Future Incidence of Malignant Mesothelioma in South Korea: Updated Projection to 2038
Abstract
1. Introduction
2. Materials and Methods
2.1. Data on MM Incidence
2.2. Data on Asbestos Consumption
2.3. Data on Population
2.4. APC Model
- Rap: Incidence rate for age group a in calendar period p;
- Aa: Age component for age group a;
- D: Common drift parameter which summarizes the linear component of trend;
- Pp: Non-linear period component of period p;
- Cc: Non-linear cohort component in cohort c.
2.5. Poisson Regression Model Based on Asbestos Consumption
- a: Age group (0–39, 40–49, 50–59, 60–69, 70–79, 80+);
- Y: Year of occurrence;
- Ma,Y: Age-specific incident cases of MM for each year;
- C: Asbestos consumption per capita (kg) for each year;
- d: Data available years for each age group;
- e: Exposed year (from birth to occurrence, with latency);
- Sex: Dummy variable of sex (0: women, 1: men);
- Agei: Dummy variables of age group for each age group (baseline: 0–39 years of age);
- Sex·Agei: Interaction variables for sex and age;
- Lt: Latent period, 40 years;
- β0: Intercept;
- β1,β2, βi1, βi2: Regression coefficients;
- εa,Y: Errors.
2.6. Statistical Analysis
3. Results
3.1. Prediction for 2014–2018 and Empirical Validation
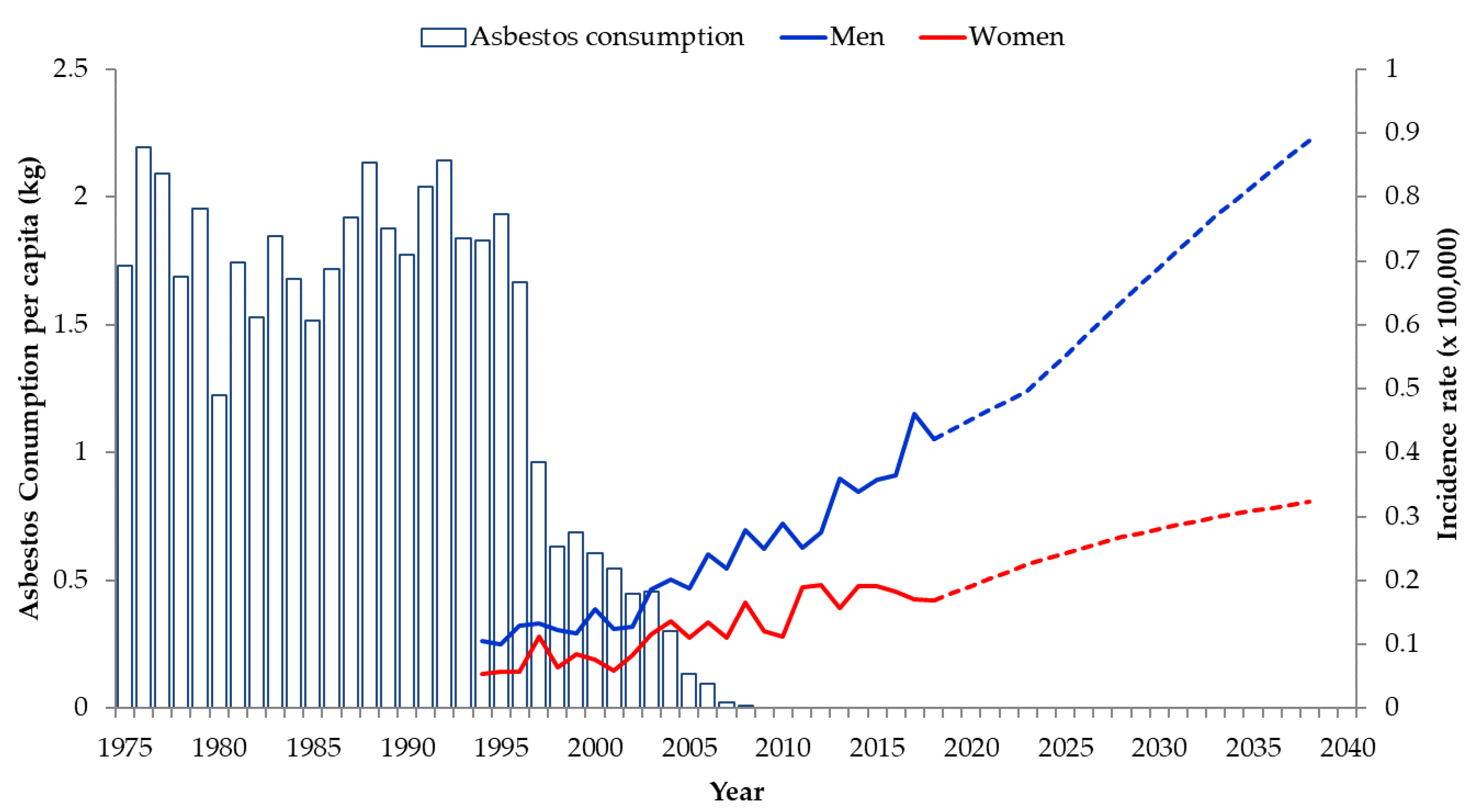
3.2. Predictions for 2019–2038 Based on the APC Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, B.M. Malignant pleural mesothelioma: An epidemiological perspective. Ann. Cardiothorac. Surg. 2012, 1, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Bianchi, T. Malignant Mesothelioma: Global Incidence and Relationship with Asbestos. Ind. Health 2007, 4, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Choi, S.; Ryu, K.; Park, J.; Paik, N. Trends in Occupational Asbestos Exposure and Asbestos Consumption over Recent Decades in Korea. Int. J. Occup. Environ. Health 2008, 14, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.M.; Paek, D.; Hwang, S.-S.; Ju, Y.-S. Estimated future incidence of malignant mesothelioma in South Korea: Projection from 2014 to 2033. PLoS ONE 2017, 12, e0183404. [Google Scholar] [CrossRef] [PubMed]
- Global Health Estimates 2020: Disease Burden by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/global-health-estimates-leading-causes-of-dalys (accessed on 18 May 2021).
- Health and Safety Executive (HSE). RR1074 Costs to Britain of Work-Related Cancer. Available online: http://www.hse.gov.uk/research/rrhtm/rr1074.htm (accessed on 18 May 2021).
- Furuya, S.; Chimed-Ochir, O.; Takahashi, K.; David, A.; Takala, J. Global Asbestos Disaster. Int. J. Environ. Res. Public Health 2018, 15, 1000. [Google Scholar] [CrossRef]
- Peto, J.; Matthews, F.; Hodgson, J.; Jones, J. Continuing increase in mesothelioma mortality in Britain. Lancet 1995, 345, 535–539. [Google Scholar] [CrossRef]
