A Review of Breast Imaging for Timely Diagnosis of Disease
Abstract
1. Introduction
2. Morphological Imaging
3. Advent of Screening Mammography
4. Digital Mammography
5. Digital Breast Tomosynthesis
6. Ultrasound
7. Elastosonography
8. Functional Breast Imaging
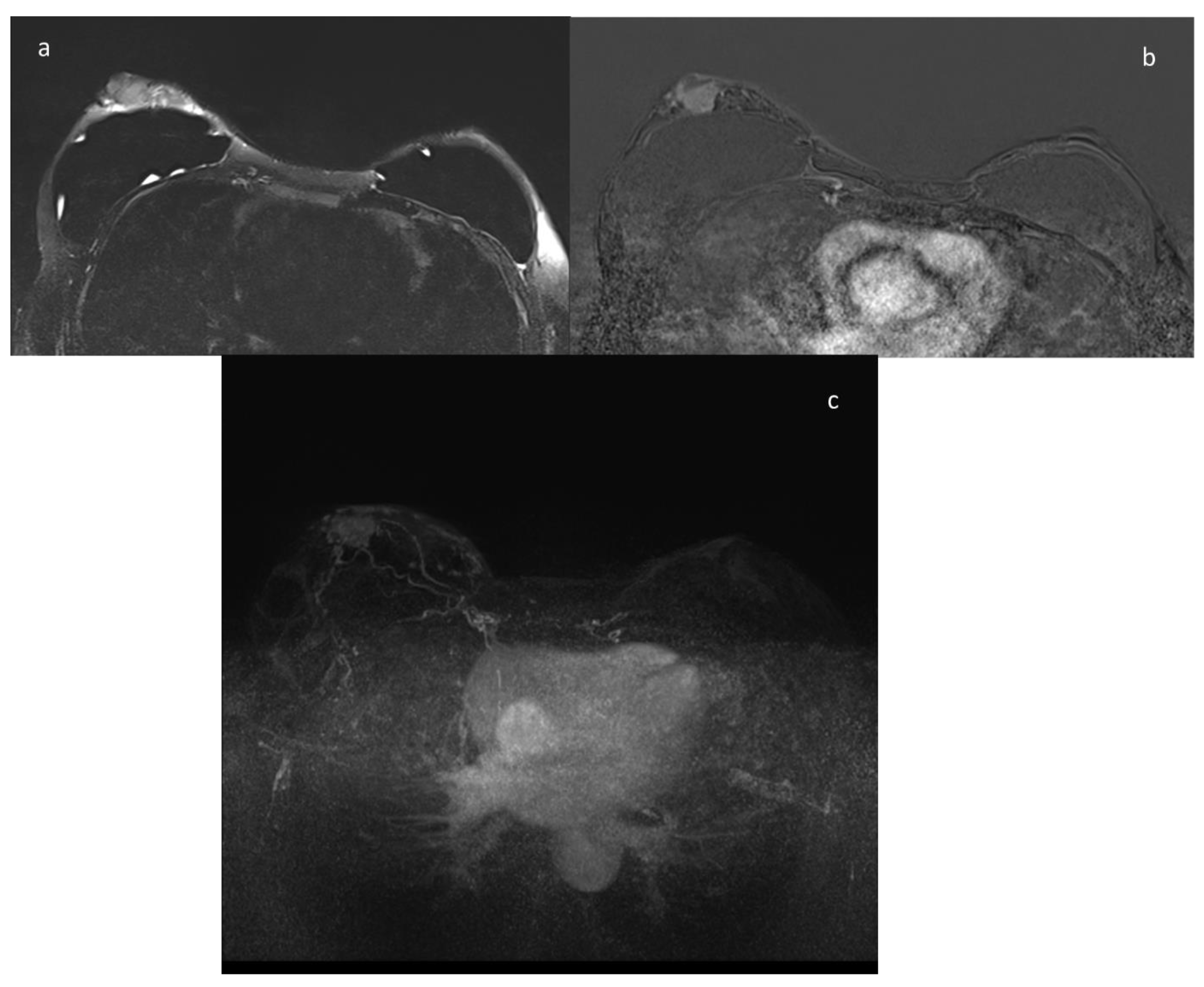
9. ce-MRI
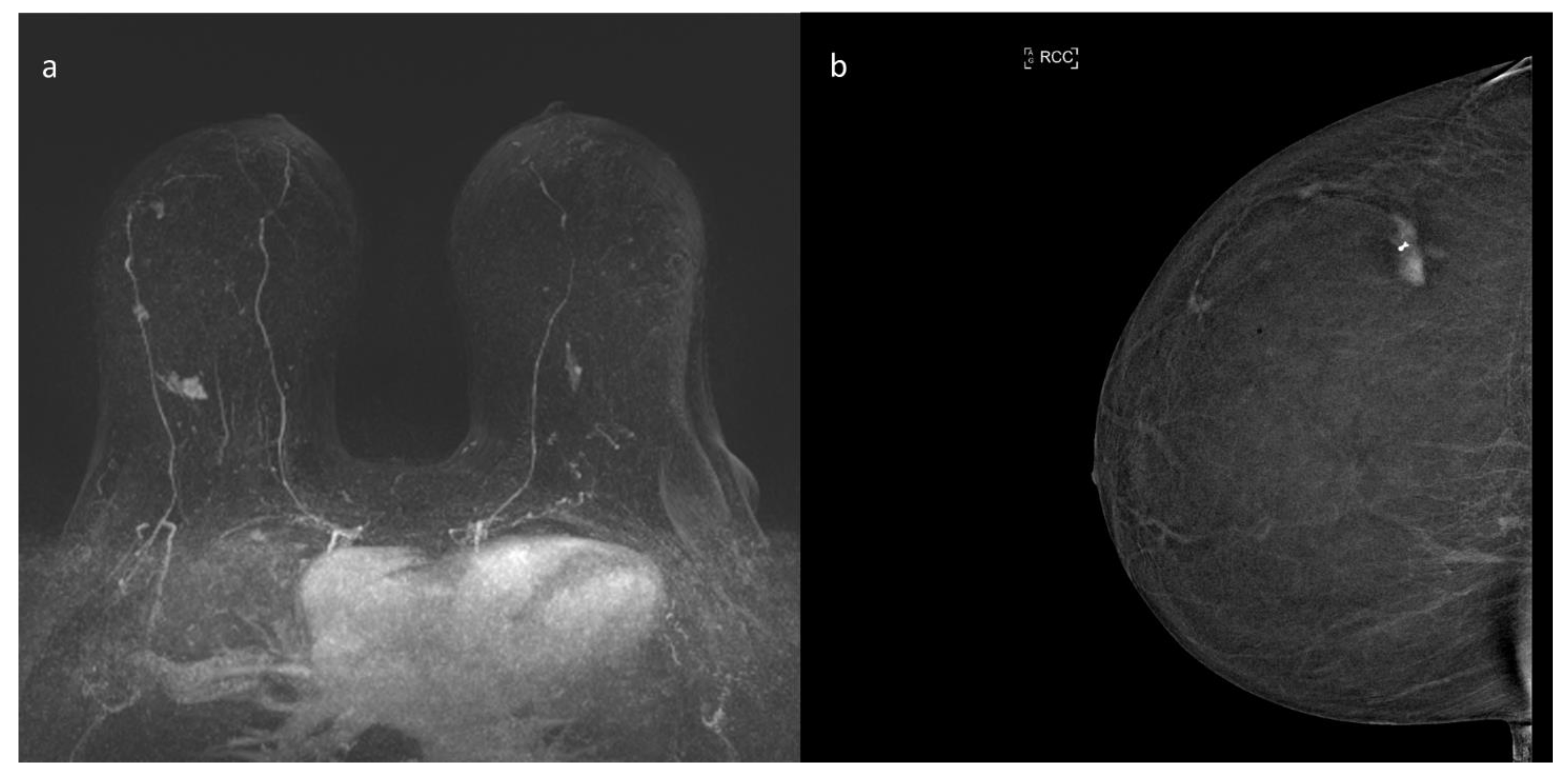
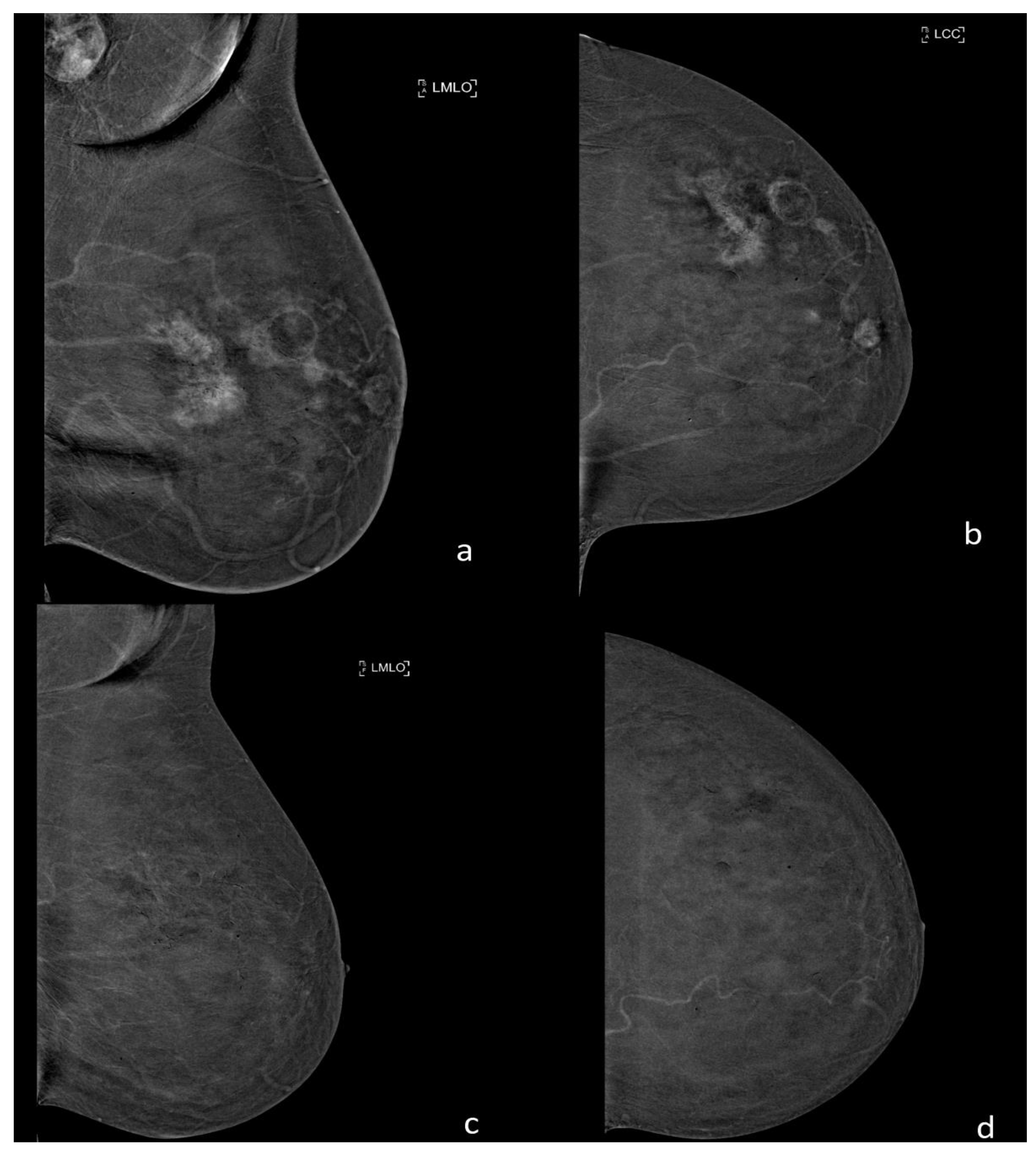
10. Contrast Enhanced Mammography (CEM)
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- The Lancet. GLOBOCAN 2018: Counting the toll of cancer. Lancet 2018, 392, 985, Erratum in 2018, 392, 1196. [Google Scholar] [CrossRef]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Pediconi, F.; Galati, F.; Bernardi, D.; Belli, P.; Brancato, B.; Calabrese, M.; Camera, L.; Carbonaro, L.A.; Caumo, F.; Clauser, P.; et al. Breast imaging and cancer diagnosis during the COVID-19 pandemic: Recommendations from the Italian College of Breast Radiologists by SIRM. Radiol. Med. 2020, 125, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.W.; Tabár, L.; Yen, A.M.; Dean, P.B.; Smith, R.A.; Jonsson, H.; Törnberg, S.; Chen, S.L.; Chiu, S.Y.; Fann, J.C.; et al. Mammography screening reduces rates of advanced and fatal breast cancers: Results in 549,091 women. Cancer 2020, 126, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- Carioli, G.; Malvezzi, M.; Rodriguez, T.; Bertuccio, P.; Negri, E.; La Vecchia, C. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast 2017, 36, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Joe, B.N.; Sickles, E.A. The evolution of breast imaging: Past to present. Radiology 2014, 273 (Suppl. S2), S23–S44. [Google Scholar] [CrossRef] [PubMed]
- Strax, P.; Venet, L.; Shapiro, S. Value of mammography in reduction of mortality from breast cancer in mass screening. Am. J. Roentgenol. Radium. 1973, 117, 686–689. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Fontham, E.T.; Etzioni, R.; Herzig, A.; Michaelson, J.S.; Shih, Y.C.; Walter, L.C.; Church, T.R.; Flowers, C.R.; LaMonte, S.J.; et al. American Cancer Society. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update from the American Cancer Society. JAMA 2015, 314, 1599–1614, Erratum in 2016, 315, 1406. [Google Scholar] [CrossRef]
- Shapiro, S.; Strax, P.; Venet, L. Periodic breast cancer screening in reducing mortality from breast cancer. JAMA 1971, 215, 1777–1785. [Google Scholar] [CrossRef]
- Tabár, L.; Fagerberg, C.J.; Gad, A.; Baldetorp, L.; Holmberg, L.H.; Gröntoft, O.; Ljungquist, U.; Lundström, B.; Månson, J.C.; Eklund, G.; et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet 1985, 1, 829–832. [Google Scholar] [CrossRef]
- Tabár, L.; Vitak, B.; Chen, T.H.; Yen, A.M.; Cohen, A.; Tot, T.; Chiu, S.Y.; Chen, S.L.; Fann, J.C.; Rosell, J.; et al. Swedish two-county trial: Impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011, 260, 658–663. [Google Scholar] [CrossRef]
