Biotic and Abiotic Factors Influencing Arsenic Biogeochemistry and Toxicity in Fluvial Ecosystems: A Review
Abstract
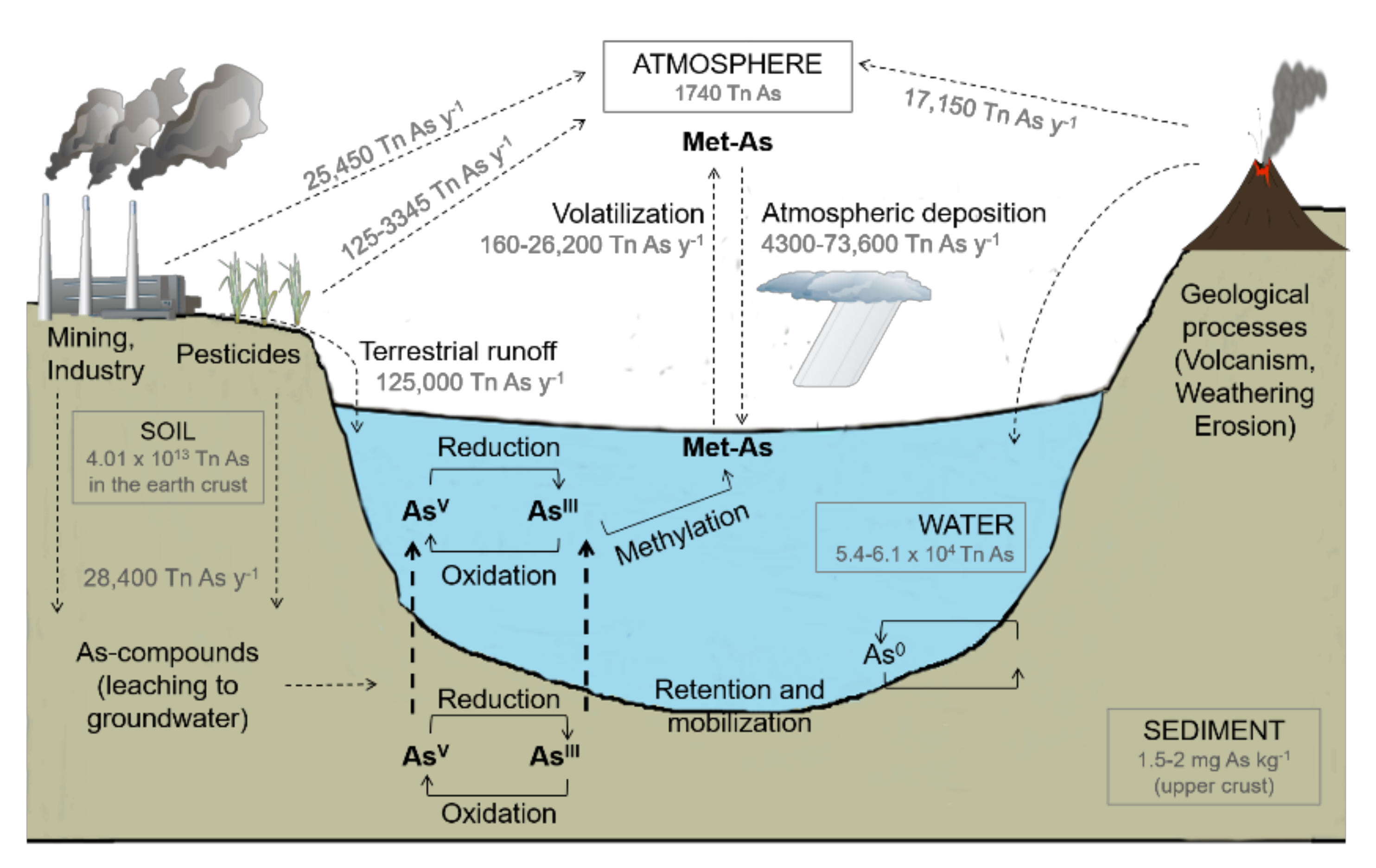
1. Arsenic Occurrence and Fate in Freshwater Environments
2. Arsenic Sources
3. Arsenic Speciation in Freshwater Ecosystems
4. Arsenic in Sediments and Sediment-Water Interactions
4.1. Precipitation/Dissolution
4.2. Oxidation/Reduction
4.3. Adsorption/Desorption
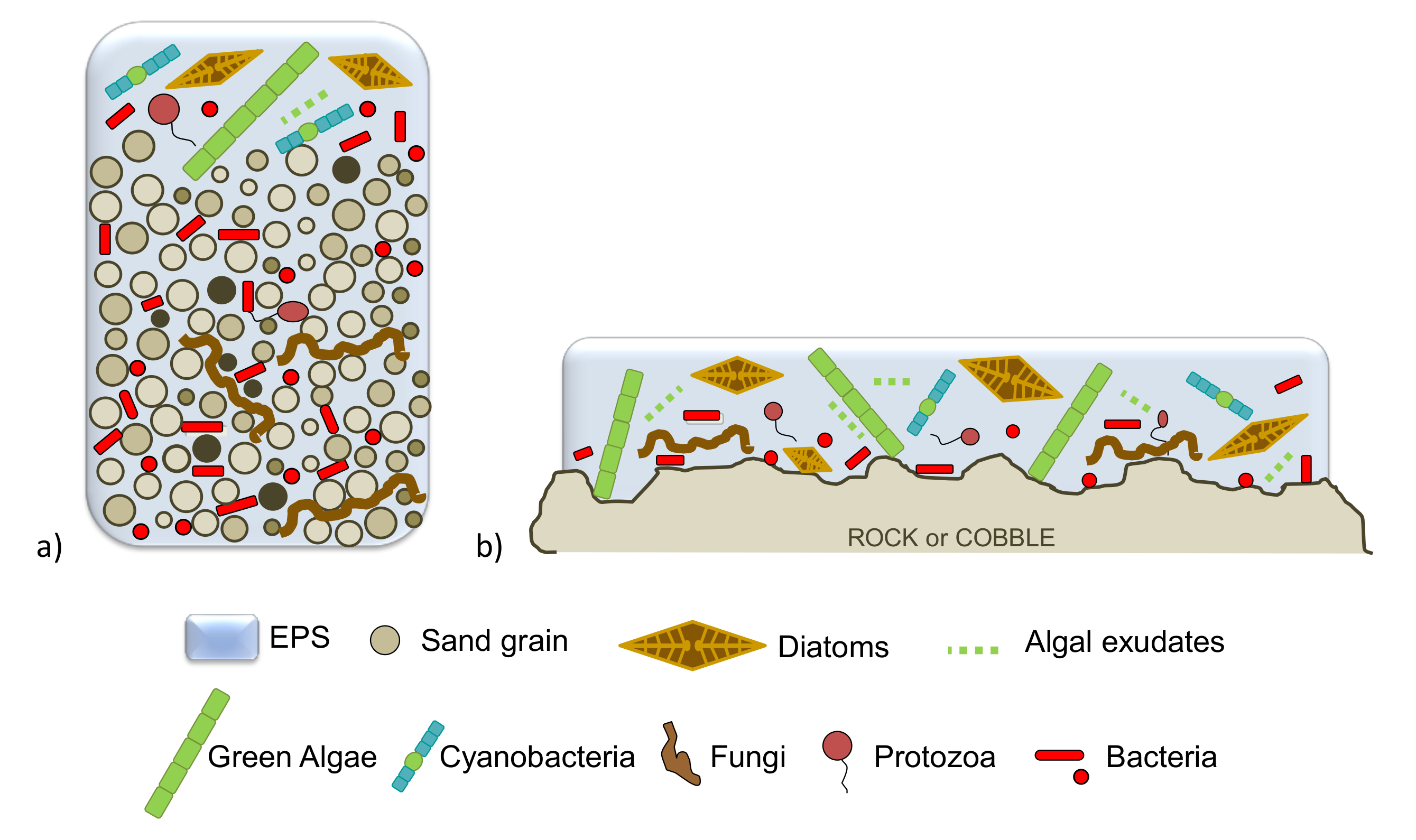
5. Microbial Biofilms in Freshwater Systems
6. Arsenic Biosorption Mediated by Biofilms
7. Effects of Arsenic Toxicity in Microorganisms and in Trophic Interactions
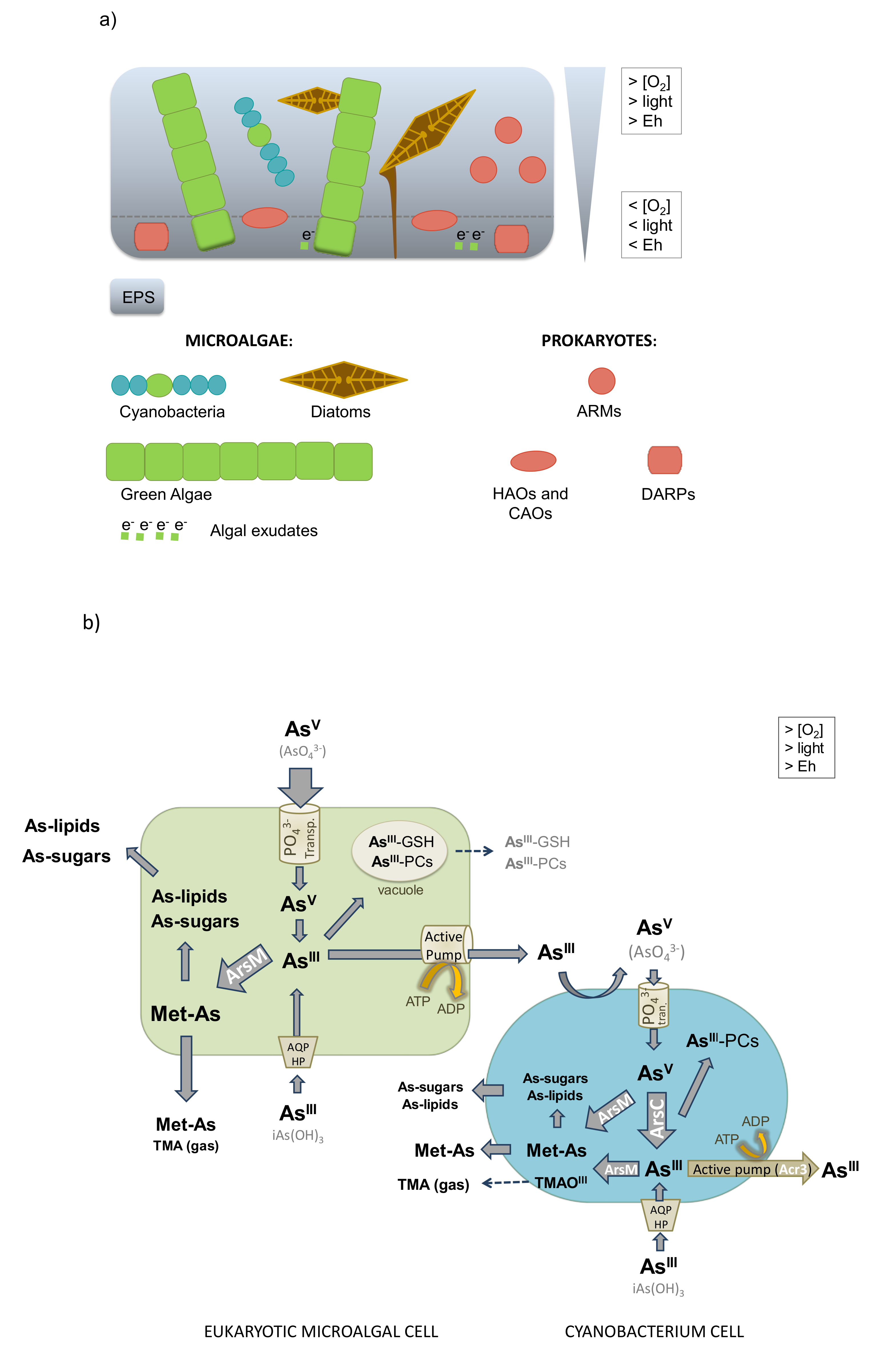
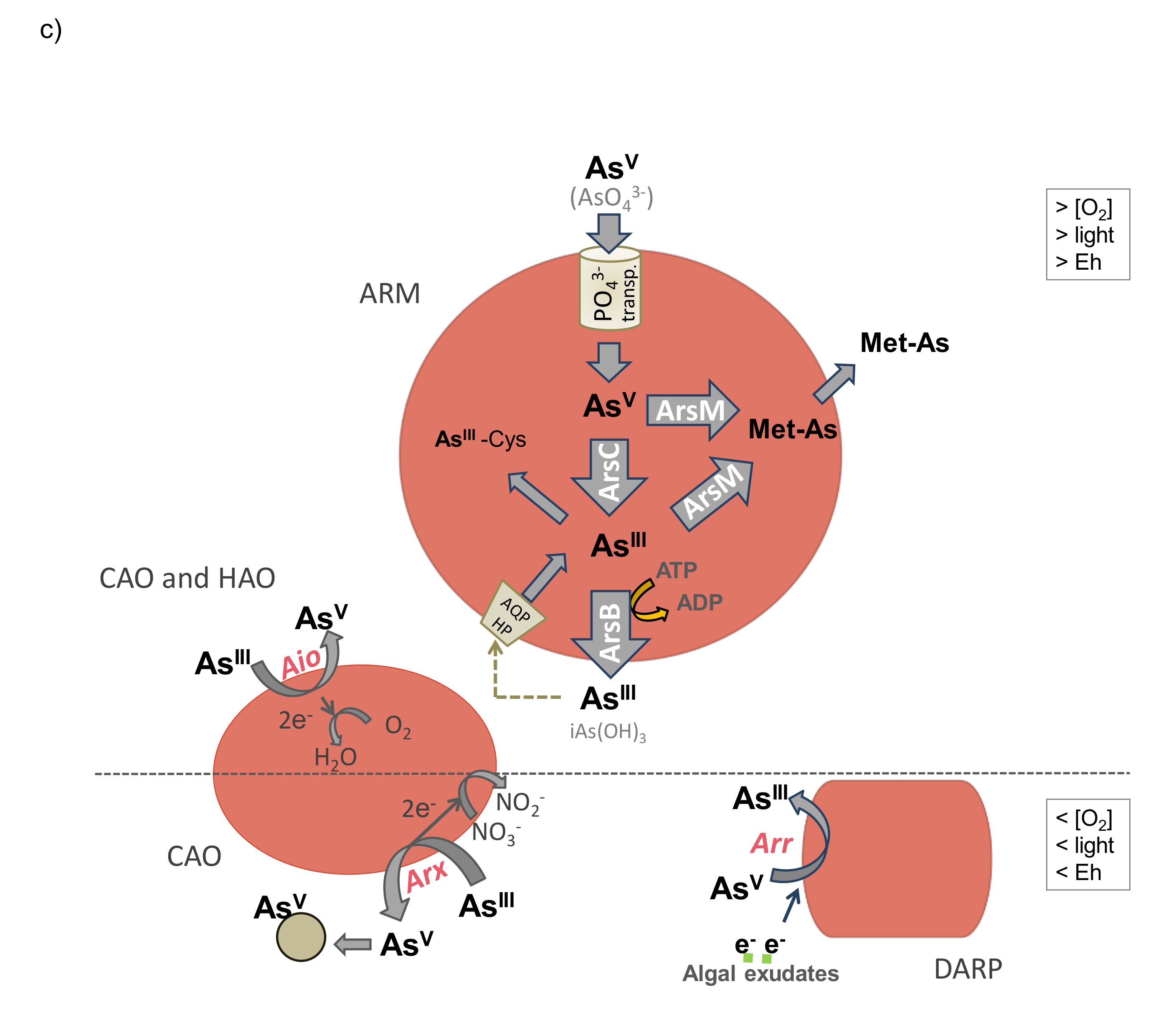
8. Changes in Arsenic Toxicity through Microbiological Biospeciation
8.1. Arsenite Oxidation
8.2. Arsenate Reduction
8.3. Arsenic Methylation and Demethylation
8.4. Synthesis of Arsenosugars and Arsenolipids
9. Cases of Arsenic-Impacted Sites: Current Knowledge and Future Research Needs
9.1. Pampean Streams: Effects of Naturally-Occurring Arsenate in Surface Waters
9.2. The Anllóns River: Polluted Sediments Resulting from Former Mining Activities
9.3. Future Research Needs
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Garelick, H.; Jones, H.; Dybowska, A.; Valsami-Jones, E. Arsenic Pollution Sources. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 197, pp. 17–60. ISBN 9780387792835. [Google Scholar]
- National Research Council (US) Committee on Medical and Biological Effects of Environmental Pollutants. Arsenic: Medical and Biologic Effects of Environmental Pollutants; National Academy of Sciences: Washington, DC, USA, 1977; ISBN 0309019273. [Google Scholar]