- Ilg, A.G.; Bignon, J.; Valleron, A.J. Estimation of the past and future burden of mortality from mesothelioma in France. Occup. Environ. Med. 1998, 55, 760–765. [Google Scholar] [CrossRef]
- Kjærgaard, J.; Andersson, M. Incidence rates of malignant mesothelioma in Denmark and predicted future number of cases among men. Scand. J. Work. Environ. Health 2000, 26, 112–117. [Google Scholar] [CrossRef]
- Segura, O.; Burdorf, A.; Looman, C. Update of predictions of mortality from pleural mesothelioma in the Netherlands. Occup. Environ. Med. 2003, 60, 50–55. [Google Scholar] [CrossRef]
- Price, B.; Ware, A. Mesothelioma Trends in the United States: An Update Based on Surveillance, Epidemiology, and End Results Program Data for 1973 through 2003. Am. J. Epidemiol. 2004, 159, 107–112. [Google Scholar] [CrossRef]
- Pitarque, S.; Clèries, R.; Martínez, J.M.; Lopez-Abente, G.; Kogevinas, M.; Benavides, F.G. Mesothelioma mortality in men: Trends during 1977–2001 and projections for 2002–2016 in Spain. Occup. Environ. Med. 2008, 65, 279–282. [Google Scholar] [CrossRef]
- Mensi, C.; De Matteis, S.; Dallari, B.; Riboldi, L.; Bertazzi, P.A.; Consonni, D. Incidence of mesothelioma in Lombardy, Italy: Exposure to asbestos, time patterns and future projections. Occup. Environ. Med. 2016, 73, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Huuskonen, M.S.; Tossavainen, A.; Higashi, T.; Okubo, T.; Rantanen, J. Ecological Relationship between Mesothelioma Incidence/Mortality and Asbestos Consumption in Ten Western Countries and Japan. J. Occup. Health 1999, 41, 8–11. [Google Scholar] [CrossRef]
- Tossavainen, A. Global Use of Asbestos and the Incidence of Mesothelioma. Int. J. Occup. Environ. Health 2004, 10, 22–25. [Google Scholar] [CrossRef]
- Lin, R.T.; Takahashi, K.; Karjalainen, A.; Hoshuyama, T.; Wilson, D.; Kameda, T.; Chan, C.C.; Wen, C.P.; Furuya, S.; Higashi, T.; et al. Ecological association between asbestos-related diseases and historical asbestos consumption: An international analysis. Lancet 2007, 369, 844–849. [Google Scholar] [CrossRef]
- Park, E.K.; Takahashi, K.; Hoshuyama, T.; Cheng, T.J.; Delgermaa, V.; Le, G.V.; Sorahan, T. Global Magnitude of Reported and Unreported Mesothelioma. Environ. Health Perspect. 2011, 119, 514–518. [Google Scholar] [CrossRef]
- Bahk, J.W. The Global Spread of Asbestos Ban Policy and It’s Impacts and Implications on Environmental Health and Safety. Ph.D. Thesis, Seoul National University, Seoul, Korea, August 2013. [Google Scholar]
- Hodgson, J.T.; McElvenny, D.M.; Darnton, A.J.; Price, M.J.; Peto, J. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br. J. Cancer 2005, 92, 587–593. [Google Scholar] [CrossRef]
- Korean Statistical Information Service. Cancer Incident Cases and Incidence Rates by Site, Sex, Age Group. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=117&tblId=DT_117N_A0024&conn_path=I2 (accessed on 17 February 2021).