- Nyström, L.; Andersson, I.; Bjurstam, N.; Frisell, J.; Nordenskjöld, B.; Rutqvist, L.E. Long-term effects of mammography screening: Updated overview of the Swedish randomised trials. Lancet 2002, 359, 909–919. [Google Scholar] [CrossRef]
- Bjurstam, N.; Björneld, L.; Warwick, J.; Sala, E.; Duffy, S.W.; Nyström, L.; Walker, N.; Cahlin, E.; Eriksson, O.; Hafström, L.O.; et al. The Gothenburg Breast Screening Trial. Cancer 2003, 97, 2387–2396. [Google Scholar] [CrossRef]
- Broeders, M.; Moss, S.; Nyström, L.; EUROSCREEN Working Group; Njor, S.; Jonsson, H.; Paap, E.; Massat, N.; Duffy, S.; Lynge, E.; et al. The impact of mammographic screening on breast cancer mortality in Europe: A review of observational studies. J. Med. Screen. 2012, 19 (Suppl. S1), 14–25. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Duffy, S.W.; Gabe, R.; Tabar, L.; Yen, A.M.; Chen, T.H. The randomized trials of breast cancer screening: What have we learned? Radiol. Clin. N. Am. 2004, 42, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Alexander, F.E.; Anderson, T.J.; Brown, H.K.; Forrest, A.P.; Hepburn, W.; Kirkpatrick, A.E.; Muir, B.B.; Prescott, R.J.; Smith, A. 14 years of follow-up from the Edinburgh randomised trial of breast-cancer screening. Lancet 1999, 353, 1903–1908. [Google Scholar] [CrossRef]
- Coldman, A.; Phillips, N.; Wilson, C.; Decker, K.; Chiarelli, A.M.; Brisson, J.; Zhang, B.; Payne, J.; Doyle, G.; Ahmad, R. Pan-Canadian study of mammography screening and mortality from breast cancer. J. Natl. Cancer Inst. 2014, 106, dju261, Erratum in 2015, 107, dju404. [Google Scholar] [CrossRef]
- Zuley, M. How to transition to digital mammography. J. Am. Coll. Radiol. 2007, 4, 178–183. [Google Scholar] [CrossRef]
- Pisano, E.D.; Gatsonis, C.; Hendrick, E.; Yaffe, M.; Baum, J.K.; Acharyya, S.; Conant, E.F.; Fajardo, L.L.; Bassett, L.; D’Orsi, C.; et al. Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group.; Diagnostic performance of digital versus film mammography for breast-cancer screening. N. Engl. J. Med. 2005, 353, 1773–1783, Erratum in 2006, 355, 1840. [Google Scholar] [CrossRef] [PubMed]
- Sickles, E.A. Breast imaging: From 1965 to the present. Radiology 2000, 215, 1–16. [Google Scholar] [CrossRef]
- American College of Radiology. BI-RADS Atlas, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Kopans, D.B. Standardized mammography reporting. Radiol. Clin. N. Am. 1992, 30, 257–264. [Google Scholar]
- Carney, P.A.; Miglioretti, D.L.; Yankaskas, B.C.; Kerlikowske, K.; Rosenberg, R.; Rutter, C.M.; Geller, B.M.; Abraham, L.A.; Taplin, S.H.; Dignan, M.; et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann. Intern. Med. 2003, 138, 168–175, Erratum in 2003, 138, 771. [Google Scholar] [CrossRef] [PubMed]