- Wang, Y.; Wang, S.; Xu, P.; Liu, C.; Liu, M.; Wang, Y.; Wang, C.; Zhang, C.; Ge, Y. Review of arsenic speciation, toxicity and metabolism in microalgae. Rev. Environ. Sci. Biotechnol. 2015, 14, 427–451. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, D.; Sahu, A.P. Arsenic in the environment: Effects on human health and possible prevention. J. Environ. Biol. 2007, 28, 359–365. [Google Scholar] [PubMed]
- Azizur Rahman, M.; Hasegawa, H. Arsenic in freshwater systems: Influence of eutrophication on occurrence, distribution, speciation, and bioaccumulation. Appl. Geochemistry 2012, 27, 304–314. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. Source and behaviour of arsenic in natural waters. Available online: http://www.who.int/water_sanitation_health/dwq/arsenicun1.pdf (accessed on 21 March 2016).
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochemistry 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H.; Lim, R.P. Bioaccumulation, biotransformation and trophic transfer of arsenic in the aquatic food chain. Environ. Res. 2012, 116, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Vasken Aposhian, H.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef]
- Henke, K. Arsenic: Environmental Chemistry, Health Threats and Waste Treatment; Wiley: Hoboken, NJ, USA, 2009; ISBN 9780470741139. [Google Scholar]
- EPA, U.S. National recommended water quality criteria: Aquatic life criteria; EPA: Washington, DC, USA, 2014.
- EPA, U.S. Arsenic and Clarifications to Compliance and New Source Monitoring Rule: A Quick Reference Guide; EPA: Washington, WA, USA, 2001.
