In-Treatment Changes in Quality of Life-Related Variables in Therapeutic Communities for Cocaine Abusers: Are These Changes Associated with Clinical Outcomes?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Participants
2.3. Measurement Instruments
2.4. Procedures
2.5. Statistical Analysis
3. Results
3.1. Baseline Comparison: Patients Who Reached the 3-Month Assessment versus Early Dropouts
3.2. Comparative Analysis: QoL-Related Variables from Baseline to the 3-Month Assessment
3.3. Comparison Analysis: Scores on the QoL-Related Instruments at the 3-Month Assessment and Clinical Impression of Treatment Outcomes at Discharge
3.4. Comparison Analysis: Reliable Changes in QoL-Related Variables from Baseline to the 3-Month Assessment and Clinical Impression of Treatment Outcomes at Discharge
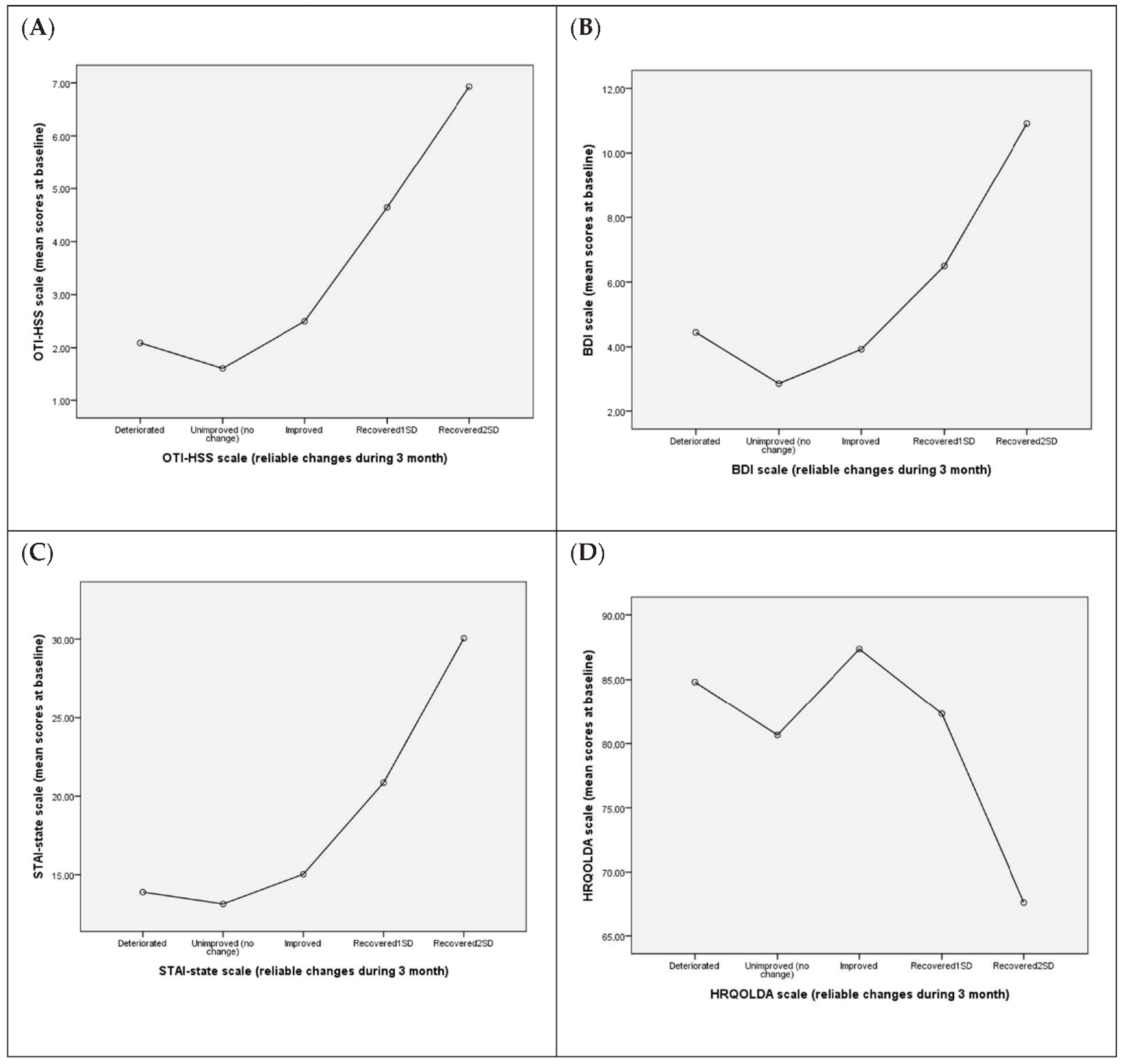
3.5. Association between Reliable Changes in QoL-Related Variables at the 3-Month Assessment and Mean Values at Baseline
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Cheong, J.; Setlow, B.; Cottler, L.B. Cocaine and Marijuana Polysubstance Use and Cocaine Use Disorder: Investigating Mediated Effects through Patterns of Cocaine Use. J. Dual Diagn. 2021, 17, 23–33. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report 2020: Key Issues. Available online: https://www.emcdda.europa.eu/publications/edr/key-issues/2020_en (accessed on 1 July 2021).
