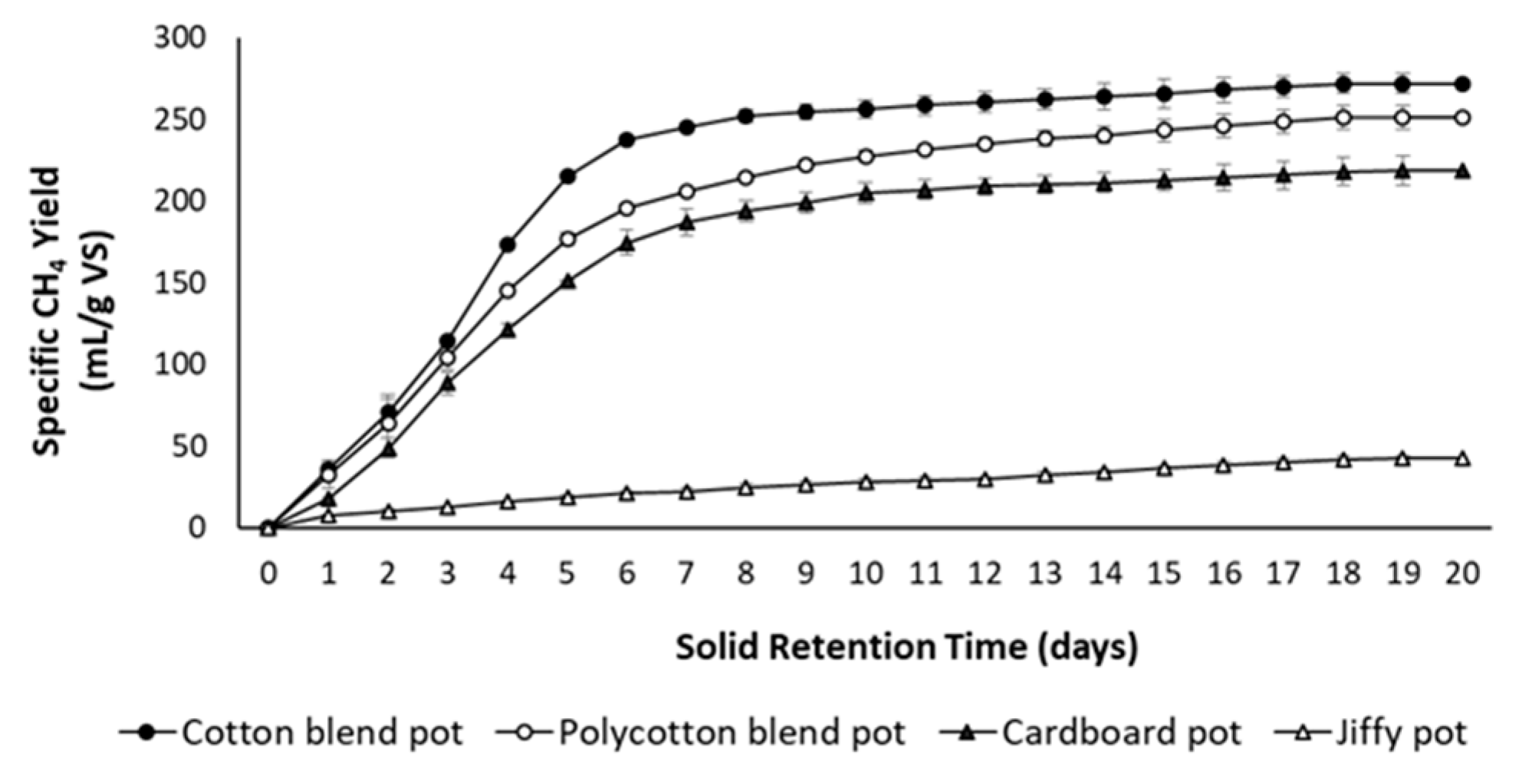
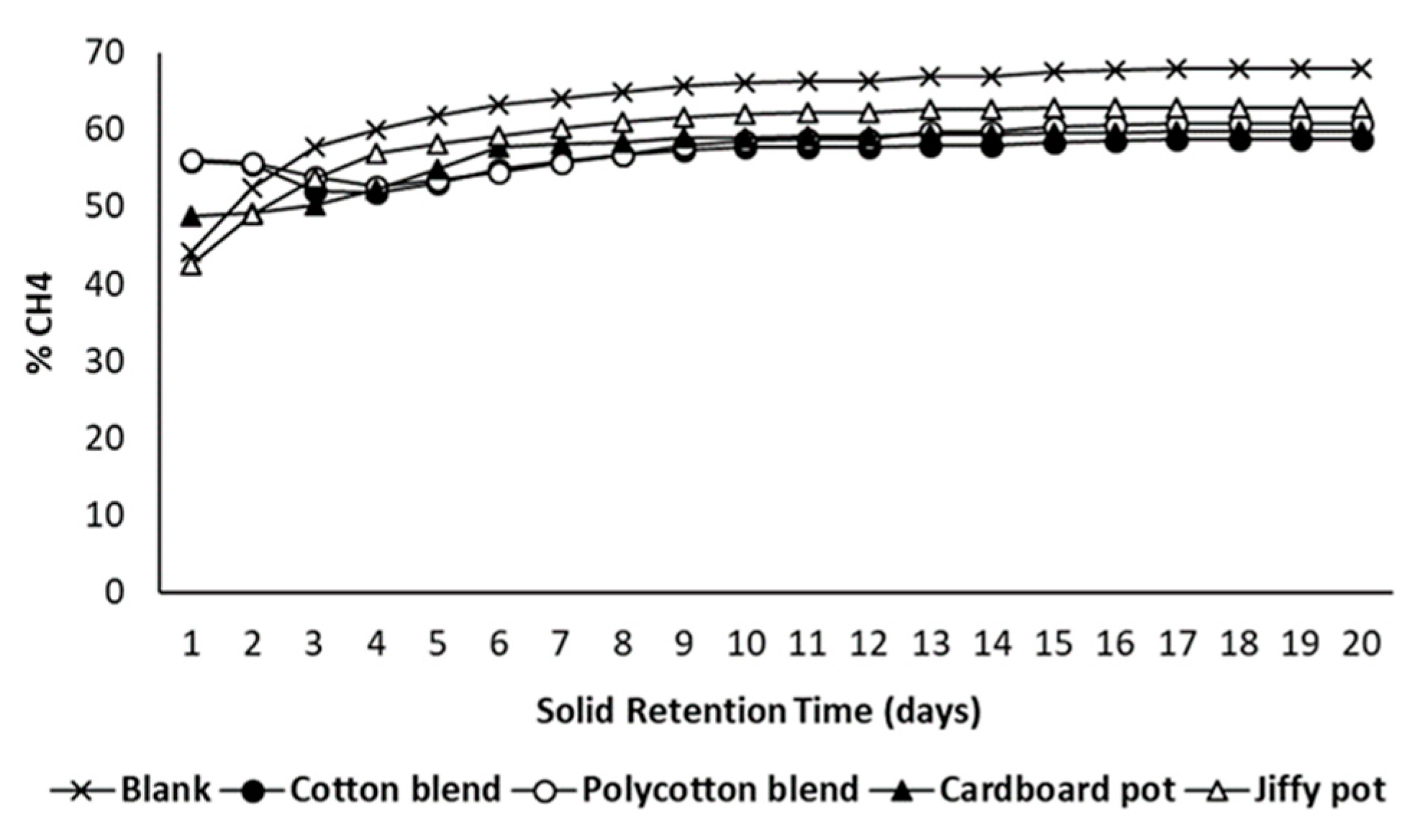
3.4. Anaerobic Biodegradability of Seedling Pots
3.5. Soil Burial Test
3.6. Germination Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Claudio, L. Waste couture: Environmental impact of the clothing industry. Environ. Health Perspect. 2007, 115, A448–A454. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y. Fiber and textile waste utilization. Waste Biomass Valorization 2010, 1, 135–143. [Google Scholar] [CrossRef]
- Lin, S.D. Recycled fiber industry facing opportunities and challenges in the new situation. Resour. Recycl. 2012, 11, 44–47. [Google Scholar]
- Bukhari, M.A.; Carrasco-Gallego, R.; Ponce-Cueto, E. Developing a national programme for textiles and clothing recovery. Waste Manag. Res. 2018, 36, 321–331. [Google Scholar] [CrossRef]
- Textile World. Man-Made Fibers Continue to Grow. 2015. Available online: https://www.textileworld.com/textile-world/fiber-world/2015/02/man-made-fiberscontinue-to-grow/ (accessed on 15 December 2019).