- EU. C.D. 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. Eur. Communities 1998, 330, 32–54. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality, 2nd ed.; WHO: Geneva, Switzerland, 1993; ISBN 964-7445-88-1. [Google Scholar]
- Ahmad, A.; van der Wens, P.; Baken, K.; de Waal, L.; Bhattacharya, P.; Stuyfzand, P. Arsenic reduction to <1 µg/L in Dutch drinking water. Environ. Int. 2020, 134, 105253. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Rosen, B.P.; Phung, L.T.; Silver, S. Microbial arsenic: From geocycles to genes and enzymes. FEMS Microbiol. Rev. 2002, 26, 311–325. [Google Scholar] [CrossRef]
- Sultana, M.; Sanyal, S.K.; Hossain, M.A. Arsenic Pollution in the Environment. Handb. Res. Uncovering New Methods Ecosyst. Manag. through Bioremediation 2015, 92–119. [Google Scholar]
- Safiuddin, M. Arsenic contamination of groundwater in Bangladesh: A review. Int. J. Phys. Sci. 2011, 6. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef]
- Bundschuh, J.; Litter, M.I.; Parvez, F.; Román-Ross, G.; Nicolli, H.B.; Jean, J.-S.; Liu, C.-W.; López, D.; Armienta, M.A.; Guilherme, L.R.G.; et al. One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries. Sci. Total Environ. 2012, 429, 2–35. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lado, L.; Sun, G.; Berg, M.; Zhang, Q.; Xue, H.; Zheng, Q.; Johnson, C.A. Groundwater arsenic contamination throughout China. Science 2013, 341, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.L.; Latorre, S.; Castillo, E.; Brandão, P.F.B. Environmental occurrence of arsenic in Colombia: A review. Environ. Pollut. 2014, 186, 272–281. [Google Scholar] [CrossRef]
- Matschullat, J. Arsenic in the geosphere—A review. Sci. Total Environ. 2000, 249, 297–312. [Google Scholar] [CrossRef]
- Oremland, R.S.; Stolz, J.F. The ecology of arsenic. Science 2003, 300, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, D.K. Effects of microbiological and geochemical interactions in mine drainage. In Environmental Aspects of Mine Wastes; Jambor, J.L., Blowes, D.W., Ritchie, A.I.M., Eds.; Mineralogical Society of Canada: Québec, QC, Canada, 2003; ISBN 092129431X. [Google Scholar]
- Hall, G.E.M.; Pelchat, J.C.; Gauthier, G. Stability of inorganic arsenic (III) and arsenic (V) in water samples. J. Anal. At. Spectrom. 1999, 14, 205–213. [Google Scholar] [CrossRef]
- Francesconi, K.A.; Kuehnelt, D. Determination of arsenic species: A critical review of methods and applications, 2000–2003. Analyst 2004, 129, 373–395. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.J.; O’Reilly, J.; Marcilla, A.L.; Shaw, R.A.; Ward, N.I. Field based speciation of arsenic in UK and Argentinean water samples. Environ. Geochem. Health 2010, 32, 479–490. [Google Scholar] [CrossRef]
- Oremland, R.S.; Stolz, J.F. Arsenic, microbes and contaminated aquifers. Trends Microbiol. 2005, 13, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Páez-Espino, D.; Tamames, J.; de Lorenzo, V.; Cánovas, D. Microbial responses to environmental arsenic. BioMetals 2009, 22, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H. Impact of microorganisms on arsenic biogeochemistry: A review. Water. Air. Soil Pollut. 2014, 225. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Lin, J.-G. Bioleaching of heavy metals from contaminated sediment by indigenous sulfur-oxidizing bacteria in an air-lift bioreactor: Effects of sulfur concentration. Water Res. 2004, 38, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Brannon, J.M.; Patrick, W.H. Fixation, transformation, and mobilization of arsenic in sediments. Environ. Sci. Technol. 1987, 21, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Förstner, U.; Salomons, W. Mobilization of Metals from Sediments. In Metals and their compounds in the environment; VCH: Weihneim, Germany, 1991; pp. 379–398. [Google Scholar]
- Linge, K.L. Methods for investigating trace element binding in sediments. Crit. Rev. Environ. Sci. Technol. 2008, 38, 165–196. [Google Scholar] [CrossRef]
- Cappuyns, V.; Swennen, R.; Devivier, A. Dredged river sediments: Potential chemical time bombs? A case study. Water Air Soil Pollut. 2006, 171, 49–66. [Google Scholar] [CrossRef]
- Anawar, H.M.; Akai, J.; Sakugawa, H. Mobilization of arsenic from subsurface sediments by effect of bicarbonate ions in groundwater. Chemosphere 2004, 54, 753–762. [Google Scholar] [CrossRef]
- Fendorf, S.; Herbel, M.J.; Tufano, K.J.; Kocar, B.D. Biogeochemical processes controlling the cycling of arsenic in soils and sediments. Biophys. Process. Heavy Met. Met. Soil Environ. 2007, 313–338. [Google Scholar]
- Matera, V.; LeHécho, I. Arsenic behavior in contaminated soils: Mobility and speciation. In Heavy Metals Release in Soils; Selim, H.M., Sparks, D.L., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2001; p. 248. [Google Scholar]
- Sahuquillo, A.; López-Sánchez, J.F.; Rauret, G.; Ure, A.M.; Muntau, H. Sequential extraction procedures for sediment analysis. In Methodologies for Soil and Sediment Fractionation Studies. Single and sequential extraction procedures; European Commission: Brussels, Belgium, 2002; p. 180. [Google Scholar]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Drahota, P.; Filippi, M. Secondary arsenic minerals in the environment: A review. Environ. Int. 2009, 35, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Menció, A.; Guasch, H.; Soler, D.; Canelles, A.; Zamorano, M.; Brusi, D. Influence of regional hydrogeological systems at a local scale: Analyzing the coupled effects of hydrochemistry and biological activity in a Fe and CO2 rich spring. Sci. Total Environ. 2016, 569, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.N.; Ficklin, W.H.; Johns, C. Partitioning of arsenic and metals in reducing sulfidic sediments. Environ. Sci. Technol. 1988, 22, 432–437. [Google Scholar] [CrossRef]
- Oremland, R.; Newman, D.; Kail, B.; Stolz, J.F. Bacterial respiration of arsenate and its significance in the environment. In Environmental Chemistry of Arsenic; Frankenberger, W., Ed.; Marcel Dekker: New York, NY, USA, 2002; pp. 273–295. [Google Scholar]
- Bose, P.; Sharma, A. Role of iron in controlling speciation and mobilization of arsenic in subsurface environment. Water Res. 2002, 36, 4916–4926. [Google Scholar] [CrossRef]
- Aggett, J.; Kriegman, M.R. The extent of formation of arsenic(III) in sediment interstitial waters and its release to hypolimnetic waters in Lake Ohakuri. Water Res. 1988, 22, 407–411. [Google Scholar] [CrossRef]
- Azcue, J.M.; Nriagu, J.O. Impact of abandoned mine tailings on the arsenic concentrations in Moira Lake, Ontario. J. Geochemical Explor. 1995, 52, 81–89. [Google Scholar] [CrossRef]
- Widerlund, A.; Ingri, J. Early diagenesis of arsenic in sediments of the Kalix River estuary, northern Sweden. Chem. Geol. 1995, 125, 185–196. [Google Scholar] [CrossRef]
- Tingzong, G.; DeLaune, R.D.; Patrick, W.H. The influence of sediment redox chemistry on chemically active forms of arsenic, cadmium, chromium, and zinc in estuarine sediment. Environ. Int. 1997, 23, 305–316. [Google Scholar] [CrossRef]
- De Vitre, R.; Belzile, N.; Tessier, A. Speciation and adsorption of arsenic on diagenetic iron oxyhydroxides. Limnol. Oceanogr. 1991, 36, 1480–1485. [Google Scholar] [CrossRef]
- Sposito, G. The Chemistry of Soils, 2nd ed.; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Rubinos, D.; Barral, M.T.; Ruiz, B.; Ruiz, M.; Rial, M.E.; Álvarez, M.; Diaz-Fierros, F. Phosphate and arsenate retention in sediments of the Anllóns river (northwest Spain). Water Sci. Technol. 2003, 48, 159–166. [Google Scholar] [CrossRef]
- Bostick, B.C.; Chen, C.; Fendorf, S. Arsenite Retention Mechanisms within Estuarine Sediments of Pescadero, CA. Environ. Sci. Technol. 2004, 38, 3299–3304. [Google Scholar] [CrossRef]
- Stollenwerk, K.G.; Breit, G.N.; Welch, A.H.; Yount, J.C.; Whitney, J.W.; Foster, A.L.; Uddin, M.N.; Majumder, R.K.; Ahmed, N. Arsenic attenuation by oxidized aquifer sediments in Bangladesh. Sci. Total Environ. 2007, 379, 133–150. [Google Scholar] [CrossRef]
- Borgnino, L.; De Pauli, C.P.; Depetris, P.J. Arsenate adsorption at the sediment–water interface: Sorption experiments and modelling. Environ. Earth Sci. 2011, 65, 441–451. [Google Scholar] [CrossRef]
- Mandal, S.K.; Majumder, N.; Chowdhury, C.; Ganguly, D.; Dey, M.; Jana, T.K. Adsorption kinetic control of As(III & V) mobilization and sequestration by Mangrove sediment. Environ. Earth Sci. 2011, 65, 2027–2036. [Google Scholar]
- Sullivan, K.A.; Aller, R.C. Diagenetic cycling of arsenic in Amazon shelf sediments. Geochim. Cosmochim. Acta 1996, 60, 1465–1477. [Google Scholar] [CrossRef]
- Dixit, S.; Hering, J.G. Comparison of Arsenic(V) and Arsenic(III) Sorption onto Iron Oxide Minerals: Implications for Arsenic Mobility. Environ. Sci. Technol. 2003, 37, 4182–4189. [Google Scholar] [CrossRef] [PubMed]
- Millero, F.; Huang, F.; Zhu, X.; Liu, X.; Zhang, J.-Z. Adsorption and desorption of phosphate on calcite and aragonite in seawater. Aquat. Geochemistry 2001, 7, 33–56. [Google Scholar] [CrossRef]
- Thanabalasingam, P.; Pickering, W.F. Arsenic sorption by humic acids. Environ. Pollut. Ser. B, Chem. Phys. 1986, 12, 233–246. [Google Scholar] [CrossRef]
- Lin, H.-T.; Wang, M.C.; Li, G.-C. Complexation of arsenate with humic substance in water extract of compost. Chemosphere 2004, 56, 1105–1112. [Google Scholar] [CrossRef]
- Yan, L.; Yin, H.; Zhang, S.; Leng, F.; Nan, W.; Li, H. Biosorption of inorganic and organic arsenic from aqueous solution by Acidithiobacillus ferrooxidans BY-3. J. Hazard. Mater. 2010, 178, 209–217. [Google Scholar] [CrossRef]
- Prasad, K.S.; Srivastava, P.; Subramanian, V.; Paul, J. Biosorption of As(III) Ion on Rhodococcussp. WB-12: Biomass Characterization and Kinetic Studies. Sep. Sci. Technol. 2011, 46, 2517–2525. [Google Scholar] [CrossRef]
- Giri, A.K.; Patel, R.K.; Mahapatra, S.S.; Mishra, P.C. Biosorption of arsenic (III) from aqueous solution by living cells of Bacillus cereus. Environ. Sci. Pollut. Res. 2012, 20, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Masscheleyn, P.H.; Delaune, R.D.; Patrick, W.H. Effect of Redox Potential and pH on Arsenic Speciation and Solubility in a Contaminated Soil. Environ. Sci. Technol. 1991, 25, 1414–1419. [Google Scholar] [CrossRef]