- Mandelson, M.T.; Oestreicher, N.; Porter, P.L.; White, D.; Finder, C.A.; Taplin, S.H.; White, E. Breast density as a predictor of mammographic detection: Comparison of interval- and screen-detected cancers. J. Natl. Cancer Inst. 2000, 92, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Bhargavan-Chatfield, M.; Burnside, E.S.; Nagy, P.; Sickles, E.A. The National Mammography Database: Preliminary Data. AJR Am. J. Roentgenol. 2016, 206, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Miglioretti, D.L.; Ichikawa, L.; Smith, R.A.; Bassett, L.W.; Feig, S.A.; Monsees, B.; Parikh, J.R.; Rosenberg, R.D.; Sickles, E.A.; Carney, P.A. Criteria for identifying radiologists with acceptable screening mammography interpretive performance on basis of multiple performance measures. AJR Am. J. Roentgenol. 2015, 204, W486–W491. [Google Scholar] [CrossRef]
- Mainiero, M.B.; Lourenco, A.; Mahoney, M.C.; Newell, M.S.; Bailey, L.; Barke, L.D.; D’Orsi, C.; Harvey, J.A.; Hayes, M.K.; Huynh, P.T.; et al. ACR Appropriateness Criteria Breast Cancer Screening. J. Am. Coll. Radiol. 2016, 126, 200–205. [Google Scholar] [CrossRef]
- Carbonaro, L.A.; Rizzo, S.S.; Schiaffino, S.; Pisani Mainini, A.; Berger, N.; Trimboli, R.M.; Sardanelli, F. Biennial screening mammography: How many women ask for more? Estimate of the interval mammogram rate in an organised population-based screening programme. Radiol. Med. 2021, 126, 200–205. [Google Scholar] [CrossRef]
- Chong, A.; Weinstein, S.P.; McDonald, E.S.; Conant, E.F. Digital Breast Tomosynthesis: Concepts and Clinical Practice. Radiology 2019, 292, 1–14. [Google Scholar] [CrossRef]
- Seo, M.; Chang, J.M.; Kim, S.A.; Kim, W.H.; Lim, J.H.; Lee, S.H.; Bae, M.S.; Koo, H.R.; Cho, N.; Moon, W.K. Addition of Digital Breast Tomosynthesis to Full-Field Digital Mammography in the Diagnostic Setting: Additional Value and Cancer Detectability. J. Breast Cancer 2016, 19, 438–446. [Google Scholar] [CrossRef]
- Skaane, P.; Sebuødegård, S.; Bandos, A.I.; Gur, D.; Østerås, B.H.; Gullien, R.; Hofvind, S. Performance of breast cancer screening using digital breast tomosynthesis: Results from the prospective population-based Oslo Tomosynthesis Screening Trial. Breast Cancer Res. Treat. 2018, 169, 489–496. [Google Scholar] [CrossRef]
- Bernardi, D.; Macaskill, P.; Pellegrini, M.; Valentini, M.; Fantò, C.; Ostillio, L.; Tuttobene, P.; Luparia, A.; Houssami, N. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): A population-based prospective study. Lancet Oncol. 2016, 17, 1105–1113. [Google Scholar] [CrossRef]
- Moger, T.A.; Swanson, J.O.; Holen, Å.S.; Hanestad, B.; Hofvind, S. Cost differences between digital tomosynthesis and standard digital mammography in a breast cancer screening programme: Results from the To-Be trial in Norway. Eur. J. Health Econ. 2019, 20, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N. Overdiagnosis of breast cancer in population screening: Does it make breast screening worthless? Cancer Biol. Med. 2017, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Caumo, F.; Romanucci, G.; Hunter, K.; Zorzi, M.; Brunelli, S.; Macaskill, P.; Houssami, N. Comparison of breast cancers detected in the Verona screening program following transition to digital breast tomosynthesis screening with cancers detected at digital mammography screening. Breast Cancer Res. Treat. 2018, 170, 391–397. [Google Scholar] [CrossRef]