- United Nations Office on Drugs and Crime. World Drug Report 2020; United Nations Publication. Available online: https://wdr.unodc.org/wdr2020/en/drug-use-health.html (accessed on 1 July 2021).
- Afful, S.E.; Strickland, J.R.; Cottler, L.; Bierut, L.J. Exposure to trauma: A comparison of cocaine dependent cases and a community-matched sample. Drug Alcohol Depend. 2010, 112, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.E.; O’Leary, C.C.; Striley, C.W.; Abdallah, A.B.; Bradford, S.; Cottler, L.B. Effects of major depression on crack use and arrests among women in drug court. Addiction 2011, 106, 1279–1286. [Google Scholar] [CrossRef] [Green Version]
- John, W.S.; Wu, L.T. Trends and correlates of cocaine use and cocaine use disorder in the United States from 2011 to 2015. Drug Alcohol Depend. 2017, 180, 376–384. [Google Scholar] [CrossRef]
- Butler, J.; Rehm, J.; Fischer, B. Health outcomes associated with crack-cocaine use: Systematic review and meta-analyses. Drug Alcohol Depend. 2017, 180, 401–416. [Google Scholar] [CrossRef]
- Jones, A.A.; Gerke, T.; Ennis, N.; Striley, C.W.; Crecelius, R.; Sullivan, J.E.; Cottler, L.B. Order in the court? The association between substance use, exposure to violence, risky sexual behaviors & observed court behaviors among women involved in the criminal justice system. J. Natl. Med. Assoc. 2019, 111, 134–147. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration (SAMHSA). Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. Available online: https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/2019NSDUHFFRPDFWHTML/2019NSDUHFFR090120.htm (accessed on 1 July 2021).
- Crits-Cristoph, P.; Siqueland, L.; Blaine, J.; Frank, A.; Luborsky, L.; Onken, L.; Muenz, L.; Thase, M.; Weiss, R.; Gastfriend, D.; et al. Psychosocial treatments for cocaine dependence. Arch. Gen. Psychiatry 1999, 56, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Blanken, P.; Da Silveira, D.; Gallassi, A.; Goldner, E.M.; Rehm, J.; Tyndall, M.; Wood, E. Effectiveness of secondary prevention and treatment interventions for crack-cocaine abuse: A comprehensive narrative overview of English-language studies. Int. J. Drug Policy 2015, 26, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Moragues, E.; Verdejo-García, A.; Lozano, O.M.; Santiago-Ramajo, S.; González-Saiz, F.; Betanzos Espinosa, P.; Pérez García, M. Association between executive function and outcome measure of treatment in therapeutic community among cocaine dependent individuals. J. Subst. Abus. Treat. 2017, 78, 45–55. [Google Scholar] [CrossRef]
- De Crescenzo, F.; Ciabattini, M.; D’Aló, G.L.; De Giorgi, R.; Del Giovane, C.; Cassar, C.; Janiri, L.; Clark, N.; Ostacher, M.J.; Cipriani, A. Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: A systematic review and network meta-analysis. PLoS Med. 2018, 15, e1002715. [Google Scholar] [CrossRef]
- De Giorgi, R.; Cassar, C.; Loreto D’Aló, G.L.; Ciabattini, M.; Minozzi, S.; Economou, A.; Tambelli, R.; Lucchese, F.; Saulle, R.; Amato, L.; et al. Psychosocial interventions in stimulant use disorders: A systematic review and qualitative synthesis of randomized controlled trials. Riv. Psichiatr. 2018, 53, 233–255. [Google Scholar] [CrossRef]