- Masue, Y.; Loeppert, R.H.; Kramer, T.A. Arsenate and arsenite adsorption and desorption behavior on coprecipitated aluminum:iron hydroxides. Environ. Sci. Technol. 2007, 41, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Martiñá-Prieto, D.; Cancelo-González, J.; Barral, M.T. Arsenic Mobility in As-Containing Soils from Geogenic Origin: Fractionation and Leachability. J. Chem. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Antelo, J.; Avena, M.; Fiol, S.; López, R.; Arce, F. Effects of pH and ionic strength on the adsorption of phosphate and arsenate at the goethite-water interface. J. Colloid Interface Sci. 2005, 285, 476–486. [Google Scholar] [CrossRef]
- Rubinos, D.; Iglesias, L.; Devesa-Rey, R.; Díaz-Fierros, F.; Barral, M.T. Arsenic release from river sediments in a gold-mining area (Anllons River basin, Spain): Effect of time, pH and phosphorous concentration. Eur. J. Mineral. 2010, 22, 665–678. [Google Scholar] [CrossRef]
- Manning, B.A.; Goldberg, S. Modeling Competitive Adsorption of Arsenate with Phosphate and Molybdate on Oxide Minerals. Soil Sci. Soc. Am. J. 1996, 60, 121–131. [Google Scholar] [CrossRef]
- Reynolds, J.G.; Naylor, D.V.; Fendorf, S.E. Arsenic Sorption in Phosphate-Amended Soils during Flooding and Subsequent Aeration. Soil Sci. Soc. Am. J. 1999, 63, 1149–1156. [Google Scholar]
- Rubinos, D.A.; Iglesias, L.; Díaz-Fierros, F.; Barral, M.T. Interacting Effect of pH, Phosphate and Time on the Release of Arsenic from Polluted River Sediments (Anllóns River, Spain). Aquat. Geochemistry 2011, 17, 281–306. [Google Scholar] [CrossRef]
- Kaplan, D.I.; Knox, A.S. Enhanced Contaminant Desorption Induced by Phosphate Mineral Additions to Sediment. Environ. Sci. Technol. 2004, 38, 3153–3160. [Google Scholar] [CrossRef]
- Bauer, M.; Blodau, C. Mobilization of arsenic by dissolved organic matter from iron oxides, soils and sediments. Sci. Total Environ. 2006, 354, 179–190. [Google Scholar] [CrossRef]
- Peryea, F.J. Phosphate-Induced Release of Arsenic from Soils Contaminated with Lead Arsenate. Soil Sci. Soc. Am. J. 1991, 55, 1301–1306. [Google Scholar] [CrossRef]
- Peryea, F.J.; Kammereck, R. Phosphate-enhanced movement of arsenic out of lead arsenate-contaminated topsoil and through uncontaminated subsoil. Water Air Soil Pollut. 1997, 93, 243–254. [Google Scholar] [CrossRef]
- Jain, A.; Loeppert, R.H. Effect of Competing Anions on the Adsorption of Arsenate and Arsenite by Ferrihydrite. J. Environ. Qual. 2000, 29, 1422–1430. [Google Scholar] [CrossRef]
- Violante, A.; Pigna, M. Competitive Sorption of Arsenate and Phosphate on Different Clay Minerals and Soils. Soil Sci. Soc. Am. J. 2002, 66, 1788–1796. [Google Scholar] [CrossRef]
- Pigna, M.; Krishnamurti, G.S.R.; Violante, A. Kinetics of Arsenate Sorption-Desorption from Metal Oxides. Soil Sci. Soc. Am. J. 2006, 70, 2017–2027. [Google Scholar] [CrossRef]
- Luxton, T.; Eick, M.; Rimstidt, D. The role of silicate in the adsorption/desorption of arsenite on goethite. Chem. Geol. 2008, 252, 125–135. [Google Scholar] [CrossRef]
- Xu, H.; Allard, B.; Grimvall, A. Effects of acidification and natural organic materials on the mobility of arsenic in the environment. Water. Air. Soil Pollut. 1991, 57, 269–278. [Google Scholar] [CrossRef]
- Grafe, M.; Eick, M.J.; Grossl, P.R. Adsorption of Arsenate (V) and Arsenite (III) on Goethite in the Presence and Absence of Dissolved Organic Carbon. Soil Sci. Soc. Am. J. 2001, 65, 1680–1687. [Google Scholar] [CrossRef]
- Grafe, M.; Eick, M.J.; Grossl, P.R.; Saunders, A.M. Adsorption of Arsenate and Arsenite on Ferrihydrite in the Presence and Absence of Dissolved Organic Carbon. J. Environ. Qual. 2002, 31, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Redman, A.D.; Macalady, D.L.; Ahmann, D. Natural organic matter affects Arsenic speciation and sorption onto hematite. Environ. Sci. Technol. 2002, 36, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Rubinos, D.A.; Iglesias, L.; Devesa-Rey, R.; Barral, M.T. Combined effect of solid/liquid ratio and phosphate, ionic strength and inorganic anions on the mobilization of arsenic from river sediments (Anllóns River, NW Spain). In Proceedings of the 11th International Conference on the Biogeochemistry of Trace Elements, Florence, Italy, 3–8 July 2011. [Google Scholar]
- Romaní, A. Freshwater Biofilms. In Biofouling; Dürr, S., Thomason, J., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2010; pp. 137–153. [Google Scholar]
- Mora-Gómez, J.; Freixa, A.; Perujo, N.; Barral-Fraga, L. Limits of the biofilm concept and types of aquatic biofilms. In Aquatic Biofilms: Ecology Water Quality and Wastewater Treatment; Caister Academic Press: Rofo, UK, 2016; pp. 3–28. [Google Scholar]
- Neury-Ormanni, J.; Vedrenne, J.; Morin, S. Who eats who in biofilms? Exploring the drivers of microalgal and micro-meiofaunal abundance. Bot. Lett. 2016, 163, 83–92. [Google Scholar] [CrossRef]
- Karatan, E.; Watnick, P. Signals, Regulatory Networks, and Materials That Build and Break Bacterial Biofilms. Microbiol. Mol. Biol. Rev. 2009, 73, 310–347. [Google Scholar] [CrossRef]
- Romani, A.M.; Sabater, S. Structure and Activity of Rock and Sand Biofilms in a Mediterranean Stream. Ecology 2001, 82, 3232. [Google Scholar] [CrossRef]
- Graba, M.; Sauvage, S.; Moulin, F.Y.; Urrea, G.; Sabater, S.; Sanchez-Pérez, J.M. Interaction between local hydrodynamics and algal community in epilithic biofilm. Water Res. 2013, 47, 2153–2163. [Google Scholar] [CrossRef]
- Kulp, T.R.; Hoeft, S.E.; Oremland, R.S. Redox Transformations of Arsenic Oxyanions in Periphyton Communities. Appl. Environ. Microbiol. 2004, 70, 6428–6434. [Google Scholar] [CrossRef] [PubMed]
- Yanan, Z.; Li, H.; Kai, Y.; Yiqun, G. The Role of Dissolved Organic Matter in the Competitive Adsorption to Goethite, during Arsenic Mobilization. Procedia Earth Planet. Sci. 2017, 17, 424–427. [Google Scholar] [CrossRef]
- Luís, A.T.; Coelho, H.; Almeida, S.F.P.; da Silva, E.F.; Serôdio, J. Photosynthetic activity and ecology of benthic diatom communities from streams affected by Acid Mine Drainage (AMD) in pyritic mines. Fundam. Appl. Limnol. / Arch. für Hydrobiol. 2013, 182, 47–59. [Google Scholar] [CrossRef]
- Luís, A.T.; Durães, N.; de Almeida, S.F.P.; da Silva, E.F. Integrating geochemical (surface waters, stream sediments) and biological (diatoms) approaches to assess AMD environmental impact in a pyritic mining area: Aljustrel (Alentejo, Portugal). J. Environ. Sci. 2016, 42, 215–226. [Google Scholar] [CrossRef]
- Morin, S.; Gómez, N.; Tornés, E.; Licursi, M.; Rosebery, J. Benthic Diatom Monitoring and Assessment of Freshwater Environments: Standard Methods and Future Challenges. Aquat. Biofilms Ecol. Water Qual. Wastewater Treat. 2016, 111–124. [Google Scholar]
- Pandey, L.K.; Bergey, E.A. Metal toxicity and recovery response of riverine periphytic algae. Sci. Total Environ. 2018, 642, 1020–1031. [Google Scholar] [CrossRef]
- Joo, J.H.; Hassan, S.H.A.; Oh, S.E. Comparative study of biosorption of Zn2+ by Pseudomonas aeruginosa and Bacillus cereus. Int. Biodeterior. Biodegrad. 2010, 64, 734–741. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, J.; Tang, J.; Zhu, Y.; Wu, Y. Arsenic removal by periphytic biofilm and its application combined with biochar. Bioresour. Technol. 2018, 248, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Schmitt, J.; Marshall, K.C. Sorption Properties of Biofilms. In Sediments and Toxic Substances; Calmano, F., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 115–130. [Google Scholar]
- Gräfe, M.; Beattie, D.A.; Smith, E.; Skinner, W.M.; Singh, B. Copper and arsenate co-sorption at the mineral-water interfaces of goethite and jarosite. J. Colloid Interface Sci. 2008, 322, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.; Shchukarev, A.; Sjöberg, S.; Lövgren, L. Composition and solubility of precipitated copper(II) arsenates. Appl. Geochemistry 2011, 26, 696–704. [Google Scholar] [CrossRef]
- Nelson, H.; Sjöberg, S.; Lövgren, L. Surface complexation modelling of arsenate and copper adsorbed at the goethite/water interface. Appl. Geochemistry 2013, 35, 64–74. [Google Scholar] [CrossRef]
- Liu, G.; Cai, Y. Complexation of arsenite with dissolved organic matter: Conditional distribution coefficients and apparent stability constants. Chemosphere 2010, 81, 890–896. [Google Scholar] [CrossRef]
- Zagury, G.J.; Samson, R.; Deschênes, L. Occurrence of Metals in Soil and Ground Water Near Chromated Copper Arsenate-Treated Utility Poles. J. Environ. Qual. 2003, 32, 507–514. [Google Scholar] [CrossRef]
- Prieto, D.M.; Devesa-Rey, R.; Rubinos, D.A.; Díaz-Fierros, F.; Barral, M.T. Arsenate retention by epipsammic biofilms developed on streambed sediments: Influence of phosphate. Biomed Res. Int. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Levy, J.L.; Stauber, J.L.; Adams, M.S.; Maher, W.A.; Kirby, J.K.; Jolley, D.F. Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp and Monoraphidium arcuatum). Environ. Toxicol. Chem. 2005, 24, 2630–2639. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.X.; Li, Y.; Deng, X.H.; Miao, A.J.; Ji, R.; Yang, L.Y. Toxicity and bioaccumulation kinetics of arsenate in two freshwater green algae under different phosphate regimes. Water Res. 2013, 47, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Gong, Y.; Wang, C.; Liu, X.; Liu, J. Arsenic speciation and effect of arsenate inhibition in a Microcystis aeruginosa culture medium under different phosphate regimes. Environ. Toxicol. Chem. 2011, 30, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Lourdes, C. Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: Role of oxidative stress and inflammatory responses. Neurotoxicology 2016, 53, 223–235. [Google Scholar] [CrossRef]
- Bertin, P.N.; Heinrich-Salmeron, A.; Pelletier, E.; Goulhen-Chollet, F.; Arsène-Ploetze, F.; Gallien, S.; Lauga, B.; Casiot, C.; Calteau, A.; Vallenet, D.; et al. Metabolic diversity among main microorganisms inside an arsenic-rich ecosystem revealed by meta- and proteo-genomics. ISME J. 2011, 5, 1735–1747. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hassler, C. Is arsenic biotransformation a detoxification mechanism for microorganisms? Aquat. Toxicol. 2014, 146, 212–219. [Google Scholar] [CrossRef]
- Hellweger, F.L.; Farley, K.J.; Lall, U.; Di Toro, D.M. Greedy algae reduce arsenate. Limnol. Oceanogr. 2003, 48, 2275–2288. [Google Scholar] [CrossRef]
- Rodriguez Castro, M.C.; Urrea, G.; Guasch, H. Influence of the interaction between phosphate and arsenate on periphyton’s growth and its nutrient uptake capacity. Sci. Total Environ. 2015, 503, 122–132. [Google Scholar] [CrossRef]
- Pesticide Action Network, North America, PAN Pesticide Database. Chemical Toxicity Studies on Aquatic Organisms. Available online: http://www.pesticideinfo.org (accessed on 8 February 2016).
