Metabolic and Molecular Subacute Effects of a Single Moderate-Intensity Exercise Bout, Performed in the Fasted State, in Obese Male Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
2.2. High-Fat Diet and Experimental Groups
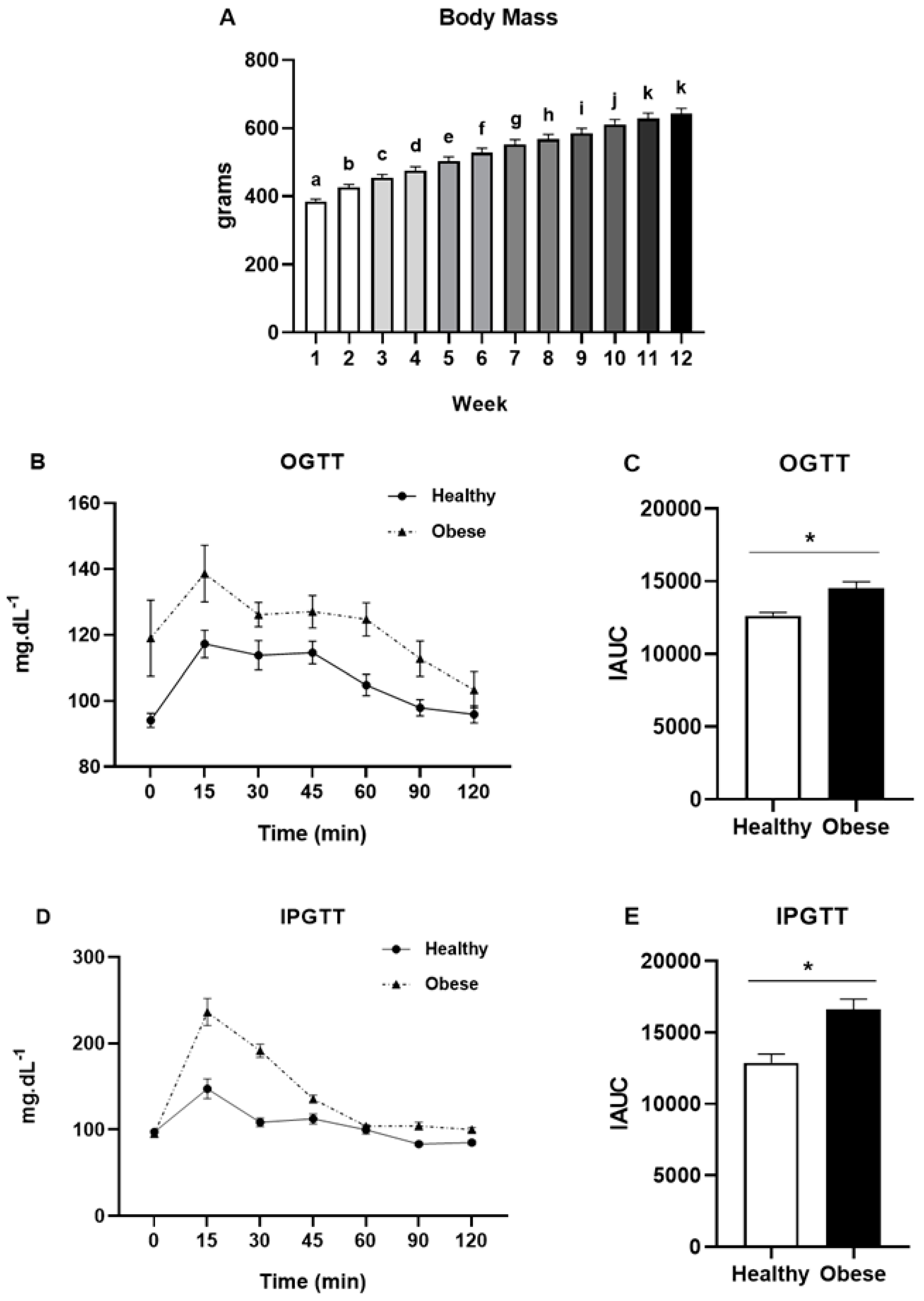
2.3. Glucose Tolerance Tests
2.4. Exercise Protocol and Experimental Design
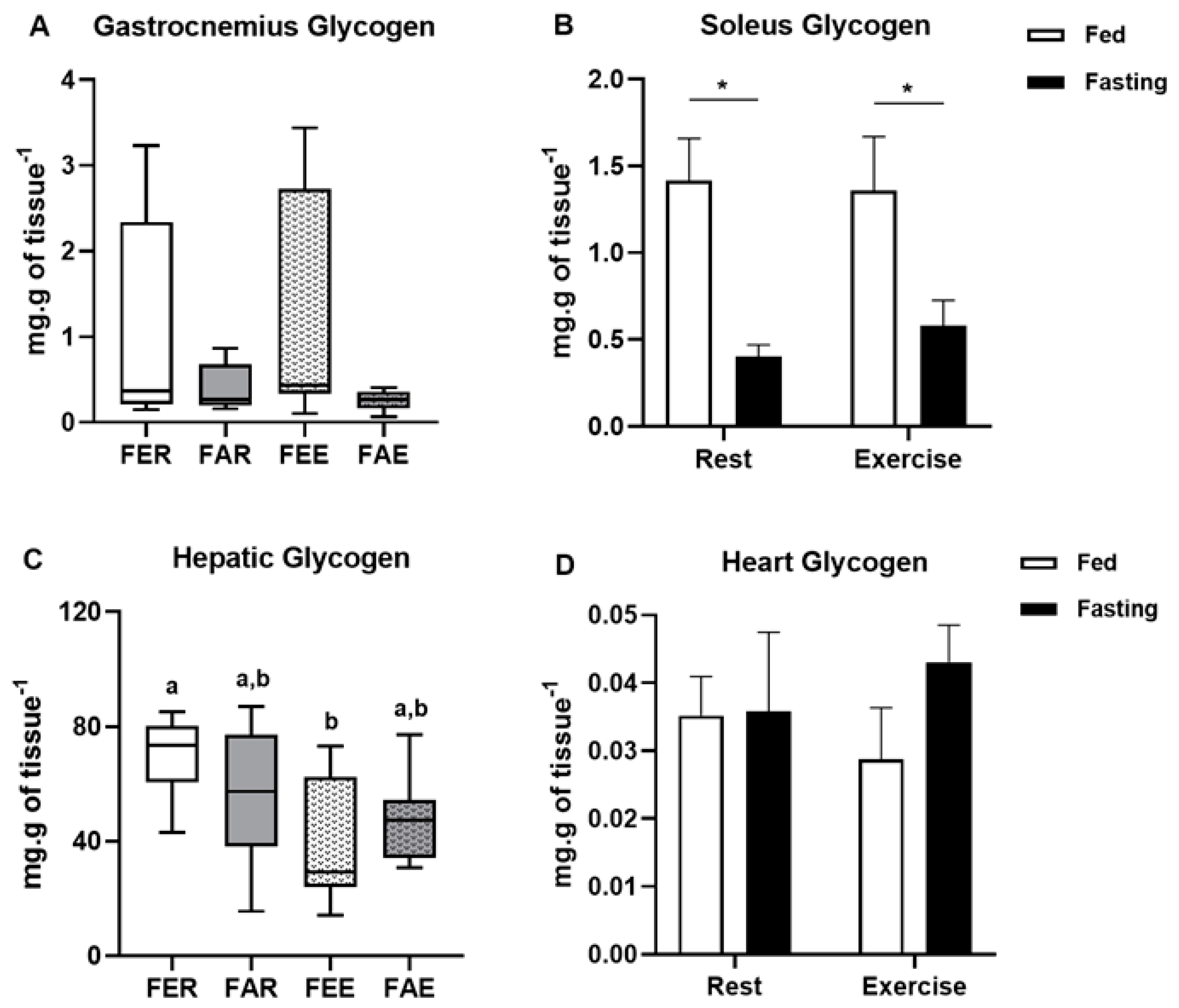
2.5. Biochemical Analyses
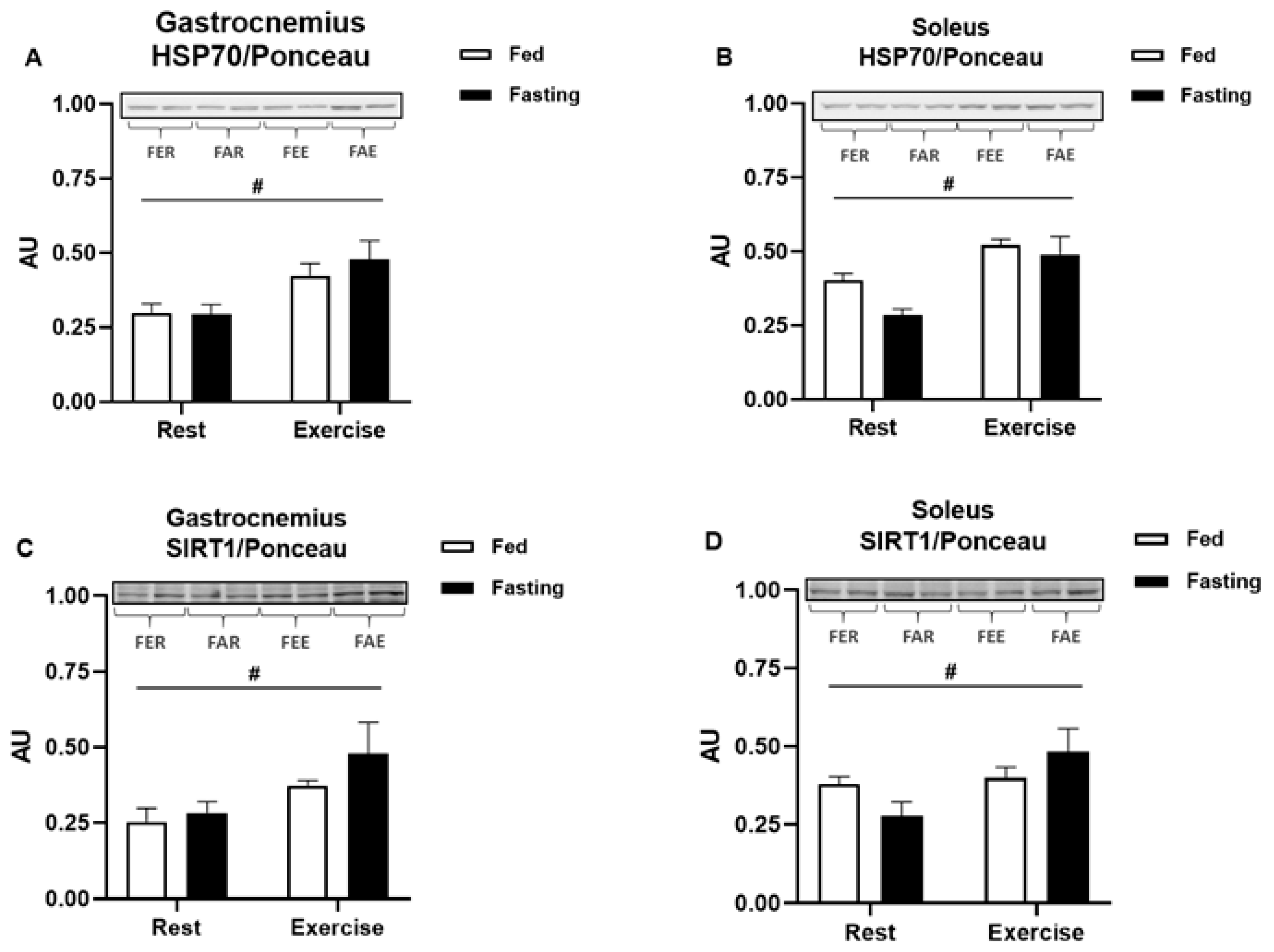
2.6. SDS-PAGE and Immunoblotting Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, E.; NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Oussaada, S.M.; van Galen, K.A.; Cooiman, M.I.; Kleinendorst, L.; Hazebroek, E.J.; van Haelst, M.M.; Ter Horst, K.W.; Serlie, M.J. The pathogenesis of obesity. Metabolism 2019, 92, 26–36. [Google Scholar] [CrossRef]
- James, W.P. WHO recognition of the global obesity epidemic. Int. J. Obes. 2008, 32 (Suppl. S7), S120–S126. [Google Scholar] [CrossRef]
- Cheung, W.W.; Mao, P. Recent advances in obesity: Genetics and beyond. ISRN Endocrinol. 2012, 2012, 536905. [Google Scholar] [CrossRef]
- Muller, C.H.L.M.; de Matos, J.R.; Grigolo, G.B.; Schroeder, H.T.; Rodrigues-Krause, J.C.; Krause, M. Exercise Training for the Elderly: Inflammaging and the Central Role for HSP70. J. Sci. Sport Exerc. 2019, 1, 10–25. [Google Scholar]
- Chung, J.; Nguyen, A.K.; Henstridge, D.C.; Holmes, A.G.; Chan, M.H.; Mesa, J.L.; Lancaster, G.I.; Southgate, R.J.; Bruce, C.R.; Duffy, S.J.; et al. HSP72 protects against obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA 2008, 105, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Hevener, A.; Lancaster, G.I.; Febbraio, M.A. Ciliary neurotrophic factor prevents acute lipid-induced insulin resistance by attenuating ceramide accumulation and phosphorylation of c-Jun N-terminal kinase in peripheral tissues. Endocrinology 2006, 147, 2077–2085. [Google Scholar] [CrossRef]
- Newsholme, P.a.M.K. Diet, Obesity, and Reactive Oxygen Species–Implications for Diabetes and Aging. Syst. Biol. Free Radic. Antioxid. 2014, 1, 3361–3374. [Google Scholar]
- Rahati, S.; Shahraki, M.; Arjomand, G.; Shahraki, T. Food pattern, lifestyle and diabetes mellitus. Int. J. High Risk Behav. Addict. 2014, 3, e8725. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Lauritzen, L.; Tholstrup, T.; Haldorssoni, T.; Riserus, U.; Uusitupa, M.; Becker, W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: A systematic review. Food Nutr. Res. 2014, 58. [Google Scholar] [CrossRef]