- Brem, R.F.; Lenihan, M.J.; Lieberman, J.; Torrente, J. Screening breast ultrasound: Past, present, and future. AJR Am. J. Roentgenol. 2015, 204, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kolb, T.M.; Lichy, J.; Newhouse, J.H. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology 2002, 225, 165–175. [Google Scholar] [CrossRef]
- Berg, W.A.; Blume, J.D.; Cormack, J.B.; Mendelson, E.B.; Lehrer, D.; Böhm-Vélez, M.; Pisano, E.D.; Jong, R.A.; Evans, W.P.; Morton, M.J. ACRIN 6666 Investigators. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008, 299, 2151–2163. [Google Scholar] [CrossRef]
- Kaplan, S.S. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology 2001, 221, 641–649. [Google Scholar] [CrossRef]
- Zanotel, M.; Bednarova, I.; Londero, V.; Linda, A.; Lorenzon, M.; Girometti, R.; Zuiani, C. Automated breast ultrasound: Basic principles and emerging clinical applications. Radiol. Med. 2018, 123, 1–12. [Google Scholar] [CrossRef]
- Kaplan, S.S. Automated whole breast ultrasound. Radiol. Clin. N. Am. 2014, 52, 539–546. [Google Scholar] [CrossRef]
- Schiaffino, S.; Gristina, L.; Tosto, S.; Massone, E.; De Giorgis, S.; Garlaschi, A.; Tagliafico, A.; Calabrese, M. The value of coronal view as a stand-alone assessment in women undergoing automated breast ultrasound. Radiol. Med. 2021, 126, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.M.; Dean, J.; Comulada, W.S.; Lee, S.J. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur. Radiol. 2010, 20, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, N.; De Giorgis, S.; Zawaideh, J.; Rossi, F.; Calabrese, M.; Tagliafico, A.S. Comparison between execution reading time of 3DABUS versus, H.H.U.S. Radiol. Med. 2020, 125, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Schäfgen, B.; Juskic, M.; Radicke, M.; Hertel, M.; Barr, R.; Pfob, A.; Togawa, R.; Nees, J.; von Au, A.; Fastner, S.; et al. Evaluation of the FUSION-X-US-II prototype to combine automated breast ultrasound and tomosynthesis. Eur. Radiol. 2020, 44, 734–742. [Google Scholar] [CrossRef]
- Larson, E.D.; Lee, W.M.; Roubidoux, M.A.; Goodsitt, M.M.; Lashbrook, C.; Davis, C.E.; Kripfgans, O.D.; Carson, P.L. Preliminary Clinical Experience with a Combined Automated Breast Ultrasound and Digital Breast Tomosynthesis System. Ultrasound Med. Biol. 2018, 44, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef]
- Parker, K.J.; Huang, S.R.; Musulin, R.A.; Lerner, R.M. Tissue response to mechanical vibrations for “sonoelasticity imaging”. Ultrasound Med. Biol. 1990, 16, 241–246. [Google Scholar] [CrossRef]
- Giuseppetti, G.M.; Martegani, A.; Di Cioccio, B.; Baldassarre, S. Elastosonography in the diagnosis of the nodular breast lesions: Preliminary report. Radiol. Med. 2005, 110, 69–76. [Google Scholar]
- Lee, S.H.; Chang, J.M.; Kim, W.H.; Bae, M.S.; Cho, N.; Yi, A.; Koo, H.R.; Kim, S.J.; Kim, J.Y.; Moon, W.K. Differentiation of benign from malignant solid breast masses: Comparison of two-dimensional and three-dimensional shear-wave elastography. Eur. Radiol. 2013, 23, 1015–1026. [Google Scholar] [CrossRef]