- Agency, U.S.E.P. Ecotox Knowledgebase. Available online: https://cfpub.epa.gov/ecotox/ (accessed on 8 February 2016).
- Tuulaikhuu, B.-A. Influences of toxicants on freshwater biofilms and fish: From experimental approaches to statistical models. PhD Dissertation, Universitat de Girona, Girona, Spain, 2016. [Google Scholar]
- Barral-Fraga, L.; Martiñá-Prieto, D.; Barral, M.T.; Morin, S.; Guasch, H. Mutual interaction between arsenic and biofilm in a mining impacted river. Sci. Total Environ. 2018, 636, 985–998. [Google Scholar] [CrossRef]
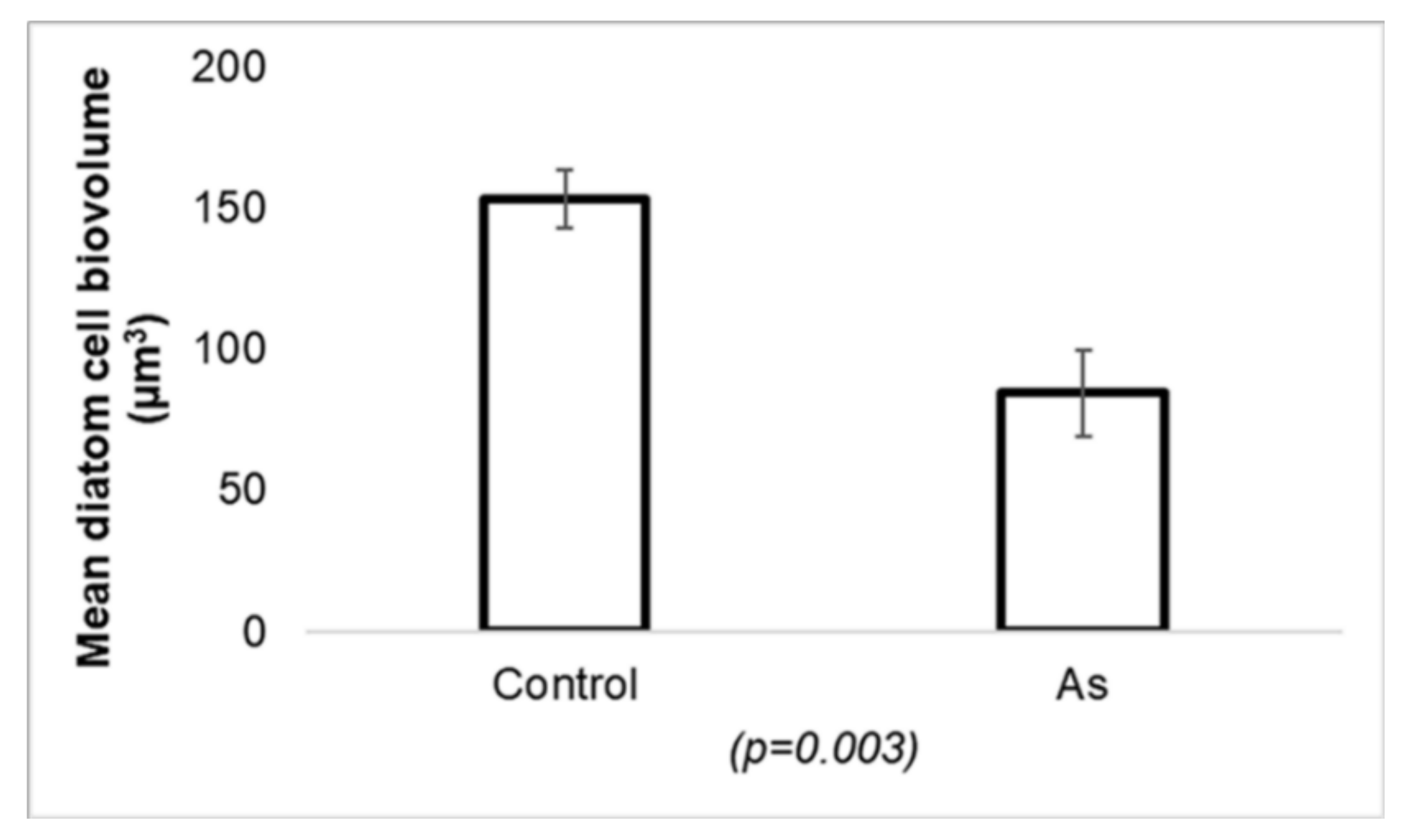
- Barral-Fraga, L.; Morin, S.; Rovira, M.D.M.; Urrea, G.; Magellan, K.; Guasch, H. Short-term arsenic exposure reduces diatom cell size in biofilm communities. Environ. Sci. Pollut. Res. 2016, 23, 4257–4270. [Google Scholar] [CrossRef]
- Navarro, E.; Guasch, H.; Sabater, S. Use of microbenthic algal communities in ecotoxicological tests for the assessment of water quality: The Ter river case study. J. Appl. Phycol. 2002, 14, 41–48. [Google Scholar] [CrossRef]
- McClellan, K.; Altenburger, R.; Schmitt-Jansen, M. Pollution-induced community tolerance as a measure of species interaction in toxicity assessment. J. Appl. Ecol. 2008, 45, 1514–1522. [Google Scholar] [CrossRef]
- Morin, S.; Cordonier, A.; Lavoie, I.; Arini, A.; Blanco, S.; Duong, T.T.; Tornés, E.; Bonet, B.; Corcoll, N.; Faggiano, L.; et al. Consistency in Diatom Response to Metal-Contaminated Environments. Handb. Environ. Chem. 2012, 117–146. [Google Scholar]
- Kelly, M.G.; Cazaubon, A.; Coring, E.; Dell’Uomo, A.; Ector, L.; Goldsmith, B.; Guasch, H.; Hurlimann, J.; Jarlman, A.; Kawecka, B.; et al. Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J. Appl. Phycol. 1998, 10, 215–224. [Google Scholar] [CrossRef]
- Prygiel, J.; Carpentier, P.; Almeida, S.; Coste, M.; Druart, J.C.; Ector, L.; Guillard, D.; Honoré, M.A.; Iserentant, R.; Ledeganck, P.; et al. Determination of the biological diatom index (IBD NF T 90-354): Results of an intercomparison exercise. J. Appl. Phycol. 2002, 14, 27–39. [Google Scholar] [CrossRef]
- Pandey, L.K.; Bergey, E.A.; Lyu, J.; Park, J.; Choi, S.; Lee, H.; Depuydt, S.; Oh, Y.T.; Lee, S.M.; Han, T. The use of diatoms in ecotoxicology and bioassessment: Insights, advances and challenges. Water Res. 2017, 118, 39–58. [Google Scholar] [CrossRef]
- Guanzon, N.G.; Nakahara, H.; Nishimura, K. Accumulation of Copper, Zinc, Cadmium, and Their Combinations by Three Freshwater Microalgae. Fish. Sci. 1995, 61, 149–156. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Control of Cd Concentrations in a Coastal Diatom by Interactions among Free Ionic Cd, Zn, and Mn in Seawater. Environ. Sci. Technol. 1998, 32, 2961–2968. [Google Scholar] [CrossRef]
- Chang, S.I.; Reinfelder, J.R. Bioaccumulation, Subcellular Distribution, and Trophic Transfer of Copper in a Coastal Marine Diatom. Environ. Sci. Technol. 2000, 34, 4931–4935. [Google Scholar] [CrossRef]
- Wang, W.-X.; Dei, R.C.H. Metal uptake in a coastal diatom influenced by major nutrients (N, P, and Si). Water Res. 2001, 35, 315–321. [Google Scholar] [CrossRef]
- Hill, W. Effects of Light. In Algal Ecology: Freshwater benthic ecosystems; Stevenson, R., Bothwell, M., Lowe, R., Eds.; Elsevier Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Corcoll, N.; Bonet, B.; Leira, M.; Montuelle, B.; Tlili, A.; Guasch, H. Light History Influences the Response of Fluvial Biofilms to Zn Exposure. J. Phycol. 2012, 48, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.M.; Martín-Liñares, V.; Piñeiro, V.; Barral, M.T. Arsenic Transfer from As-Rich Sediments to River Water in the Presence of Biofilms. J. Chem. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Cattaneo, A.; Asioli, A.; Comoli, P.; Manca, M. Organisms’ response in a chronically polluted lake supports hypothesized link between stress and size. Limnol. Oceanogr. 1998, 43, 1938–1943. [Google Scholar] [CrossRef]
- Cattaneo, A.; Couillard, Y.; Wunsam, S.; Courcelles, M. Diatom taxonomic and morphological changes as indicators of metal pollution and recovery in Lac Dufault (Québec, Canada). J. Paleolimnol. 2004, 32, 163–175. [Google Scholar] [CrossRef]
- Morin, S.; Coste, M. Metal-induced shifts in the morphology of diatoms from the Riou Mort and Riou Viou streams ( South West France ). Use algae Monit. rivers VI 2006, 91–106. [Google Scholar]
- Luís, A.T.; Teixeira, P.; Almeida, S.F.P.; Matos, J.X.; da Silva, E.F. Environmental impact of mining activities in the Lousal area (Portugal): Chemical and diatom characterization of metal-contaminated stream sediments and surface water of Corona stream. Sci. Total Environ. 2011, 409, 4312–4325. [Google Scholar] [CrossRef]
- Magellan, K.; Barral-Fraga, L.; Rovira, M.; Srean, P.; Urrea, G.; García-Berthou, E.; Guasch, H. Behavioural and physical effects of arsenic exposure in fish are aggravated by aquatic algae. Aquat. Toxicol. 2014, 156, 116–124. [Google Scholar] [CrossRef]
- Stevenson, R.J. An Introduction to Algal Ecology in Freshwater Benthic Habitats. In Algal Ecology; Academic Press: Cambridge, MA, USA, 1996; pp. 3–30. [Google Scholar]
- Guasch, H.; Artigas, J.; Bonet, B.; Bonnineau, C.; Canals, O.; Corcoll, N.; Foulquier, A.; López-Doval, J.; Kim-Tiam, S.; Morin, S.; et al. The Use of Biofilms to Assess the Effects of Chemicals on Freshwater Ecosystems. In Aquatic Biofilms: Ecology Water Quality and Wastewater Treatment; Caister Academic Press: Rofo, UK, 2016; pp. 125–144. [Google Scholar]
- Proia, L.; Cassió, F.; Pascoal, C.; Tlili, A.; Romaní, A.M. The Use of Attached Microbial Communities to Assess Ecological Risks of Pollutants in River Ecosystems: The Role of Heterotrophs. In Handbook of Environmental Chemistry Series; Springer: Berlin/Heidelberg, Germany, 2012; pp. 55–83. [Google Scholar]
- Tuulaikhuu, B.-A.; Romaní, A.M.; Guasch, H. Arsenic toxicity effects on microbial communities and nutrient cycling in indoor experimental channels mimicking a fluvial system. Aquat. Toxicol. 2015, 166, 72–82. [Google Scholar] [CrossRef]