- Hameed, I.; Masoodi, S.R.; Mir, S.A.; Nabi, M.; Ghazanfar, K.; Ganai, B.A. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J. Diabetes 2015, 6, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Flavell, R.A. Innate sensors of pathogen and stress: Linking inflammation to obesity. J. Allergy Clin. Immunol. 2013, 132, 287–294. [Google Scholar] [CrossRef]
- Li, H.; Bao, Y.; Zhang, X.; Yu, Y. Free fatty acids induce endothelial dysfunction and activate protein kinase C and nuclear factor-kappaB pathway in rat aorta. Int. J. Cardiol. 2015, 152, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Bessesen, D.H.; Van Gaal, L.F. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018, 6, 237–248. [Google Scholar] [CrossRef]
- Montegut, L.; Lopez-Otin, C.; Magnan, C.; Kroemer, G. Old Paradoxes and New Opportunities for Appetite Control in Obesity. Trends Endocrinol. Metab. 2021, 32, 264–294. [Google Scholar] [CrossRef] [PubMed]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metabolism 2019, 92, 163–169. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.F.; Costa, R.R.; Macedo, R.C.; Coconcelli, L.; Kruel, L.F. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: A systematic review and meta-analysis. Br. J. Nutr. 2016, 116, 1153–1164. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Bertholdt, L.; Gudiksen, A.; Stankiewicz, T.; Villesen, I.; Tybirk, J.; van Hall, G.; Bangsbo, J.; Plomgaard, P.; Pilegaard, H. Impact of training state on fasting-induced regulation of adipose tissue metabolism in humans. J. Appl. Physiol. 2018, 124, 729–740. [Google Scholar] [CrossRef]
- Van Proeyen, K.; Szlufcik, K.; Nielens, H.; Ramaekers, M.; Hespel, P. Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J. Appl. Physiol. 2010, 110, 236–245. [Google Scholar] [CrossRef]
- Curi, R.; Newsholme, P.; Marzuca-Nassr, G.N.; Takahashi, H.K.; Hirabara, S.M.; Cruzat, V.; Krause, M.; de Bittencourt, P.I., Jr. Regulatory principles in metabolism-then and now. Biochem. J. 2016, 473, 1845–1857. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Modulation of carbohydrate and fat utilization by diet, exercise and environment. Biochem. Soc. Trans. 2003, 31, 1270–1273. [Google Scholar] [CrossRef]