- Andersson, H.W.; Wenaas, M.; Nordfjærn, T. Relapse after inpatient substance use treatment: A prospective cohort study among users of illicit substances. Addict. Behav. 2019, 90, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Dacosta-Sánchez, D.; González-Ponce, B.M.; Fernández-Calderón, F.; Rojas-Tejada, A.J.; Ordóñez-Carrasco, J.L.; Lozano-Rojas, O.M. Profiles of patients with cocaine and alcohol use disorder based on cognitive domains and their relationship with relapse. Drug Alcohol Depend. 2021, 218, 108349. [Google Scholar] [CrossRef] [PubMed]
- Malivert, M.; Fatséas, M.; Denis, C.; Langlois, E.; Auriacombe, M. Effectiveness of therapeutic communities: A systematic review. Eur. Addict. Res. 2012, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, M.L.; Podus, D.; Chang, E.; Urada, D. The effectiveness of drug abuse treatment: A meta-analysis of comparison group studies. Drug Alcohol Depend. 2002, 61, 53–72. [Google Scholar] [CrossRef]
- Smith, L.A.; Gates, S.; Foxcroft, D. Therapeutic communities for substance related disorders. Cochrane Database Syst. Rev. 2006, 1, CD005338. [Google Scholar] [CrossRef] [PubMed]
- Vanderplasschen, W.; Colpaert, K.; Autrique, M.; Rapp, R.C.; Pearce, S.; Broekaert, E.; Vandevelde, S. Therapeutic communities for addictions: A review of their effectiveness from a recovery-oriented perspective. Sci. World J. 2013, 1–22. [Google Scholar] [CrossRef]
- Magor-Blatch, L.; Bhullar, N.; Thomson, B.; Thorsteinsson, E. A systematic review of studies examining effectiveness of therapeutic communities. Ther. Communities 2014, 35, 168–184. [Google Scholar] [CrossRef]
- González-Saiz, F.; Ballesta Gomez, R.; Acedos Bilbao, I.; Lozano Rojas, O.M.; Gutiérrez Ortega, J. Methadone-treated patients after switching to buprenorphine in residential therapeutic communities: An addiction-specific assessment of quality of life. Heroin Addict. Relat. Clin. Probl. 2009, 11, 9–19. [Google Scholar]
- Gonzalez-Saiz, F.; Lozano Rojas, O.M.; Martin Esteban, J.; Acedos Bilbao, I.; Ballesta Gomez, R.; Gutierrez Ortega, J. Psychiatric comorbidity in a sample of opiate-dependent patient treated with sublingual buprenorphine in a therapeutic community regime. Rev. Psiquiatr. Salud Ment. 2011, 4, 81–87. [Google Scholar] [CrossRef]
- Johnson, K.W.; Young, L.; Shamblen, S.; Suresh, G.; Browne, T.; Chookhare, K.W. Evaluation of the therapeutic community treatment model in Thailand: Policy Implications for compulsory and prison-based treatment. Subst. Use Misuse 2012, 47, 889–909. [Google Scholar] [CrossRef]
- Snyder, M.; Schactman, L.; Young, S. Rates and correlations of change in three dimensions of recovery within a recovery model oriented therapeutic community. Psychiatr. Q. 2015, 86, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, E.; De Maeyer, J.; Vandevelde, S.; Vanderplasschen, W.; Claes, C.; Colpaert, K.; Walgraeve, M. Quality of life in therapeutic communities for addictions: A positive search for wellbeing and happiness. J. Groups Addict. Recovery 2017, 12, 207–221. [Google Scholar] [CrossRef]
- Holland, S. Measuring process in drug abuse treatment research. In Therapeutic Communities for Addictions; De Leon, G., Ziegenfuss, J.T., Eds.; Charles C. Thomas: Springfield, IL, USA, 1986; pp. 169–181. [Google Scholar]