- Andres, J.; Arsène-Ploetze, F.; Barbe, V.; Brochier-Armanet, C.; Cleiss-Arnold, J.; Coppée, J.-Y.; Dillies, M.-A.; Geist, L.; Joublin, A.; Koechler, S.; et al. Life in an Arsenic-Containing Gold Mine: Genome and Physiology of the Autotrophic Arsenite-Oxidizing Bacterium Rhizobium sp. NT-26. Genome Biol. Evol. 2013, 5, 934–953. [Google Scholar] [CrossRef]
- Yan, C.; Che, F.; Zeng, L.; Wang, Z.; Du, M.; Wei, Q.; Wang, Z.; Wang, D.; Zhen, Z. Spatial and seasonal changes of arsenic species in Lake Taihu in relation to eutrophication. Sci. Total Environ. 2016, 563, 496–505. [Google Scholar] [CrossRef]
- Baker, J.; Wallschläger, D. The role of phosphorus in the metabolism of arsenate by a freshwater green alga, Chlorella vulgaris. J. Environ. Sci. 2016, 49, 169–1178. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.D.; Wong, P.T.S.; Chau, Y.K.; Mayfield, C.I.; Inniss, W.E. Methylation of Arsenic by Freshwater Green Algae. Can. J. Fish. Aquat. Sci. 1983, 40, 1254–1257. [Google Scholar] [CrossRef]
- Hasegawa, H.; Sohrin, Y.; Seki, K.; Sato, M.; Norisuye, K.; Naito, K.; Matsui, M. Biosynthesis and release of methylarsenic compounds during the growth of freshwater algae. Chemosphere 2001, 43, 265–272. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Yoshinaga, M.; Zhao, F.-J.; Rosen, B.P. Earth Abides Arsenic Biotransformations. Annu. Rev. Earth Planet. Sci. 2014, 42, 443–467. [Google Scholar] [CrossRef]
- Nagvenkar, G.S.; Ramaiah, N. Arsenite tolerance and biotransformation potential in estuarine bacteria. Ecotoxicology 2009, 19, 604–613. [Google Scholar] [CrossRef]
- Stolz, J.F.; Basu, P.; Santini, J.M.; Oremland, R.S. Arsenic and Selenium in Microbial Metabolism. Annu. Rev. Microbiol. 2006, 60, 107–130. [Google Scholar] [CrossRef]
- Hudson-Edwards, K.; Santini, J. Arsenic-Microbe-Mineral Interactions in Mining-Affected Environments. Minerals 2013, 3, 337–351. [Google Scholar] [CrossRef]
- Qin, J.; Lehr, C.R.; Yuan, C.; Le, X.C.; McDermott, T.R.; Rosen, B.P. Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga. Proc. Natl. Acad. Sci. 2009, 106, 5213–5217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Rensing, C.; Zhu, Y. Cyanobacteria-mediated arsenic redox dynamics is regulated by phosphate in aquatic environments. Environ. Sci. Technol. 2013, 48, 994–1000. [Google Scholar] [CrossRef]
- Kulp, T.R.; Hoeft, S.E.; Asao, M.; Madigan, M.T.; Hollibaugh, J.T.; Fisher, J.C.; Stolz, J.F.; Culbertson, C.W.; Miller, L.G.; Oremland, R.S. Arsenic(III) fuels anoxygenic photosynthesis in hot spring biofilms from Mono Lake, California. Science 2008, 321, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Amend, J.P.; Saltikov, C.; Lu, G.-S.; Hernandez, J. Microbial arsenic metabolism and reaction energetics. Rev. Mineral. Geochemistry 2014, 79, 391–433. [Google Scholar] [CrossRef]
- López-Maury, L.; Florencio, F.J.; Reyes, J.C. Arsenic Sensing and Resistance System in the Cyanobacterium Synechocystis sp. Arsenic Sensing and Resistance System in the Cyanobacterium. J. Bacteriol. 2003, 185, 5363–5371. [Google Scholar] [PubMed]
- Hervás, M.; López-Maury, L.; León, P.; Sánchez-Riego, A.M.; Florencio, F.J.; Navarro, J.A. ArsH from the CyanobacteriumSynechocystissp. PCC 6803 Is an Efficient NADPH-Dependent Quinone Reductase. Biochemistry 2012, 51, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Kaise, T.; Ogura, M.; Nozaki, T.; Saitoh, K.; Sakurai, T.; Matsubara, C.; Watanabe, C.; Hanaoka, K. Biomethylation of Arsenic in an Arsenic-rich Freshwater Environment. Appl. Organomet. Chem. 1997, 11, 297–304. [Google Scholar] [CrossRef]
- Mestrot, A.; Planer-Friedrich, B.; Feldmann, J. Biovolatilisation: A poorly studied pathway of the arsenic biogeochemical cycle. Environ. Sci. Process. Impacts 2013, 15, 1639. [Google Scholar] [CrossRef]
- Prieto, D.M.; Rubinos, D.A.; Piñeiro, V.; Díaz-Fierros, F.; Barral, M.T. Influence of epipsammic biofilm on the biogeochemistry of arsenic in freshwater environments. Biogeochemistry 2016, 129, 291–306. [Google Scholar] [CrossRef]
- Yin, X.; Wang, L.; Duan, G.; Sun, G. Characterization of arsenate transformation and identification of arsenate reductase in a green alga Chlamydomonas reinhardtii. J. Environ. Sci. 2011, 23, 1186–1193. [Google Scholar] [CrossRef]
- Miiyashita, S.; Fujiwara, S.; Tsuzuki, M.; Kaise, T. Rapid Biotransformation of Arsenate into Oxo-Arsenosugars by a Freshwater Unicellular Green Alga, Chlamydomonas reinhardtii. Biosci. Biotechnol. Biochem. 2011, 75, 522–530. [Google Scholar] [CrossRef]
- Miyashita, S.; Fujiwara, S.; Tsuzuki, M.; Kaise, T. Cyanobacteria produce arsenosugars. Environ. Chem. 2012, 9, 474. [Google Scholar] [CrossRef]
- Xue, X.-M.; Raber, G.; Foster, S.; Chen, S.-C.; Francesconi, K.A.; Zhu, Y.-G. Biosynthesis of arsenolipids by the cyanobacterium Synechocystis sp. PCC 6803. Environ. Chem. 2014, 11, 506. [Google Scholar] [CrossRef]
- Meyer, S.; Schulz, J.; Jeibmann, A.; Taleshi, M.S.; Ebert, F.; Francesconi, K.A.; Schwerdtle, T. Arsenic-containing hydrocarbons are toxic in the in vivo model Drosophila melanogaster. Metallomics 2014, 6, 2010–2014. [Google Scholar] [CrossRef] [PubMed]
- Viglizzo, E.; Jobbágy, E.G. Expansión de la frontera agropecuaria en Argentina y su impacto ecológico-ambiental; Ediciones INTA Buenos Aires: Buenos Aires, Argentina, 2010; ISBN 9871623836. [Google Scholar]
- Farias, S.; Casa, V.; Vázquez, C.; Ferpozzi, L.; Pucci, G.; Cohen, I. Natural contamination with arsenic and other trace elements in ground waters of Argentine Pampean Plain1. Sci. Total Environ. 2003, 309, 187–199. [Google Scholar] [CrossRef]
- Smedley, P.L.; Nicolli, H.B.; Macdonald, D.M.J.; Barros, A.J.; Tullio, J.O. Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259–284. [Google Scholar] [CrossRef]
- Bundschuh, J.; Farias, B.; Martin, R.; Storniolo, A.; Bhattacharya, P.; Cortes, J.; Bonorino, G.; Albouy, R. Groundwater arsenic in the Chaco-Pampean Plain, Argentina. Appl. Geochem. 2004, 19, 231–243. [Google Scholar] [CrossRef]
- Nicolli, H.B.; Bundschuh, J.; García, J.W.; Falcón, C.M.; Jean, J.-S. Sources and controls for the mobility of arsenic in oxidizing groundwaters from loess-type sediments in arid/semi-arid dry climates – Evidence from the Chaco–Pampean plain (Argentina). Water Res. 2010, 44, 5589–5604. [Google Scholar] [CrossRef] [PubMed]