- Krause, M.; Gerchman, F.; Friedman, R. Coronavirus infection (SARS-CoV-2) in obesity and diabetes comorbidities: Is heat shock response determinant for the disease complications? Diabetol. Metab. Syndr. 2020, 12, 63. [Google Scholar] [CrossRef]
- Krause, M.; Heck, T.G.; Bittencourt, A.; Scomazzon, S.P.; Newsholme, P.; Curi, R.; Homem de Bittencourt, P.I., Jr. The chaperone balance hypothesis: The importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediat. Inflamm. 2015, 2015, 249205. [Google Scholar] [CrossRef]
- Newsholme, P.; de Bittencourt, P.I., Jr. The fat cell senescence hypothesis: A mechanism responsible for abrogating the resolution of inflammation in chronic disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.S.; He, J.R.; Calderwood, S.; Hasday, J.D. A high affinity HSF-1 binding site in the 5′-untranslated region of the murine tumor necrosis factor-alpha gene is a transcriptional repressor. J. Biol. Chem. 2002, 277, 4981–4988. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Hu, C.; Huang, M.; Jiang, M.; Lu, L.; Tang, J. Heat shock transcription factor 1 attenuates TNFalpha-induced cardiomyocyte death through suppression of NFkappaB pathway. Gene 2013, 527, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.A.; Sandstrom, M.E.; Lovell, R.J.; McNaughton, L. Inducible heat shock protein 70 and its role in preconditioning and exercise. Amino Acids 2008, 34, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Bock, P.M.; Takahashi, H.K.; Homem De Bittencourt, P.I., Jr.; Newsholme, P. The regulatory roles of NADPH oxidase, intra- and extra-cellular HSP70 in pancreatic islet function, dysfunction and diabetes. Clin. Sci. 2015, 128, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Atalay, M.; Oksala, N.K.; Laaksonen, D.E.; Khanna, S.; Nakao, C.; Lappalainen, J.; Roy, S.; Hanninen, O.; Sen, C.K. Exercise training modulates heat shock protein response in diabetic rats. J. Appl. Physiol. 2004, 97, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Kurucz, I.; Morva, A.; Vaag, A.; Eriksson, K.F.; Huang, X.; Groop, L.; Koranyi, L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes 2002, 51, 1102–1109. [Google Scholar] [CrossRef]
- De Bock, K.; Richter, E.A.; Russell, A.P.; Eijnde, B.O.; Derave, W.; Ramaekers, M.; Koninckx, E.; Leger, B.; Verhaeghe, J.; Hespel, P. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J. Physiol. 2005, 564, 649–660. [Google Scholar] [CrossRef]