- De Leon, G.; Inciardi, J.A.; Martin, S.S. Residential drug abuse treatment research: Are conventional control designs appropriate for assessing treatment effectiveness? J. Psychoact. Drugs 1995, 27, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.D.; Savage, L.J.; Lloyd, M.R. Follow-up evaluation of treatment of drug abuse during 1969 to 1972. Arch. Gen. Psychiatry 1979, 36, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.D. Treatment for drug abuse: Follow-up outcomes and length of time spent. Arch. Gen. Psychiatry 1981, 38, 875–880. [Google Scholar] [CrossRef]
- Bale, R.N.; Van Stone, W.W.; Kuldau, J.M.; Engelsing, T.M.J.; Elashoff, R.M.; Zarcone, V.P. Therapeutic communities vs. methadone maintenance. A prospective study of narcotic addiction treatment: Design and one year follow-up results. Arch. Gen. Psychiatry 1980, 37, 179–193. [Google Scholar] [CrossRef]
- De Leon, G. The therapeutic community for substance abuse: Perspective and approach. In Therapeutic Communities for Addictions; De Leon, G., Ziegenfuss, J.T., Eds.; Charles C. Thomas: Springfield, IL, USA, 1986. [Google Scholar]
- Bleiberg, J.L.; Devlin, P.; Croan, J.; Briscoe, R. Relationship between treatment length and outcome in a therapeutic community. Int. J. Addict. 1994, 29, 729–740. [Google Scholar] [CrossRef]
- Condelli, W.S.; Hubbard, R.L. Client outcomes from therapeutic communities. In Therapeutic Community: Advances in Research and Application; Tims, F.M., De Leon, G., Jainchill, N., Eds.; NIDA Research Monographs: Rockville, MD, USA, 1994; p. 144. [Google Scholar]
- Llorente del Pozo, J.M.; Fernández Gómez, C. Comunidades terapéuticas. Situación actual y perspectivas de futuro. Adicciones 1999, 11, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Brunette, M.F.; Drake, R.E.; Woods, M.; Hartnett, T. A comparison of long-term and short-term residential treatment programs for dual diagnosis patients. Psychiatr. Serv. 2001, 52, 526–528. [Google Scholar] [CrossRef]
- Greenfield, L.; Burgdorf, K.; Chen, X.; Porowski, A.; Roberts, T.; Herrell, J. Effectiveness of long-term residential substance abuse treatment for women: Findings from three national studies. Am. J. Drug Alcohol Abus. 2004, 30, 537–550. [Google Scholar] [CrossRef]
- González-Saiz, F.; Vergara-Moragues, E.; Verdejo-García, A.; Fernández-Calderón, F.; Lozano-Rojas, O. Impact of psychiatric comorbidity on the in-treatment outcomes of cocaine-dependent patients on therapeutic community. Subst. Abus. 2014, 35, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Moos, R.H.; King, M.J. Participation in community residential treatment and substance abuse patients’ outcomes at discharge. J. Subst. Abus. Treat. 1997, 14, 71–80. [Google Scholar] [CrossRef]
- Hser, Y.I.; Evans, E.; Huang, D.; Anglin, D.M. Relationship between drug treatment services, retention, and outcomes. Psychiatr. Serv. 2004, 55, 767–774. [Google Scholar] [CrossRef]
- De Leon, G. Retention in drug-free therapeutic communities. In Improving Drug Abuse Treatment; Pickens, R.W., Leukefeld, C.G., Schuster, C.R., Eds.; NIDA Research Monograph: Rockville, MD, USA, 1991. [Google Scholar]