- Galindo, G.; Sainato, C.; Dapeña, C.; Fernández-Turiel, J.L.; Gimeno, D.; Pomposiello, M.C.; Panarello, H.O. Surface and groundwater quality in the northeastern region of Buenos Aires Province, Argentina. J. South Am. Earth Sci. 2007, 23, 336–345. [Google Scholar] [CrossRef]
- Schenone, N.; Volpedo, A.V.; Cirelli, A.F. Trace metal contents in water and sediments in Samborombón Bay wetland, Argentina. Wetl. Ecol. Manag. 2007, 15, 303–310. [Google Scholar] [CrossRef]
- Puntoriero, M.L.; Volpedo, A.V.; Fernández Cirelli, A. Arsenic, Fluoride, and Vanadium in surface water (Chasicó Lake, Argentina). Front. Environ. Sci. 2014, 2. [Google Scholar] [CrossRef]
- Rosso, J.J.; Troncoso, J.J.; Fernández Cirelli, A. Geographic Distribution of Arsenic and Trace Metals in Lotic Ecosystems of the Pampa Plain, Argentina. Bull. Environ. Contam. Toxicol. 2010, 86, 129–132. [Google Scholar] [CrossRef]
- Rodríguez Castro, M.C. Capacidad de depuración de sustancias bioaprovechables en arroyos de llanura y su relación con el arsénico. PhD Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2015. [Google Scholar]
- Feijoo, C.; Lombardo, R. Baseline water quality and macrophyte assemblages in Pampean streams: A regional approach. Water Res. 2007, 41, 1399–1410. [Google Scholar] [CrossRef]
- Frenguelli, J. Rasgos generales de la hidrogeografía de la provincia de Buenos Aires; La Plata: Buenos Aires, Argentina, 1956. [Google Scholar]
- Smedley, P.L.; Kinniburgh, D.G.; Macdonald, D.M.J.; Nicolli, H.B.; Barros, A.J.; Tullio, J.O.; Pearce, J.M.; Alonso, M.S. Arsenic associations in sediments from the loess aquifer of La Pampa, Argentina. Appl. Geochem. 2005, 20, 989–1016. [Google Scholar] [CrossRef]
- Rodríguez Castro, M.C.; Vilches, C.; Torremorell, A.; Vázquez, C.; Giorgi, A. Total reflection X ray fluorescence in environmental and geochemical studies: Unveiling solute provenance in streams during a rain episode. X-Ray Spectrom. 2016, 45, 225–232. [Google Scholar] [CrossRef]
- Schenone, N.F.; Avigliano, E.; Goessler, W.; Fernández Cirelli, A. Toxic metals, trace and major elements determined by ICPMS in tissues of Parapimelodus valenciennis and Prochilodus lineatus from Chascomus Lake, Argentina. Microchem. J. 2014, 112, 127–131. [Google Scholar] [CrossRef]
- Rosso, J.J.; Fernández Cirelli, A. Effects of land use on environmental conditions and macrophytes in prairie lotic ecosystems. Limnologica 2013, 43, 18–26. [Google Scholar] [CrossRef]
- Rodríguez Castro, M.C.; Marcó P, L.; Ranieri, M.C.; Vázquez, C.; Giorgi, A. Arsenic in the health of ecosystems: Spatial distribution in water, sediment and aquatic biota of Pampean streams. Environ. Monit. Assess. 2017, 189, 542. [Google Scholar] [CrossRef]
- Al Sayegh Petkovšek, S.; Mazej Grudnik, Z.; Pokorny, B. Heavy metals and arsenic concentrations in ten fish species from the Šalek lakes (Slovenia): Assessment of potential human health risk due to fish consumption. Environ. Monit. Assess. 2011, 184, 2647–2662. [Google Scholar] [CrossRef]
- Noël, L.; Chekri, R.; Millour, S.; Merlo, M.; Leblanc, J.-C.; Guérin, T. Distribution and relationships of As, Cd, Pb and Hg in freshwater fish from five French fishing areas. Chemosphere 2013, 90, 1900–1910. [Google Scholar] [CrossRef]
- Varol, M.; Kaya, G.K.; Sünbül, M.R. Evaluation of health risks from exposure to arsenic and heavy metals through consumption of ten fish species. Environ. Sci. Pollut. Res. 2019, 26, 33311–33320. [Google Scholar] [CrossRef]
- Buschmann, J.; Kappeler, A.; Lindauer, U.; Kistler, D.; Berg, M.; Sigg, L. Arsenite and arsenate binding to dissolved humic acids: Influence of pH, type of humic acid, and aluminum. Environ. Sci. Technol. 2006, 40, 6015–6020. [Google Scholar] [CrossRef]
- Sharma, P.; Kappler, A. Desorption of arsenic from clay and humic acid-coated clay by dissolved phosphate and silicate. J. Contam. Hydrol. 2011, 126, 216–225. [Google Scholar] [CrossRef]
- Chakraborty, P.; Jayachandran, S.; Babu, P.V.R.; Karri, S.; Tyadi, P.; Yao, K.M.; Sharma, B.M. Intra-annual variations of arsenic totals and species in tropical estuary surface sediments. Chem. Geol. 2012, 322, 172–180. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Paradelo, R.; Díaz-Fierros, F.; Barral, M.T. Fractionation and Bioavailability of Arsenic in the Bed Sediments of the Anllóns River (NW Spain). Water. Air. Soil Pollut. 2008, 195, 189–199. [Google Scholar] [CrossRef]
- Nespereira, X. Síntesis sobre los yacimientos auríferos gallegos. Braña 1978, 1, 18–49. [Google Scholar]
- Boixet, L.; Gleeson, C.F.; García, J. The Corcoesto gold deposit. In Proceedings of the 23rd International Applied Geochemistry Symposium; Oviedo, Spain, 14–19 June 2007.
- DIN 38414 – S4. German standards procedure: Determination of the leachability by water. German standards methods for the estimation of water, wastewater and sludge: Sludge and sediments (Group S); DIN 38414 part4; Fachgruppe Wasserchemie in der GDCh, Normausschuss asserwesen in DIN, Eds.; VCH: Weinheim, Germany, 1984. [Google Scholar]
- Costas, M.; Prego, R.; Filgueiras, A.V.; Bendicho, C. Land–ocean contributions of arsenic through a river–estuary–ria system (SW Europe) under the influence of arsenopyrite deposits in the fluvial basin. Sci. Total Environ. 2011, 412, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.L.; Devesa-Rey, R.; Pérez-Moreira, R.; Díaz-Fierros, F.; Barral, M.T. Phosphorus transfer across boundaries: From basin soils to river bed sediments. J. Soils Sediments 2011, 11, 1125–1134. [Google Scholar] [CrossRef]
- Barral, M.T.; Devesa-Rey, R.; Ruiz, B.; Díaz-Fierros, F. Evaluation of Phosphorus Species in the Bed Sediments of an Atlantic Basin: Bioavailability and Relation with Surface Active Components of the Sediment. Soil Sediment Contam. An Int. J. 2012, 21, 1–18. [Google Scholar] [CrossRef]
- Rial, M. Investigación dos procesos que regulan o caudal e a calidade das augas na bacía do Río Anllóns. PhD Dissertation, Universidade de Santiago de Compostela, Santiago de Compostela, Spain, 2007. [Google Scholar]