- Horowitz, J.F. Regulation of lipid mobilization and oxidation during exercise in obesity. Exerc. Sport Sci. Rev. 2001, 29, 42–46. [Google Scholar] [CrossRef]
- Iwayama, K.; Kawabuchi, R.; Nabekura, Y.; Kurihara, R.; Park, I.; Kobayashi, M.; Ogata, H.; Kayaba, M.; Omi, N.; Satoh, M.; et al. Exercise before breakfast increases 24-h fat oxidation in female subjects. PLoS ONE 2017, 12, e0180472. [Google Scholar] [CrossRef]
- Iwayama, K.; Kawabuchi, R.; Park, I.; Kurihara, R.; Kobayashi, M.; Hibi, M.; Oishi, S.; Yasunaga, K.; Ogata, H.; Nabekura, Y.; et al. Transient energy deficit induced by exercise increases 24-h fat oxidation in young trained men. J. Appl. Physiol. 2015, 118, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Iwayama, K.; Kurihara, R.; Nabekura, Y.; Kawabuchi, R.; Park, I.; Kobayashi, M.; Ogata, H.; Kayaba, M.; Satoh, M.; Tokuyama, K. Exercise Increases 24-h Fat Oxidation Only When It Is Performed before Breakfast. EBioMedicine 2016, 2, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Yamamoto, Y.; Iwayama, K.; Nakamura, K.; Yamaguchi, S.; Hibi, M.; Nabekura, Y.; Tokuyama, K. Effects of post-absorptive and postprandial exercise on 24 h fat oxidation. Metabolism 2013, 62, 793–800. [Google Scholar] [CrossRef]
- Vicente-Salar, N.; Urdampilleta Otegui, A.; Roche Collado, E. Endurance Training in Fasting Conditions: Biological Adaptations and Body Weight Management. Nutr. Hosp. 2015, 32, 2409–2420. [Google Scholar] [CrossRef] [PubMed]
- Vilaca-Alves, J.; Muller, F.; Rosa, C.; Payan-Carreira, R.; Lund, R.; Matos, F.; Garrido, N.; Saavedra, F.J.; Machado Reis, V. Cardiorespiratory, enzymatic and hormonal responses during and after walking while fasting. PLoS ONE 2018, 13, e0193702. [Google Scholar] [CrossRef] [PubMed]
- Aird, T.P.; Davies, R.W.; Carson, B.P. Effects of fasted vs fed-state exercise on performance and post-exercise metabolism: A systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2018, 28, 1476–1493. [Google Scholar] [CrossRef]
- Rosenkilde, M.; Nordby, P.; Nielsen, L.B.; Stallknecht, B.M.; Helge, J.W. Fat oxidation at rest predicts peak fat oxidation during exercise and metabolic phenotype in overweight men. Int. J. Obes. 2010, 34, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.C.D. Metabolism during Fasting and Starvation: Understanding the Basics to Glimpse New Boundaries. J. Nutr. Diet. 2017, 1, e02. [Google Scholar]