- Condelli, W.S.; De Leon, G. Fixed and dynamic predictors of client retention in therapeutic communities. J. Subst. Abus. Treat. 1993, 10, 11–16. [Google Scholar] [CrossRef]
- Hubbard, R.L.; Craddock, S.G.; Flynn, P.M.; Anderson, J.; Etheridge, R.M. Overview of 1-year follow-up outcomes in the Drug Abuse Treatment Outcome Study (DATOS). Psychol. Addict. Behav. 1997, 11, 261–278. [Google Scholar] [CrossRef]
- Messina, N.; Wish, E.; Nemes, S. Predictors of treatment outcomes in men and women admitted to a therapeutic community. Am. J. Drug Alcohol Abus. 2000, 26, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Mulder, R.T.; Frampton, C.M.; Peka, H.; Hampton, G.; Marsters, T. Predictors of 3-month retention in a drug treatment therapeutic community. Drug Alcohol Rev. 2009, 28, 366–371. [Google Scholar] [CrossRef]
- Vergara-Moragues, E.; González-Saiz, F.; Lozano, O.M.; Verdejo-García, A. Psychiatric profile of three-month retention in cocaine-dependent patients treated in a therapeutic community. J. Stud. Alcohol Drugs 2013, 74, 452–459. [Google Scholar] [CrossRef]
- Egelko, S.; Galanter, M. Impact of social anxiety in a “therapeutic community”-oriented cocaine treatment clinic. Am. J. Addict. 1998, 7, 136–141. [Google Scholar] [CrossRef]
- Egelko, S.; Galanter, M.; Dermatis, H.; Jurewicz, E.; Jamison, A.; Dingle, S.; De Leon, G. Improved psychological status in a modified therapeutic community for homeless MICA men. J. Addict. Dis. 2002, 21, 75–92. [Google Scholar] [CrossRef]
- Polimeni, A.M.; Moore, S.M.; Gruenert, S. Mental health improvement of substance-dependent clients after 4 months in a therapeutic community. Drug Alcohol Rev. 2010, 29, 546–550. [Google Scholar] [CrossRef]
- McKee, S.A.; Harris, G.T.; Cormier, C.A. Implementing residential integrated treatment for co-occurring disorders. J. Dual Diagn. 2013, 9, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, N.S.; Truax, P. Clinical significance: A statistical approach to denning meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 1991, 59, 12–19. [Google Scholar] [CrossRef]
- Arenas, F.; del Valle, M.; López, R.; Martín, J.; Tirado, P. Programa de Intervención. Comunidad Terapéutica en Andalucía; Consejería de Asuntos Sociales. Comisionado para las Drogodependencias; Junta de Andalucía: Sevilla, Spain, 2003. [Google Scholar]
- American Psychiatric Association (APA). Diagnosis Statistical Manual of Mental Disorders (DSM-IV-R), 4th ed.; American Psychiatry Association: Washington, DC, USA, 2004. [Google Scholar]
- Nunes, E.V.; Liu, X.; Samet, S.; Matseoane, K.; Hasin, D. Independent versus substance-induced mayor depressive disorders in substance-dependent patients: Observational study of course during follow-up. J. Clin. Psychiatry 2006, 67, 1561–1567. [Google Scholar] [CrossRef]
- Lozano, O.M.; Rojas, A.; Pérez, C.; Apraiz, B.; Sánchez, F.; Marín, A. Test para la Evaluación de la Calidad de Vida en Adictos a Sustancias (TECVASP): Estudios de fiabilidad y validez. Trastor. Adict. 2007, 9, 99–109. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, A.T.; Rush, A.J.; Shaw, B.F.; Emery, G. Cognitive Therapy of Depression; Guilford: New York, NY, USA, 1979. [Google Scholar]