- Rubinos, D.A.; Calvo, V.; Iglesias, L.; Barral, M.T. Acute toxicity of arsenic to Aliivibrio fischeri (Microtox® bioassay) as influenced by potential competitive—Protective agents. Environ. Sci. Pollut. Res. 2014, 21, 8631–8644. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Moldes, A.B.; Díaz-Fierros, F.; Barral, M.T. Study of phytopigments in river bed sediments: Effects of the organic matter, nutrients and metal composition. Environ. Monit. Assess. 2008, 153, 147–159. [Google Scholar] [CrossRef]
- Prieto, D.M.; Devesa-Rey, R.; Paradelo, R.; Penalta-Rodríguez, M.; Díaz-Fierros, F.; Barral, M.T. Monitoring benthic microflora in river bed sediments: A case study in the Anllóns River (Spain). J. Soils Sediments 2016, 16, 1825–1839. [Google Scholar] [CrossRef]
- Prieto, D.M.; Devesa-Rey, R.; Rubinos, D.A.; Díaz-Fierros, F.; Barral, M.T. Biofilm Formation on River Sediments Under Different Light Intensities and Nutrient Inputs: A Flume Mesocosm Study. Environ. Eng. Sci. 2016, 33, 250–260. [Google Scholar] [CrossRef]
- Dzombak, D.A.; Morel, F.M.M. Surface Complexation Modeling: Hydrous Ferric Oxide; John Wiley & Sons: Hoboken, NJ, USA, 1990; ISBN 0471637319. [Google Scholar]
- Bennett, W.W.; Teasdale, P.R.; Panther, J.G.; Welsh, D.T.; Jolley, D.F. Speciation of Dissolved Inorganic Arsenic by Diffusive Gradients in Thin Films: Selective Binding of AsIIIby 3-Mercaptopropyl-Functionalized Silica Gel. Anal. Chem. 2011, 83, 8293–8299. [Google Scholar] [CrossRef] [PubMed]
- Tuulaikhuu, B.A.; Bonet, B.; Guasch, H. Effects of low arsenic concentration exposure on freshwater fish in the presence of fluvial biofilms. Sci. Total Environ. 2016, 544, 467–475. [Google Scholar] [CrossRef] [PubMed]
- MacNeill, K.L. Drivers of Nutrient and Toxic Element Cycles in Tropical and Temperate Montane Streams; Cornell University: Ithaca, NY, USA, 2019. [Google Scholar]
- Lopez, A.R.; Hesterberg, D.R.; Funk, D.H.; Buchwalter, D.B. Bioaccumulation Dynamics of Arsenate at the Base of Aquatic Food Webs. Environ. Sci. Technol. 2016, 50, 6556–6564. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Médigue, C.; Koechler, S.; Barbe, V.; Barakat, M.; Talla, E.; Bonnefoy, V.; Krin, E.; Arsène-Ploetze, F.; Carapito, C.; et al. A Tale of Two Oxidation States: Bacterial Colonization of Arsenic-Rich Environments. PLoS Genet. 2007, 3, e53. [Google Scholar] [CrossRef] [PubMed]
- Arsène-Ploetze, F.; Koechler, S.; Marchal, M.; Coppée, J.-Y.; Chandler, M.; Bonnefoy, V.; Brochier-Armanet, C.; Barakat, M.; Barbe, V.; Battaglia-Brunet, F.; et al. Structure, Function, and Evolution of the Thiomonas spp. Genome. PLoS Genet. 2010, 6, e1000859. [Google Scholar] [CrossRef] [PubMed]
- Price, J.R.; Ledford, S.H.; Ryan, M.O.; Toran, L.; Sales, C.M. Wastewater treatment plant effluent introduces recoverable shifts in microbial community composition in receiving streams. Sci. Total Environ. 2018, 613, 1104–1116. [Google Scholar] [CrossRef]
- Argudo, M.; Gich, F.; Bonet, B.; Espinosa, C.; Gutiérrez, M.; Guasch, H. Responses of resident (DNA) and active (RNA) microbial communities in fluvial biofilms under different polluted scenarios. Chemosphere 2020, 242, 125108. [Google Scholar] [CrossRef]
- Pandey, S.; Rai, R.; Rai, L.C. Proteomics combines morphological, physiological and biochemical attributes to unravel the survival strategy of Anabaena sp. PCC7120 under arsenic stress. J. Proteomics 2012, 75, 921–937. [Google Scholar] [CrossRef]
- Guasch, H.; Bonet, B.; Bonnineau, C.; Barral, L. Microbial Biomarkers. In Microbial Ecotoxicology; Cravo-Laureau, C., Cagnon, C., Lauga, B., Duran, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 251–281. ISBN 9783319617947. [Google Scholar]
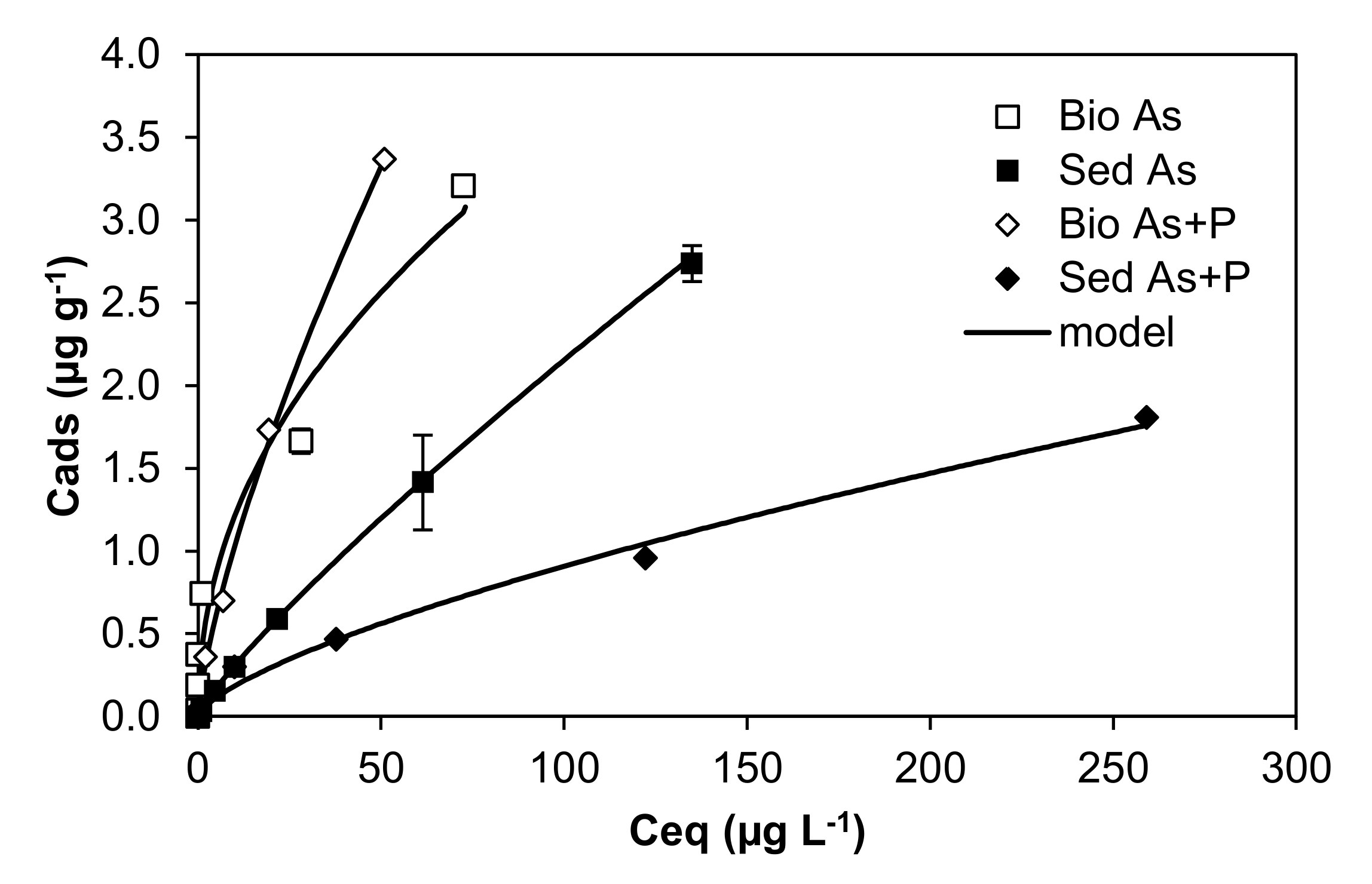
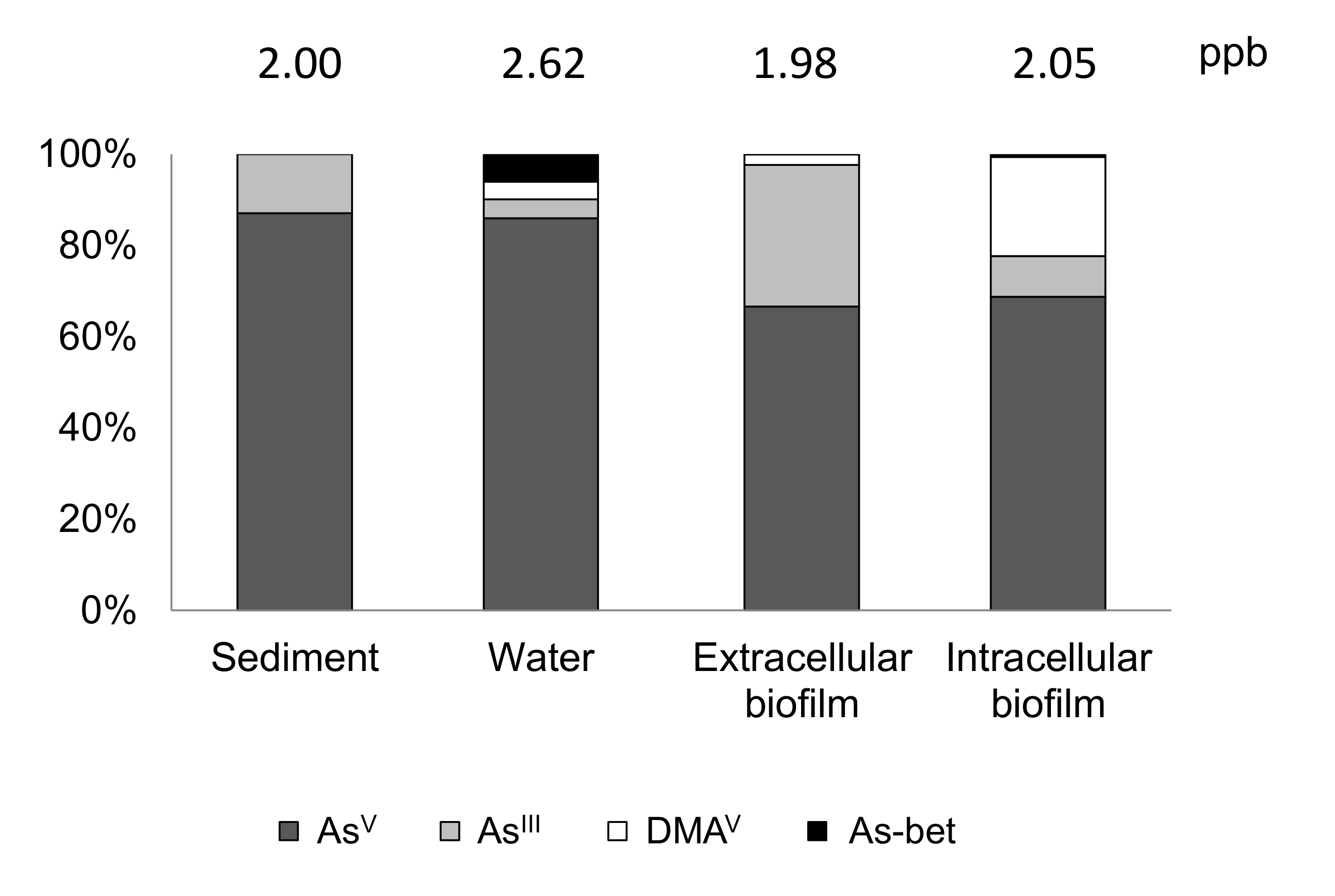
| Database | As-Exposed Organisms | Mean Toxic Dose | Concentration Units | Toxicity Endpoint | Effect Measurement |
|---|---|---|---|---|---|
| PAN | Biofilms (Periphyton) | 37.5 | µg As L−1 | IC20 | Carbon content |
| 59.9 | Nitrogen content | ||||
| 44.9 | Photosynthesis | ||||
| 30 | Photosynthesis | ||||
| 22.5 | Photosynthesis | ||||
| 22.5 | Biomass | ||||
| 15 | Diversity | ||||
| Diatoms | 60 | µg As L−1 | NR | General biochemistry | |
| 150 | General biochemistry | ||||
| 25 | General biochemistry | ||||
| 1.5 | pg cell−1 | LOEC | Abundance | ||
| 4.5 | NOEC | Abundance | |||
| ECOTOX | Algae | 79.4 | mg AsV L−1 | LC50 | Abundance |
| 1.19 | NOEC | Biomass | |||
| 8.59 | mg AsIII L−1 | NOEC | Biomass |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barral-Fraga, L.; Barral, M.T.; MacNeill, K.L.; Martiñá-Prieto, D.; Morin, S.; Rodríguez-Castro, M.C.; Tuulaikhuu, B.-A.; Guasch, H. Biotic and Abiotic Factors Influencing Arsenic Biogeochemistry and Toxicity in Fluvial Ecosystems: A Review. Int. J. Environ. Res. Public Health 2020, 17, 2331. https://doi.org/10.3390/ijerph17072331
Barral-Fraga L, Barral MT, MacNeill KL, Martiñá-Prieto D, Morin S, Rodríguez-Castro MC, Tuulaikhuu B-A, Guasch H. Biotic and Abiotic Factors Influencing Arsenic Biogeochemistry and Toxicity in Fluvial Ecosystems: A Review. International Journal of Environmental Research and Public Health. 2020; 17(7):2331. https://doi.org/10.3390/ijerph17072331
Chicago/Turabian StyleBarral-Fraga, Laura, María Teresa Barral, Keeley L. MacNeill, Diego Martiñá-Prieto, Soizic Morin, María Carolina Rodríguez-Castro, Baigal-Amar Tuulaikhuu, and Helena Guasch. 2020. "Biotic and Abiotic Factors Influencing Arsenic Biogeochemistry and Toxicity in Fluvial Ecosystems: A Review" International Journal of Environmental Research and Public Health 17, no. 7: 2331. https://doi.org/10.3390/ijerph17072331
APA StyleBarral-Fraga, L., Barral, M. T., MacNeill, K. L., Martiñá-Prieto, D., Morin, S., Rodríguez-Castro, M. C., Tuulaikhuu, B.-A., & Guasch, H. (2020). Biotic and Abiotic Factors Influencing Arsenic Biogeochemistry and Toxicity in Fluvial Ecosystems: A Review. International Journal of Environmental Research and Public Health, 17(7), 2331. https://doi.org/10.3390/ijerph17072331