- Model, J.F.A.; Lima, M.V.; Ohlweiler, R.; Lopes Vogt, E.; Rocha, D.S.; Souza, S.K.; Turck, P.; Araujo, A.; Vinagre, A.S. Liraglutide improves lipid and carbohydrate metabolism of ovariectomized rats. Mol. Cell Endocrinol. 2021, 524, 111158. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.K.; Martins, T.L.; Ferreira, G.D.; Vinagre, A.S.; Silva, R.S.; Frizzo, M.E. Metabolic effects of perinatal asphyxia in the rat cerebral cortex. Metab. Brain Dis. 2012, 28, 25–32. [Google Scholar] [CrossRef]
- Dohm, G.L.; Tapscott, E.B.; Barakat, H.A.; Kasperek, G.J. Influence of fasting on glycogen depletion in rats during exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Koubi, H.E.; Desplanches, D.; Gabrielle, C.; Cottet-Emard, J.M.; Sempore, B.; Favier, R.J. Exercise endurance and fuel utilization: A reevaluation of the effects of fasting. J. Appl. Physiol. 1991, 70, 1337–1343. [Google Scholar] [CrossRef]
- Bruce, C.R.; Lee, J.S.; Kiens, B.; Hawley, J.A. Postexercise muscle triacylglycerol and glycogen metabolism in obese insulin-resistant zucker rats. Obes. Res. 2004, 12, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Homem De Bittencourt, P.I.; O’Hagan, C.; De Vito, G.; Murphy, C.; Krause, M.S. Exercise and possible molecular mechanisms of protection from vascular disease and diabetes: The central role of ROS and nitric oxide. Clin. Sci. 2009, 118, 341–349. [Google Scholar] [CrossRef]
- Deighton, K.; Batterham, R.L.; Stensel, D.J. Appetite and gut peptide responses to exercise and calorie restriction. The effect of modest energy deficits. Appetite 2014, 81, 52–59. [Google Scholar] [CrossRef]
- Felding, P.; Fex, G. Factors responsible for the decreased plasma concentration of prealbumin during acute inflammation and fasting in the rat. Acta Physiol. Scand. 1983, 117, 377–383. [Google Scholar] [CrossRef]
- Torbert, H.C. The Effect of Fasting on the Serum Protein Concentration of the Rat: With Special Reference to the Question of the Existence of an Immediately Utilizable Circulating Protein Fraction. J. Exp. Med. 1935, 62, 1–10. [Google Scholar] [CrossRef][Green Version]
- Jamart, C.; Naslain, D.; Gilson, H.; Francaux, M. Higher activation of autophagy in skeletal muscle of mice during endurance exercise in the fasted state. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E964–E974. [Google Scholar] [CrossRef]