- Virta, R.L. Worldwide Asbestos Supply and Consumption Trends from 1900 through 2003. U.S. Geological Survey Circular 1298; U.S. Department of the Interior: Washington, DC, USA, 2006. Available online: https://pubs.usgs.gov/circ/2006/1298 (accessed on 25 March 2020).
- Korean Statistical Information Service. Resident Population by City, County, and District in Five-Year Age Groups. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B040M5&conn_path=I2 (accessed on 19 February 2021).
- Korean Statistical Information Service. Projected Population by Age Group. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1BPA001&conn_path=I2 (accessed on 19 February 2021).
- Antoni, S.; Soerjomataram, I.; Møller, B.; Bray, F.; Ferlay, J. An assessment of GLOBOCAN methods for deriving national estimates of cancer incidence. Bull. World Health Organ. 2016, 94, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.; Fairley, L.; Coupland, V.; Okello, C.; Green, M.; Forman, D.; Møller, B.; Bray, F. The future burden of cancer in England: Incidence and numbers of new patients in 2020. Br. J. Cancer 2007, 96, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- NORDPRED Software Package. Available online: https://www.kreftregisteret.no/en/Research/Projects/Nordpred/Nordpred-software (accessed on 28 February 2020).
- Mitchell, P.L.; Sheehy, J.E. Comparison of predictions and observations to assess model performance: A method of empirical validation. In Applications of Systems Approaches at the Field Level; Kropff, M.J., Teng, P.S., Aggarwal, P.K., Bouma, J., Bouman, B.A.M., Jones, J.W., van Laar, H.H., Eds.; Springer: Dordrecht, The Netherlands, 1997; Volume 2, pp. 437–451. [Google Scholar] [CrossRef]
- Park, Y.; Freedman, A.N.; Gail, M.H.; Pee, D.; Hollenbeck, A.; Schatzkin, A.; Pfeiffer, R.M. Validation of a Colorectal Cancer Risk Prediction Model among White Patients Age 50 Years and Older. J. Clin. Oncol. 2009, 27, 694–698. [Google Scholar] [CrossRef]
- Shin, H.-R.; Won, Y.-J.; Jung, K.-W.; Kong, H.-J.; Yim, S.-H.; Lee, J.-K.; Noh, H.-I.; Lee, J.-K.; Pisani, P.; Park, J.-G.; et al. Nationwide Cancer Incidence in Korea, 1999~2001; First Result Using the National Cancer Incidence Database. Cancer Res. Treat. 2005, 37, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Health and Safety Executive. Mesothelioma Statistics for Great Britain, 2020; Health and Safety Executive: London, UK, 2020. Available online: https://www.hse.gov.uk/Statistics/causdis/mesothelioma/mesothelioma.pdf (accessed on 14 March 2021).
- Sundhedsdatastyrelsen. Nye Kræfttilfælde i Danmark, Cancerregisteret 2019; Sundhedsdatastyrelsen: Copenhagen, Denmark, 2021; Available online: https://sundhedsdatastyrelsen.dk/-/media/sds/filer/find-tal-og-analyser/sygdomme/cancerregisteret/kraefttilfaelde-2019.pdf (accessed on 12 April 2021). (In Danish)
- Netherlands Cancer Registry. Mortality, Mesothelioma, Male and Female. Available online: https://www.iknl.nl/en/ncr (accessed on 11 April 2020).