- Sanz, J.; García-Vera, M.P. Análisis psicométrico de las versiones breves del “Inventario para la Depresión de Beck” de 1978 (BDI-IA). Psicol. Conduct. 2007, 15, 191–214. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the State-Trait Anxiety Inventory; Consulting Psychologist Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Buela-Casal, G.; Guillén-Riquelme, A. Short form of the Spanish adaptation of the State-Trait Anxiety Inventory. Int. J. Clin. Health Psychol. 2017, 17, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Darke, S.; Hall, W.; Wodak, A.; Heather, N.; Ward, J. Development and validation of a multidimensional instrument for assessing outcome of treatment among opiate users: The Opiate Treatment Index. Br. J. Addict. 1992, 87, 733–742. [Google Scholar] [CrossRef]
- González-Saiz, F. Estandarización de un Instrumento de Evaluación Multidimensional en los Trastornos Adictivos. Ph.D. Thesis, Universidad de Cádiz, Cádiz, Spain, 1997. [Google Scholar]
- González-Saiz, F.; García-Valderrama, T. The Opiate Treatment Index (OTI) clinical interview: New evidence of its reliability and validity. Heroin Addict Relat. Clin. Probl. 2012, 14, 19–34. [Google Scholar]
- Hasin, D.; Trautman, K.; Miele, G.; Samet, S.; Smith, M.; Endicott, J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): Reliability for substance abusers. Am. J. Psychiatry 1996, 153, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Torrens, M.; Serrano, D.; Astals, M.; Perez-Dominguez, G.; Martin-Santos, R. Diagnosing comorbid psychiatric disorders in substance abusers: Validity of the Spanish versions of the Psychiatric Research Interview for Substance and Mental Disorders and the Structured Clinical Interview for DSM-IV. Am. J. Psychiatry 2004, 161, 1231–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conners, C.K.; Erhardt, D.; Sparrow, E. Conners’ Adult ADHD Rating Scales; Multi-Health Systems: North Tonawanda, NY, USA, 1999. [Google Scholar]
- Saez-Francàs, N.; Bosch, R.; Ramos-Quiroga, J.A.; Valero, S.; Nogueira, M.; Gómez, N.; Palomar, G.; Casas, M. Validación de la versión española de la Conners’ Adult ADHD Diagnostic Interview for DSM-IV. In Proceeding of the National Congress of Psychiatry, Valencia, Spain, 3 November 2008. [Google Scholar]
- Ramos Quiroga, J.A.; Bosch, R.; Richarte, V.; Valero, S.; Gómez-Barros, N.; Nogueira, M.; Palomar, G.; Corrales, M.; Saez-Francàs, N.; Corominas, M.; et al. Validez de criterio y concurrente de la versión española de la Conners’ Adult ADHD Diagnostic Interview for DSM-IV. Rev. Psiquiatr. Salud Ment. 2012, 5, 229–235. [Google Scholar] [CrossRef]
- Diener, E.; Ryan, K. Subjective well-being: A general overview. S. Afr. J. Psychol. 2009, 39, 391–406. [Google Scholar] [CrossRef]
- Iraurgi-Castillo, I. Evaluación de resultados clínicos (y III): Índices de Cambio Fiable (ICF) como estimadores del cambio clínicamente significativo. Norte Salud Ment. 2010, 8, 105–122. [Google Scholar]
- McLellan, A.T. Have we evaluated addiction treatment correctly? Implications from a chronic care perspective. Addiction 2002, 97, 249–252. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.T.; Arndt, I.O.; Metzger, D.S.; Woody, G.E.; O’Brien, C.P. The effects of psychosocial services in substance abuse treatment. JAMA 1993, 269, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