- Krause, M.; Ludwig, M.S.; Heck, T.G.; Takahashi, H.K. Heat shock proteins and heat therapy for type 2 diabetes: Pros and cons. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 374–380. [Google Scholar] [CrossRef]
- Moreno, M.; Lombardi, A.; Silvestri, E.; Senese, R.; Cioffi, F.; Goglia, F.; Lanni, A.; de Lange, P. PPARs: Nuclear Receptors Controlled by, and Controlling, Nutrient Handling through Nuclear and Cytosolic Signaling. PPAR Res. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- de Matos, M.A.; Ottone Vde, O.; Duarte, T.C.; Sampaio, P.F.; Costa, K.B.; Fonseca, C.A.; Neves, M.P.; Schneider, S.M.; Moseley, P.; Coimbra, C.C.; et al. Exercise reduces cellular stress related to skeletal muscle insulin resistance. Cell Stress Chaperones 2013, 19, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lormes, W.; Baur, C.; Opitz-Gress, A.; Altenburg, D.; Lehmann, M.; Steinacker, J.M. Human skeletal muscle HSP70 response to physical training depends on exercise intensity. Int. J. Sports Med. 2000, 21, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Krause, J.; Krause, M.; O’Hagan, C.; De Vito, G.; Boreham, C.; Murphy, C.; Newsholme, P.; Colleran, G. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: Does fat matter? Cell Stress Chaperones 2012, 17, 293–302. [Google Scholar] [CrossRef]
- Henstridge, D.C.; Whitham, M.; Febbraio, M.A. Chaperoning to the metabolic party: The emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes. Mol. Metab. 2014, 3, 781–793. [Google Scholar] [CrossRef]
- Krause, M.; Crognale, D.; Cogan, K.; Contarelli, S.; Egan, B.; Newsholme, P.; De Vito, G. The effects of a combined bodyweight-based and elastic bands resistance training, with or without protein supplementation, on muscle mass, signaling and heat shock response in healthy older people. Exp. Gerontol. 2019, 115, 104–113. [Google Scholar] [CrossRef]
- Walker, A.K.; Yang, F.; Jiang, K.; Ji, J.Y.; Watts, J.L.; Purushotham, A.; Boss, O.; Hirsch, M.L.; Ribich, S.; Smith, J.J.; et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010, 24, 1403–1417. [Google Scholar] [CrossRef]