- Ricci, P.; Cantisani, V.; Ballesio, L.; Pagliara, E.; Sallusti, E.; Drudi, F.M.; Trippa, F.; Calascibetta, F.; Erturk, S.M.; Modesti, M.; et al. Benign and malignant breast lesions: Efficacy of real time contrast-enhanced ultrasound vs. magnetic resonance imaging. Ultraschall Med.-Eur. J. Ultrasound 2007, 28, 57–62. [Google Scholar] [CrossRef]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Lewin, J. Comparison of Contrast-Enhanced Mammography and Contrast-Enhanced Breast MR Imaging. Magn. Reson. Imaging Clin. 2018, 26, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Heywang-Köbrunner, S.H.; Sinnatamby, R.; Lebeau, A.; Lebrecht, A.; Britton, P.D.; Schreer, I.; Consensus Group. Interdisciplinary consensus on the uses and technique of MR-guided vacuum-assisted breast biopsy (VAB): Results of a European consensus meeting. Eur. J. Radiol. 2009, 72, 289–294. [Google Scholar] [CrossRef]
- Pinker, K.; Helbich, T.H.; Morris, E.A. The potential of multiparametric MRI of the breast. Br. J. Radiol. 2017, 90, 20160715, Erratum in 2017, 90, 20160715e. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Zhang, K.; Liu, Y.; Cui, J.; Tao, J.; Wang, Y.; Wang, S. Invasive ductal breast cancer: Preoperative predict Ki-67 index based on radiomics of ADC maps. Radiol. Med. 2020, 125, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Jang, M.; Kim, S.M.; La Yun, B.; Lee, S.H. Usefulness of preoperative breast magnetic resonance imaging with a dedicated axillary sequence for the detection of axillary lymph node metastasis in patients with early ductal breast cancer. Radiol. Med. 2019, 124, 1220–1228. [Google Scholar] [CrossRef]
- Sardanelli, F.; Trimboli, R.M.; Houssami, N.; Gilbert, F.J.; Helbich, T.H.; Alvarez Benito, M.; Balleyguier, C.; Bazzocchi, M.; Bult, P.; Calabrese, M.; et al. Solving the preoperative breast MRI conundrum: Design and protocol of the MIPA study. Eur. Radiol. 2020, 30, 5427–5436. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, L.; Brown, S.; Harvey, I.; Olivier, C.; Drew, P.; Napp, V.; Hanby, A.; Brown, J. Comparative effectiveness of MRI in breast cancer (COMICE) trial: A randomised controlled trial. Lancet 2010, 375, 563–571. [Google Scholar] [CrossRef]
- Peters, N.H.; van Esser, S.; van den Bosch, M.A.; Torm, R.K.; Plaisier, P.W.; van Dalen, T.; Diepstraten, S.C.; Weits, T.; Westenend, P.J.; Stapper, G.; et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: The MONET-randomised controlled trial. Eur. J. Cancer 2011, 47, 879–886. [Google Scholar] [CrossRef]
- Houssami, N.; Turner, R.M.; Morrow, M. Meta-analysis of pre-operative magnetic resonance imaging (MRI) and surgical treatment for breast cancer. Breast Cancer Res. Treat. 2017, 165, 273–283. [Google Scholar] [CrossRef]