| Total Sample (n = 226) | Early Dropout Group (n = 84) | 3-Month Follow-Up Group (n = 142) | Statistical | p-Value | |
|---|---|---|---|---|---|
| Age, M(SD) | 34.66 (7.55) | 34.73 (7.93) | 34.62 (7.35) | t(224) = 0.102 | 0.919 |
| Gender, n (%) | |||||
| Women | 20 (8.8) | 9 (10.7) | 11 (7.7) | χ2(1) = 0.576 | 0.448 |
| Men | 206 (91.2) | 75 (89.3) | 131 (92.3) | ||
| Marital status, n (%) | |||||
| Single | 129 (57.1) | 38 (45.2) | 91 (64.1) | χ2(2) = 8.869 | 0.012 |
| Married/Partner | 43 (19.0) | 23 (27.4) | 20 (14.1) | ||
| Divorced/Separated/Widowed | 54 (23.9) | 23 (27.4) | 31 (21.8) | ||
| Educational level, n (%) | |||||
| Primary school | 99 (43.8) | 40 (47.6) | 59 (41.5) | χ2(1) = 0.790 | 0.374 |
| Secondary school | 127 (56.2) | 44 (52.4) | 83 (58.5) | ||
| Work status, n (%) | |||||
| Employed | 46 (20.4) | 13 (15.5) | 33 (23.2) | ||
| Unemployed | 152 (67.3) | 55 (65.5) | 97 (68.3) | χ2(2) = 6.410 | 0.041 |
| Retired/Disabled | 28 (12.4) | 16 (19.0) | 12 (8.5) | ||
| Criminal history, n (%) | 132 (58.4) | 52 (61.9) | 80 (56.3) | χ2(1) = 0.673 | 0.412 |
| Previous treatments for SUD a, n (%) | 181 (80.1) | 67 (79.8) | 114 (80.3) | χ2(1) =0.009 | 0.925 |
| Previous treatments for MD b, n (%) | 98 (43.4) | 40 (47.6) | 58 (40.8) | χ2(1) = 0.986 | 0.321 |
| Age at start of cocaine dependency, M(SD) | 24.27 (7.02) | 23.86 (7.92) | 24.52 (6.45) | t(224) = −0.682 | 0.496 |
| Severity of cocaine dependency, M(SD) | 6.50 (0.87) | 6.36 (1.02) | 6.57 (0.75) | t(224) = −1.747 | 0.108 |
| Lifetime SUD a, n (%) | |||||
| Heroin | 152 (67.3) | 59 (70.2) | 93 (65.5) | χ2(1) = 0.540 | 0.463 |
| Alcohol | 133 (58.8) | 51 (60.7) | 82 (57.7) | χ2(1) = 0.192 | 0.661 |
| Cannabis | 134 (59.3) | 53 (63.1) | 81 (57.0) | χ2(1) = 0.801 | 0.371 |
| Sedatives | 73 (32.3) | 30 (35.7) | 43 (30.3) | χ2(1) = 0.712 | 0.399 |
| Hallucinogens | 31 (13.7) | 12 (14.3) | 19 (13.4) | χ2(1) = 0.037 | 0.848 |
| Stimulants | 32 (14.2) | 13 (15.5) | 19 (13.4) | χ2(1) = 0.191 | 0.662 |
| OTI-HSS c, M(SD) | 3.93 (3.85) | 3.98 (4.92) | 3.90 (3.07) | t(224) = 0.134 | 0.881 |
| BDI d, M(SD) | 6.67 (5.64) | 7.75 (6.64) | 6.03 (4.88) | t(224) = 2.225 | 0.041 |
| STAI-state e, M(SD) | 18.66 (11.18) | 20.94 (12.58) | 17.32 (10.06) | t(224) = 2.374 | 0.026 |
| HRQOLDA f, M(SD) | 78.58 (14.16) | 78.73 (13.86) | 78.49 (14.39) | t(224) = 0.127 | 0.900 |
| Dual diagnosis g, n (%) | 156 (69.0) | 62 (73.8) | 94 (66.2) | χ2(1) = 1.431 | 0.232 |
| Baseline Assessment | 3-Month Follow-Up | Statistical | p-Value | Cohen’s d | |
|---|---|---|---|---|---|
| OTI-HSS a, M(SD) | 3.90 (3.07) | 1.28 (1.63) | t(141) = 9.825 | 0.000 | 0.86 |
| BDI b, M(SD) | 6.03 (4.88) | 5.05 (5.41) | t(141) = 2.22 | 0.027 | 0.20 |
| STAI-state c, M(SD) | 17.32 (10.06) | 17.68 (10.95) | t(141) = −0.410 | 0.682 | 0.03 |
| HRQOLDA d, M(SD) | 78.49 (14.39) | 91.79 (12.58) | t(141) = −11.625 | 0.000 | 0.92 |
| Follow-Up Total Sample (n = 142) | Treatment Outcomes (Clinical Impression) at Discharge | Statistical | p-Value | ||
|---|---|---|---|---|---|
| Non-Clinically Relevant Outcomes (n = 41) | Clinically Relevant Outcomes (n = 101) | ||||