- Henley, S.J.; Larson, T.C.; Wu, M.; Antao, V.C.; Lewis, M.; Pinheiro, G.A.; Eheman, C. Mesothelioma incidence in 50 states and the District of Columbia, United States, 2003–2008. Int. J. Occup. Environ. Health 2013, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.P.; Baez, J.; Stern, M.E.C.; Takahashi, K.; George, F. Trends and the Economic Effect of Asbestos Bans and Decline in Asbestos Consumption and Production Worldwide. Int. J. Environ. Res. Public Health 2018, 15, 531. [Google Scholar] [CrossRef] [PubMed]
- Würtz, E.T.; Hansen, J.; Røe, O.D.; Omland, Ø. Asbestos exposure and haematological malignancies: A Danish cohort study. Eur. J. Epidemiol. 2020, 35, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Tobin, K.; Gilthorpe, M.S.; Rooney, J.; Heverin, M.; Vajda, A.; Staines, A.; Hardiman, O. Age-period-cohort analysis of trends in amyotrophic lateral sclerosis incidence. J. Neurol. 2016, 263, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
| Sex | Observed Cases | Predicted Cases for 2014–2018 | E/O Ratio (95% CI) * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1994–1998 | 1999–2003 | 2004–2008 | 2009–2013 | 2014–2018 | APC Model | Poisson Model | APC Model | Poisson Model | |
| Men | 137 | 171 | 277 | 361 | 501 | 461 | 634 | 0.920 (0.843–1.004) | 1.265 (1.159–1.381) |
| Women | 79 | 100 | 161 | 195 | 233 | 254 | 351 | 1.090 (0.999–1.119) | 1.506 (1.380–1.644) |
| Age Group | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Observed Cases | Predicted Cases | E/O Ratio (95% CI) * | Observed Cases | Predicted Cases | E/O Ratio (95% CI) * | |||||
| APC Model | Poisson Model | APC Model | Poisson Model | APC Model | Poisson Model | APC Model | Poisson Model | |||
| 0–39 | 11 | 11.6 | 17.8 | 1.055 (0.966–1.151) | 1.618 (1.483–1.766) | 15 | 19.7 | 13.5 | 1.313 (1.203–1.434) | 0.9 (0.825–0.982) |
| 40–49 | 25 | 38.8 | 95.4 | 1.552 (1.422–1.694) | 3.816 (3.496–4.165) | 20 | 13.8 | 45.9 | 0.69 (0.632–0.753) | 2.295 (2.103–2.505) |
| 50–59 | 95 | 95 | 275.1 | 1 (0.916–1.092) | 2.896 (2.653–3.161) | 50 | 61.6 | 132.1 | 1.232 (1.129–1.345) | 2.642 (2.420–2.884) |
| 60–69 | 165 | 121 | 141.0 | 0.733 (0.672–0.800) | 0.855 (0.783–0.933) | 60 | 76.8 | 87.4 | 1.28 (1.173–1.397) | 1.457 (1.335–1.590) |
| 70–79 | 157 | 163.2 | 101.5 | 1.039 (0.952–1.135) | 0.646 (0.592–0.706) | 61 | 50.4 | 56.3 | 0.826 (0.757–0.902) | 0.923 (0.846–1.007) |
| 80+ | 48 | 31.3 | 12.0 | 0.652 (0.597–0.712) | 0.25 (0.229–0.273) | 27 | 31.2 | 16.0 | 1.156 (1.059–1.261) | 0.593 (0.543–0.647) |
| 501 | 460.9 | 642.7 | 0.920 (0.843–1.004) | 1.265 (1.159–1.381) | 233 | 253.6 | 351.3 | 1.090 (0.999–1.119) | 1.506 (1.380–1.644) | |
| Sex | Observed | Predicted | ||||||
|---|---|---|---|---|---|---|---|---|
| 1999–2003 | 2004–2008 | 2009–2013 | 2014–2018 | 2019–2023 | 2024–2028 | 2029–2033 | 2034–2038 | |
| Men | 171 | 277 | 361 | 501 | 645 | 825 | 999 | 1141 |
| Women | 100 | 161 | 195 | 233 | 291 | 348 | 389 | 417 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, K.; Cho, S.-i.; Paek, D. Future Incidence of Malignant Mesothelioma in South Korea: Updated Projection to 2038. Int. J. Environ. Res. Public Health 2021, 18, 6614. https://doi.org/10.3390/ijerph18126614
Kwak K, Cho S-i, Paek D. Future Incidence of Malignant Mesothelioma in South Korea: Updated Projection to 2038. International Journal of Environmental Research and Public Health. 2021; 18(12):6614. https://doi.org/10.3390/ijerph18126614
Chicago/Turabian StyleKwak, Kyeongmin, Sung-il Cho, and Domyung Paek. 2021. "Future Incidence of Malignant Mesothelioma in South Korea: Updated Projection to 2038" International Journal of Environmental Research and Public Health 18, no. 12: 6614. https://doi.org/10.3390/ijerph18126614
APA StyleKwak, K., Cho, S.-i., & Paek, D. (2021). Future Incidence of Malignant Mesothelioma in South Korea: Updated Projection to 2038. International Journal of Environmental Research and Public Health, 18(12), 6614. https://doi.org/10.3390/ijerph18126614