- Monticciolo, D.L.; Newell, M.S.; Moy, L.; Niell, B.; Monsees, B.; Sickles, E.A. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations from the ACR. J. Am. Coll. Radiol. 2018, 15 Pt 3, 408–414. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Houssami, N.; Macaskill, P.; Sardanelli, F.; Irwig, L.; Mamounas, E.P.; von Minckwitz, G.; Brennan, M.E.; Ciatto, S. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J. Natl. Cancer Inst. 2013, 105, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Pentheroudakis, G.; Lazaridis, G.; Pavlidis, N. Axillary nodal metastases from carcinoma of unknown primary (CUPAx): A systematic review of published evidence. Breast Cancer Res. Treat. 2010, 119, 1–11. [Google Scholar] [CrossRef]
- Bennani-Baiti, B.; Bennani-Baiti, N.; Baltzer, P.A. Diagnostic Performance of Breast Magnetic Resonance Imaging in Non-Calcified Equivocal Breast Findings: Results from a Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0160346. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Mihai, G.; Hassonjee, S.E.; Raj, S.D.; Palmer, M.R.; Brook, A.; Zhang, D. Comparative Dose of Contrast-Enhanced Spectral Mammography (CESM), Digital Mammography, and Digital Breast Tomosynthesis. AJR Am. J. Roentgenol. 2018, 211, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Bicchierai, G.; Amato, F.; Vanzi, B.; De Benedetto, D.; Boeri, C.; Vanzi, E.; Di Naro, F.; Bianchi, S.; Cirone, D.; Cozzi, D.; et al. Which clinical, radiological, histological, and molecular parameters are associated with the absence of enhancement of known breast cancers with Contrast Enhanced Digital Mammography (CEDM)? Breast 2020, 54, 15–24. [Google Scholar] [CrossRef]
- Zanardo, M.; Cozzi, A.; Trimboli, R.M.; Labaj, O.; Monti, C.B.; Schiaffino, S.; Carbonaro, L.A.; Sardanelli, F. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): A systematic review. Insights Imaging 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Jochelson, M.S.; Dershaw, D.D.; Sung, J.S.; Heerdt, A.S.; Thornton, C.; Moskowitz, C.S.; Ferrara, J.; Morris, E.A. Bilateral contrast-enhanced dual-energy digital mammography: Feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013, 266, 743–751. [Google Scholar] [CrossRef]
- Łuczyńska, E.; Heinze-Paluchowska, S.; Hendrick, E.; Dyczek, S.; Ryś, J.; Herman, K.; Blecharz, P.; Jakubowicz, J. Comparison between breast MRI and contrast-enhanced spectral mammography. Med. Sci. Monit. 2015, 21, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Fallenberg, E.M.; Schmitzberger, F.F.; Amer, H.; Ingold-Heppner, B.; Balleyguier, C.; Diekmann, F.; Engelken, F.; Mann, R.M.; Renz, D.M.; Bick, U.; et al. Contrast-enhanced spectral mammography vs. mammography and MRI-clinical performance in a multi-reader evaluation. Eur. Radiol. 2017, 27, 2752–2764. [Google Scholar] [CrossRef]
- Li, L.; Roth, R.; Germaine, P.; Ren, S.; Lee, M.; Hunter, K.; Tinney, E.; Liao, L. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): A retrospective comparison in 66 breast lesions. Diagn. Interv. Imaging 2017, 98, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Ali-Mucheru, M.; Pockaj, B.; Patel, B.; Pizzitola, V.; Wasif, N.; Stucky, C.C.; Gray, R. Contrast-Enhanced Digital Mammography in the Surgical Management of Breast Cancer. Ann. Surg. Oncol. 2016, 23 (Suppl. S5), 649–655. [Google Scholar] [CrossRef] [PubMed]