- Wang, Y.; Vera, L.; Fischer, W.H.; Montminy, M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 2009, 460, 534–537. [Google Scholar] [CrossRef]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Jiang, L.Q.; Deshmukh, A.S.; Mataki, C.; Coste, A.; Lagouge, M.; Zierath, J.R.; Auwerx, J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010, 11, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Derave, W.; Eijnde, B.O.; Hesselink, M.K.; Koninckx, E.; Rose, A.J.; Schrauwen, P.; Bonen, A.; Richter, E.A.; Hespel, P. Effect of training in the fasted state on metabolic responses during exercise with carbohydrate intake. J. Appl. Physiol. 2008, 104, 1045–1055. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights from Epidemiology, Genetics, and Biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogt, É.L.; Von Dentz, M.C.; Rocha, D.S.; Argenta Model, J.F.; Kowalewski, L.S.; de Souza, S.K.; Girelli, V.d.O.; de Bittencourt, P.I.H., Jr.; Friedman, R.; Krause, M.; et al. Metabolic and Molecular Subacute Effects of a Single Moderate-Intensity Exercise Bout, Performed in the Fasted State, in Obese Male Rats. Int. J. Environ. Res. Public Health 2021, 18, 7543. https://doi.org/10.3390/ijerph18147543
Vogt ÉL, Von Dentz MC, Rocha DS, Argenta Model JF, Kowalewski LS, de Souza SK, Girelli VdO, de Bittencourt PIH Jr., Friedman R, Krause M, et al. Metabolic and Molecular Subacute Effects of a Single Moderate-Intensity Exercise Bout, Performed in the Fasted State, in Obese Male Rats. International Journal of Environmental Research and Public Health. 2021; 18(14):7543. https://doi.org/10.3390/ijerph18147543
Chicago/Turabian StyleVogt, Éverton Lopes, Maiza Cristina Von Dentz, Débora Santos Rocha, Jorge Felipe Argenta Model, Lucas Stahlhöfer Kowalewski, Samir Khal de Souza, Vitória de Oliveira Girelli, Paulo Ivo Homem de Bittencourt, Jr., Rogério Friedman, Mauricio Krause, and et al. 2021. "Metabolic and Molecular Subacute Effects of a Single Moderate-Intensity Exercise Bout, Performed in the Fasted State, in Obese Male Rats" International Journal of Environmental Research and Public Health 18, no. 14: 7543. https://doi.org/10.3390/ijerph18147543
APA StyleVogt, É. L., Von Dentz, M. C., Rocha, D. S., Argenta Model, J. F., Kowalewski, L. S., de Souza, S. K., Girelli, V. d. O., de Bittencourt, P. I. H., Jr., Friedman, R., Krause, M., & Vinagre, A. S. (2021). Metabolic and Molecular Subacute Effects of a Single Moderate-Intensity Exercise Bout, Performed in the Fasted State, in Obese Male Rats. International Journal of Environmental Research and Public Health, 18(14), 7543. https://doi.org/10.3390/ijerph18147543