| OTI-HSS a, M(SD) | 1.28 (1.63) | 1.46 (2.13) | 1.21 (1.38) | t(140) = 0.845 | 0.399 |
| BDI b, M(SD) | 5.05 (5.41) | 6.56 (5.49) | 4.44 (5.29) | t(140) = 2.145 | 0.034 |
| STAI-state c, M(SD) | 17.68 (10.95) | 21.12 (11.90) | 16.29 (10.27) | t(140) = 2.425 | 0.017 |
| HRQOLDA d, M(SD) | 91.79 (12.58) | 87.90 (15.19) | 93.37 (11.05) | t(140) = −2.384 | 0.041 |
| Follow-Up Total Sample (n = 142) | Treatment Outcome Based on Clinical Impression at Discharge | ||
|---|---|---|---|
| Non-Clinically Relevant Outcomes (n = 41) | Clinically Relevant Outcomes (n = 101) | ||
| OTI-HSS a, n (%) | |||
| Worsened b | 11 (7.7) | 5 (12.2) | 6 (5.9) |
| Unimproved c | 18 (12.7) | 6 (14.6) | 12 (11.9) |
| Improved d | 40 (28.2) | 9 (22.0) | 31 (30.7) |
| Recovered at cut-off point of 1SD e | 45 (31.7) | 10 (24.4) | 35 (34.7) |
| Recovered at cut-off point of 2SD f | 28 (19.7) | 11 (26.8) | 17 (16.8) |
| BDI g, n (%) | |||
| Worsened | 47 (33.1) | 16 (39.0) | 31 (30.7) |
| Unimproved | 15 (10.6) | 3 (7.3) | 12 (11.9) |
| Improved | 27 (19.0) | 9 (22.0) | 18 (17.8) |
| Recovered at cut-off point of 1SD | 18 (12.7) | 6 (14.6) | 12 (11.9) |
| Recovered at cut-off point of 2SD | 35 (24.6) | 7 (17.1) | 28 (27.7) |
| STAI-state h, n (%) | |||
| Worsened | 66 (46.5) | 23 (56.1) | 43 (42.6) |
| Unimproved | 7 (4.9) | 1 (2.4) | 6 (5.9) |
| Improved | 28 (19.7) | 6 (14.6) | 22 (21.8) |
| Recovered at cut-off point of 1SD | 22 (15.5) | 7 (17.1) | 15 (14.9) |
| Recovered at cut-off point of 2SD | 19 (13.4) | 4 (9.8) | 15 (14.9) |
| HRQOLDA i, n (%) | |||
| Worsened | 21 (14.8) | 7 (17.1) | 14 (13.9) |
| Unimproved | 3 (2.1) | 1 (2.4) | 2 (2.0) |
| Improved | 31 (21.8) | 10 (23.4) | 21 (20.8) |
| Recovered at cut-off point of 1SD | 36 (25.4) | 11 (26.8) | 25 (24.8) |
| Recovered at cut-off point of 2SD | 51 (35.9) | 12 (29.3) | 39 (38.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Saiz, F.; Vergara-Moragues, E. In-Treatment Changes in Quality of Life-Related Variables in Therapeutic Communities for Cocaine Abusers: Are These Changes Associated with Clinical Outcomes? Int. J. Environ. Res. Public Health 2021, 18, 7442. https://doi.org/10.3390/ijerph18147442
González-Saiz F, Vergara-Moragues E. In-Treatment Changes in Quality of Life-Related Variables in Therapeutic Communities for Cocaine Abusers: Are These Changes Associated with Clinical Outcomes? International Journal of Environmental Research and Public Health. 2021; 18(14):7442. https://doi.org/10.3390/ijerph18147442
Chicago/Turabian StyleGonzález-Saiz, Francisco, and Esperanza Vergara-Moragues. 2021. "In-Treatment Changes in Quality of Life-Related Variables in Therapeutic Communities for Cocaine Abusers: Are These Changes Associated with Clinical Outcomes?" International Journal of Environmental Research and Public Health 18, no. 14: 7442. https://doi.org/10.3390/ijerph18147442
APA StyleGonzález-Saiz, F., & Vergara-Moragues, E. (2021). In-Treatment Changes in Quality of Life-Related Variables in Therapeutic Communities for Cocaine Abusers: Are These Changes Associated with Clinical Outcomes? International Journal of Environmental Research and Public Health, 18(14), 7442. https://doi.org/10.3390/ijerph18147442