- IHS Markit. Natural and Man-Made Fibers Overview. November 2019. Available online: https://ihsmarkit.com/products/fibers-chemical-economics-handbook.html (accessed on 20 December 2019).
- GFA & BCG (Global Fashion Agenda and the Boston Consulting Group). Pulse of the Fashion Industry. 2017. Available online: https://www.globalfashionagenda.com/publications/#pulseofthefashionindustryreport (accessed on 22 December 2019).
- USEPA (U.S. Environmental Protection Agency). Advancing sustainable materials management: 2015 Factsheet. Assessing trends in material generation, recycling, composting, combustion with energy recovery and landfilling in the United States. July 2018. Available online: https://www.epa.gov/sites/production/files/2018-07/documents/2015_smm_msw_factsheet_07242018_fnl_508_002.pdf (accessed on 6 December 2019).
- Nambuthiri, S.; Fulcher, A.; Koeser, A.K.; Geneve, R.; Nui, G. Moving toward sustainability with alternative containers for greenhouse and nursery crop production. A review and research update. HortTechnology 2015, 25, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.R.; Hensley, D. Plant growth in plastic, peat and processed poultry feather fibre growing containers. HortScience 2004, 39, 1012–1014. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.R.; Taylor, M.; Kuehny, J. Physical properties of biocontainers for greenhouse crops production. HortTechnology 2010, 20, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Mahmood, T.; Bano, B. Development of bio-decomposable (Jiffy) pots for raising and transplanting nursery plants. Int. J. Agric. Biol. 2000, 2, 380–381. [Google Scholar]
- Beeks, S.A.; Evans, M.R. Physical properties of biocontainers used to grow long-term greenhouse crops in an ebb-and-flood irrigation system. HortScience 2013, 48, 732–737. [Google Scholar] [CrossRef]
- Mooney, B.P. The second green revolution? Production of plant-based biodegradable plastics. Biochem. J. 2009, 418, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, C.; Chen, Y. Properties of selected biodegradable seedling plug-trays. Sci. Hortic. 2019, 249, 177–184. [Google Scholar] [CrossRef]
- Schettini, E.; Santagata, G.; Malinconico, M.; Immirzi, B.; Mugnozza, G.S.; Vox, G. Recycled wastes of tomato and hemp fibres for biodegradable pots: Physico-chemical characterization and field performance. Resour. Conserv. Recycl. 2013, 70, 9–19. [Google Scholar] [CrossRef]
- Stone, P. Evaluation of Biosolids for Use in Biodegradable Transplant Containers. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2017. [Google Scholar]
- Jirapornvaree, I.; Suppadit, T.; Popan, A. Use of pineapple waste for production of decomposable pots. Int. J. Recycl. Org. Waste Agric. 2017, 6, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, M.; Masuda, S.; Kihara, M. Recycled pots using sweet potato distillation lees. Resour. Conserv. Recycl. 2006, 47, 183–194. [Google Scholar] [CrossRef]
- Rafee, S.N.A.M.; Lee, Y.L.; Jamalludin, M.R.; Razak, N.A.; Makhtar, N.L.; Ismail, R.I. Effect of different ratios of biomaterials to banana peels on the weight loss of biodegradable pots. Acta Technol. Agric. 2019, 1, 1–4. [Google Scholar]