- Lee-Felker, S.A.; Tekchandani, L.; Thomas, M.; Gupta, E.; Andrews-Tang, D.; Roth, A.; Sayre, J.; Rahbar, G. Newly Diagnosed Breast Cancer: Comparison of Contrast-enhanced Spectral Mammography and Breast MR Imaging in the Evaluation of Extent of Disease. Radiology 2017, 285, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Jochelson, M.S.; Pinker, K.; Dershaw, D.; Hughes, M.; Gibbons, G.F.; Rahbar, K.; Robson, M.E.; Mangino, D.A.; Goldman, D.; Moskowitz, C.S.; et al. Comparison of screening CEDM and MRI for women at increased risk for breast cancer: A pilot study. Eur. J. Radiol. 2017, 97, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Youn, I.; Lee, K.H.; Yun, J.-S.; Park, Y.L.; Park, C.H.; Moon, J.; Choi, S.H.; Choi, Y.J.; Ham, S.-Y.; et al. Diagnostic Value of Contrast-Enhanced Digital Mammography versus Contrast-Enhanced Magnetic Resonance Imaging for the Preoperative Evaluation of Breast Cancer. J. Breast Cancer 2018, 21, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Rao, H.; Zhou, L. A meta-analysis of contrast-enhanced spectral mammography versus MRI in the diagnosis of breast cancer. Thorac. Cancer 2020, 11, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, K.F.; Phillips, J.; Perry, H.; Lotfi, P.; Mehta, T.S. Contrast-enhanced Mammography: Current Applications and Future Directions. Radiographics 2019, 39, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Amato, F.; Bicchierai, G.; Cirone, D.; Depretto, C.; Di Naro, F.; Vanzi, E.; Scaperrotta, G.; Bartolotta, T.V.; Miele, V.; Nori, J. Preoperative locoregional staging of invasive lobular carcinoma with contrast-enhanced digital mammography (CEDM). Radiol. Med. 2019, 124, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Bicchierai, G.; Tonelli, P.; Piacenti, A.; De Benedetto, D.; Boeri, C.; Vanzi, E.; Bianchi, S.; Cirone, D.; Kaur Gill, M.; Miele, V.; et al. Evaluation of contrast-enhanced digital mammography (CEDM) in the preoperative staging of breast cancer: Large-scale single-center experience. Breast, J. 2020, 26, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Xiang, C.; Yang, Q. The diagnostic performance of CESM and CE-MRI in evaluating the pathological response to neoadjuvant therapy in breast cancer: A systematic review and meta-analysis. Br. J. Radiol. 2020, 93, 20200301. [Google Scholar] [CrossRef]
- Sung, J.S.; Lebron, L.; Keating, D.; D’Alessio, D.; Comstock, C.E.; Lee, C.H.; Pike, M.C.; Ayhan, M.; Moskowitz, C.S.; Morris, E.A.; et al. Performance of Dual-Energy Contrast-enhanced Digital Mammography for Screening Women at Increased Risk of Breast Cancer. Radiology 2019, 293, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Tennant, S.L.; James, J.J.; Cornford, E.J.; Chen, Y.; Burrell, H.C.; Hamilton, L.J.; Girio-Fragkoulakis, C. Contrast-enhanced spectral mammography improves diagnostic accuracy in the symptomatic setting. Clin. Radiol. 2016, 71, 1148–1155. [Google Scholar] [CrossRef]