- Koeser, A.K. Performance and environmental impacts of biocontainers in horticultural crop production systems. Ph.D. Thesis, University of Illinois, Urbana-Champaign, Champaign, IL, USA, 2013. [Google Scholar]
- Castronuovo, P.; Picuno, D.; Manera, C.; Scopa, A.; Sofo, A.; Candido, V. Biodegradable pots for pointsettia cultivation: Agronomic and technical traits. Sci. Hortic. 2015, 197, 150–156. [Google Scholar] [CrossRef]
- Juanga-Labayen, J.P.; Yuan, Q. Making Biodegradable Seedling Pots from Textile and Paper Waste—Part A: Factors Affecting Tensile Strength. Int. J. Environ. Res. Public Health 2021, 18, 6964. [Google Scholar] [CrossRef] [PubMed]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential test. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, L.; Oliveira, R.; Alves, M.M. Influence of inoculum activity on the biomethanization of a kitchen waste under different waste/inoculum ratios. Process Biochem. 2004, 39, 2019–2024. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.-M.; Kim, S.-H.; Shin, K.-S.; Kim, C.-H. Effects of substrate to inoculum ratio on the biochemical methane potential of piggery slaughterhouse wastes. Asian-Australas. J. Anim. Sci. 2014, 27, 600–607. [Google Scholar] [CrossRef]
- Juanga-Labayen, J.; Yanac, K.; Yuan, Q. Effect of substrate-to-inoculum ratio on anaerobic digestion of treated and untreated cotton textile waste. Int. J. Environ. Sci. Technol. 2021, 18, 287–296. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Rapa, M.; Darie-Nita, R.N.; Mitelut, A.C.; Popa, E.E.; Popescu, P.A.; Draghici, M.C.; Popa, M.E. Study of soil burial degradation of some PLA/CS biocomposites. Compos. Part B Eng. 2018, 142, 251–262. [Google Scholar] [CrossRef]
- Popa, E.E.; Rapa, M.; Cornea, C.P.; Popa, V.I.; Mitelut, A.C.; Popa, O.; Cristea, M.G.; Popa, M.E. PHB/cellulose fibres composites colonization and biodegradation behaviour. Mater. Plast. 2018, 55, 48–53. [Google Scholar] [CrossRef]
- Briassoulis, D.; Mistriotis, A.; Mortier, N.; De Wilde, B. Standard testing methods and specifications for biodegradation of bio-based materials in soil—A comparative analysis. In Proceedings of the International Conference of Agricultural Engineering, Zurich, Switzerland, 6–10 July 2014. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis. Part 3 Chemical Methods; Soil Science Society of America book series 5; Sparks, D.L., Ed.; SSSA and ASA: Madison, WI, USA, 1996. [Google Scholar]
- USEPA (U.S. Environmental Protection Agency). Seed germination/root elongation toxicity test. In Ecological Effects Test Guidelines; OPPTS 850.4200; USEPA: Washington, DC, USA, 1996. [Google Scholar]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Chemical treatments on plant-based natural fibre reinforced polymer composites: An overview. Compos. Part B 2012, 43, 2883–2892. [Google Scholar] [CrossRef]
- Rowell, R.M.; Young, R.A.; Rowell, J.K. Paper and Composites from Agro-Based Resources; CRC Press Inc.: Boca Raton, FL, USA, 1997. [Google Scholar]
- Nishino, T.; Matsuda, I.; Hirao, K. All-cellulose composite. Macromolecules 2004, 37, 7683–7687. [Google Scholar] [CrossRef]
- Huber, T.; Müssig, J.; Curnow, O.; Pang, S.; Bickerton, S.; Staiger, M. A critical review of all-cellulose composites. J. Mater. Sci. Lett. 2012, 47, 1171–1186. [Google Scholar] [CrossRef]
- Raj, C.S.; Arul, S.; Sendilvelan, S.; Saravanan, C.G. Biogas from textile cotton waste—An alternate fuel for diesel engines. Open Waste Manag. J. 2009, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Angelidaki, I.; Ahring, B.K. Anaerobic thermophilic digestion of manure at different ammonia loads: Effect of temperature. Water Res. 1994, 28, 727–731. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Bio/Technol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Juanga, J.P.; Visvanathan, C.; Tränkler, J. Optimization of anaerobic digestion of municipal solid waste in combined process and sequential staging. Waste Manag. Res. 2007, 25, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Yahya, S.R.; Rashid, A.A.; Azahari, B. Soil burial studies for biodegradation of natural rubber latex films. Adv. Mater. Res. 2014, 844, 406–409. [Google Scholar] [CrossRef]
- Rinaldi, P.S.; Nisa’Akromah, Z.; Ramadhan, H.; Husna, S.; Syamsudin, D.L.; Panggabean, P.B.; Murdianti, R.A.; Fatahillah, M.H.; Perala, I.; Rizqia, E.K.; et al. Physical and chemical analysis of land in forest peat swamp in resort pondok soar, Tanjung Puting National Park, Central Kalimantan. IOP Conf. Ser. Earth Environ. Sci. 2019, 394, 01203. [Google Scholar] [CrossRef]





| Bio-Composite Sheets | Substrate Composition | ||
|---|---|---|---|
| Cotton (C) blend | Cotton | Newspaper | Corrugated cardboard |
| C blend 1 | 20% | 40% | 40% |
| C blend 2 | 50% | 25% | 25% |
| C blend 3 | 80% | 10% | 10% |
| Polycotton (PC) blend | Polycotton | Newspaper | Corrugated cardboard |
| PC blend 1 | 20% | 40% | 40% |
| PC blend 2 | 50% | 25% | 25% |
| PC blend 3 | 80% | 10% | 10% |
| Substrates | Moisture Content (%) | Total Solids (%) | Volatile Solids (%) |
|---|---|---|---|
| Cotton blend pot | 4.11 | 95.89 | 95.60 |
| Polycotton blend pot | 4.20 | 95.80 | 95.80 |
| Cardboard seed starter pot | 3.53 | 96.47 | 83.94 |
| Jiffy pot | 6.18 | 93.82 | 96.90 |
| Aqueous Extracts | pH | Conductivity (µS/cm) |
|---|---|---|
| Cotton blend pot | 7.37 | 206.30 |
| Polycotton blend pot | 7.40 | 220.20 |
| Cardboard pot | 7.39 | 614.10 |
| Jiffy pot | 4.72 | 236.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juanga-Labayen, J.P.; Yuan, Q. Making Biodegradable Seedling Pots from Textile and Paper Waste—Part B: Development and Evaluation of Seedling Pots. Int. J. Environ. Res. Public Health 2021, 18, 7609. https://doi.org/10.3390/ijerph18147609
Juanga-Labayen JP, Yuan Q. Making Biodegradable Seedling Pots from Textile and Paper Waste—Part B: Development and Evaluation of Seedling Pots. International Journal of Environmental Research and Public Health. 2021; 18(14):7609. https://doi.org/10.3390/ijerph18147609
Chicago/Turabian StyleJuanga-Labayen, Jeanger P., and Qiuyan Yuan. 2021. "Making Biodegradable Seedling Pots from Textile and Paper Waste—Part B: Development and Evaluation of Seedling Pots" International Journal of Environmental Research and Public Health 18, no. 14: 7609. https://doi.org/10.3390/ijerph18147609
APA StyleJuanga-Labayen, J. P., & Yuan, Q. (2021). Making Biodegradable Seedling Pots from Textile and Paper Waste—Part B: Development and Evaluation of Seedling Pots. International Journal of Environmental Research and Public Health, 18(14), 7609. https://doi.org/10.3390/ijerph18147609