- Dilorenzo, G.; Telegrafo, M.; La Forgia, D.; tabile Ianora, A.A.; Moschetta, M. Breast MRI background parenchymal enhancement as an imaging bridge to molecular cancer sub-type. Eur. J. Radiol. 2019, 113, 148–152. [Google Scholar] [CrossRef]
- La Forgia, D.; Fanizzi, A.; Campobasso, F.; Bellotti, R.; Didonna, V.; Lorusso, V.; Moschetta, M.; Massafra, R.; Tamborra, P.; Tangaro, S.; et al. Radiomic Analysis in Contrast-Enhanced Spectral Mammography for Predicting Breast Cancer Histological Outcome. Diagnostics 2020, 10, 708. [Google Scholar] [CrossRef]
- Losurdo, L.; Basile, T.M.A.; Fanizzi, A.; Bellotti, R.; Bottigli, U.; Carbonara, R.; Dentamaro, R.; Diacono, D.; Didonna, V.; Lombardi, A.; et al. A Gradient-Based Approach for Breast DCE-MRI Analysis. Biomed. Res. Int. 2018, 9032408. [Google Scholar] [CrossRef] [PubMed]

| Trial or Data | % |
|---|---|
| HIP RCT [15] | 22 |
| Malmo RCT [10] | 22 |
| Swedish Two-Country RCT [11] | 27 |
| Edinburgh RCT [16] | 21 |
| Stockholm RCT [15] | 10 |
| Gothenburg RCT [13] | 23 |
| Canadian service screening [17] | 40 |
| European case control studies Screened vs. not screened [14] | 48 |
| Study | MRI | CEM | ||
|---|---|---|---|---|
| Sensibility | Specificity | Sensibility | Specificity | |
| Jochelson, Radiology, 2013 [69] | 96% | n.a. | 96% | n.a. |
| Łuczyńska, Med Sci Monit, 2015 [70] | 93% | n.a. | 100% | n.a. |
| Fallenberg, Eur Radiol, 2016 [71] | 76% | 88% | 72% | 95% |
| Li, Diag and Interv Imaging, 2016 [72] | 100% | n.a. | 100% | n.a. |
| Ali-Mucheru, Ann Surg Oncol, 2016 [73] | 100% | n.a. | 98% | n.a. |
| Lee-Felker, Radiology, 2017 [74] | 99% | 4% | 94% | 17% |
| Jochelson, Eur J of Radiol, 2017 [75] | n.a. | 94.1% | n.a. | 94.7% |
| Kim, J Breast Cancer, 2018 [76] | 95.2% | 73.6% | 92.9% | 81.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bicchierai, G.; Di Naro, F.; De Benedetto, D.; Cozzi, D.; Pradella, S.; Miele, V.; Nori, J. A Review of Breast Imaging for Timely Diagnosis of Disease. Int. J. Environ. Res. Public Health 2021, 18, 5509. https://doi.org/10.3390/ijerph18115509
Bicchierai G, Di Naro F, De Benedetto D, Cozzi D, Pradella S, Miele V, Nori J. A Review of Breast Imaging for Timely Diagnosis of Disease. International Journal of Environmental Research and Public Health. 2021; 18(11):5509. https://doi.org/10.3390/ijerph18115509
Chicago/Turabian StyleBicchierai, Giulia, Federica Di Naro, Diego De Benedetto, Diletta Cozzi, Silvia Pradella, Vittorio Miele, and Jacopo Nori. 2021. "A Review of Breast Imaging for Timely Diagnosis of Disease" International Journal of Environmental Research and Public Health 18, no. 11: 5509. https://doi.org/10.3390/ijerph18115509
APA StyleBicchierai, G., Di Naro, F., De Benedetto, D., Cozzi, D., Pradella, S., Miele, V., & Nori, J. (2021). A Review of Breast Imaging for Timely Diagnosis of Disease. International Journal of Environmental Research and Public Health, 18(11), 5509. https://doi.org/10.3390/ijerph18115509






