Determination of Propane-1,3-sultone in Workplace Air for Occupational Exposure Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Material and Reagents
3. Methodology
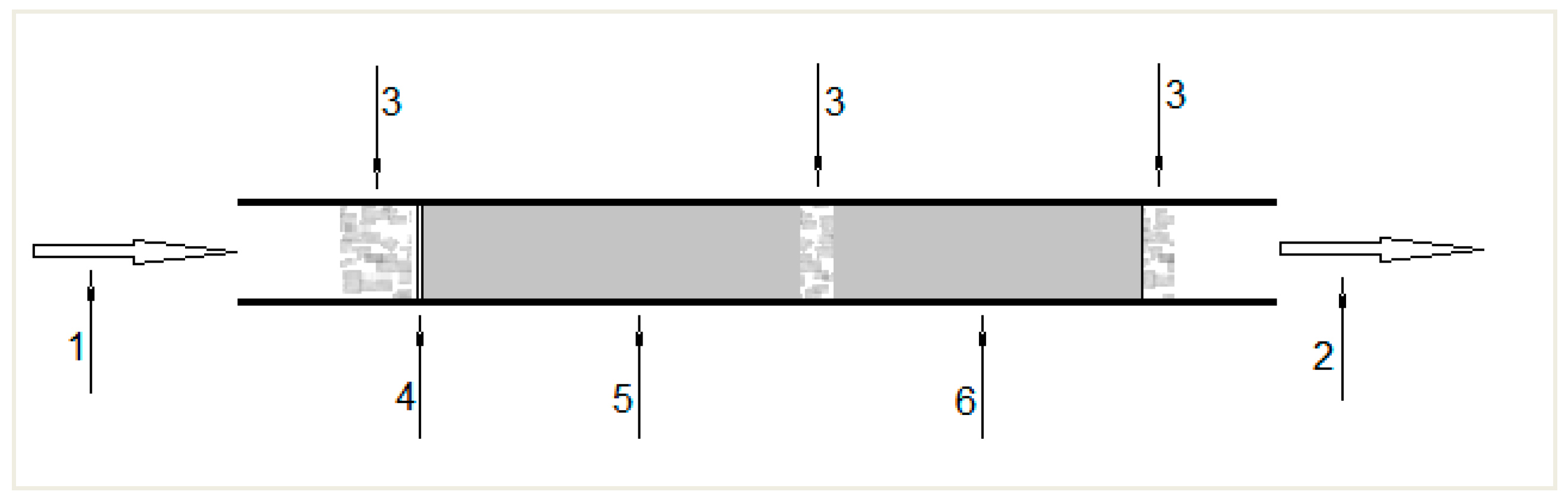
3.1. Sampling
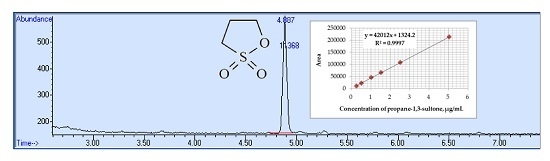
3.2. Conditions for Chromatographic Determination
3.3. Examination of Extraction Rate
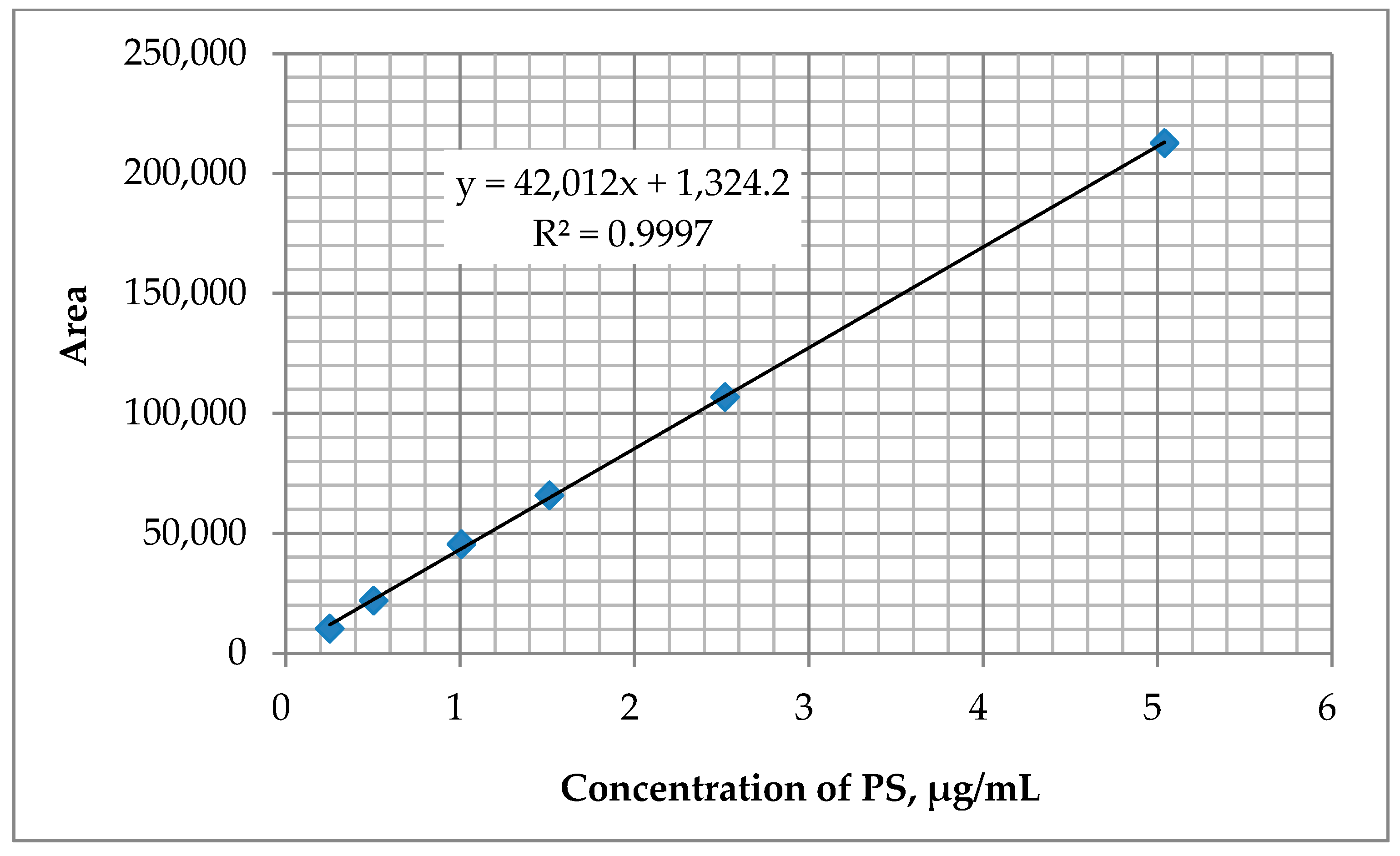
3.4. Calibration and Precision
3.5. Validation of the Method
- b—slope coefficient of the calibration curve, so—standard deviation.
- The LOQ value calculated as a multiple of the LOD value, from the following formula:
- Vp—is the precision of the sampling device (Vp = ± 5%);
- Vz—mean precision of three levels of ranges, which has been calculated using the formula:
4. Results and Discussion
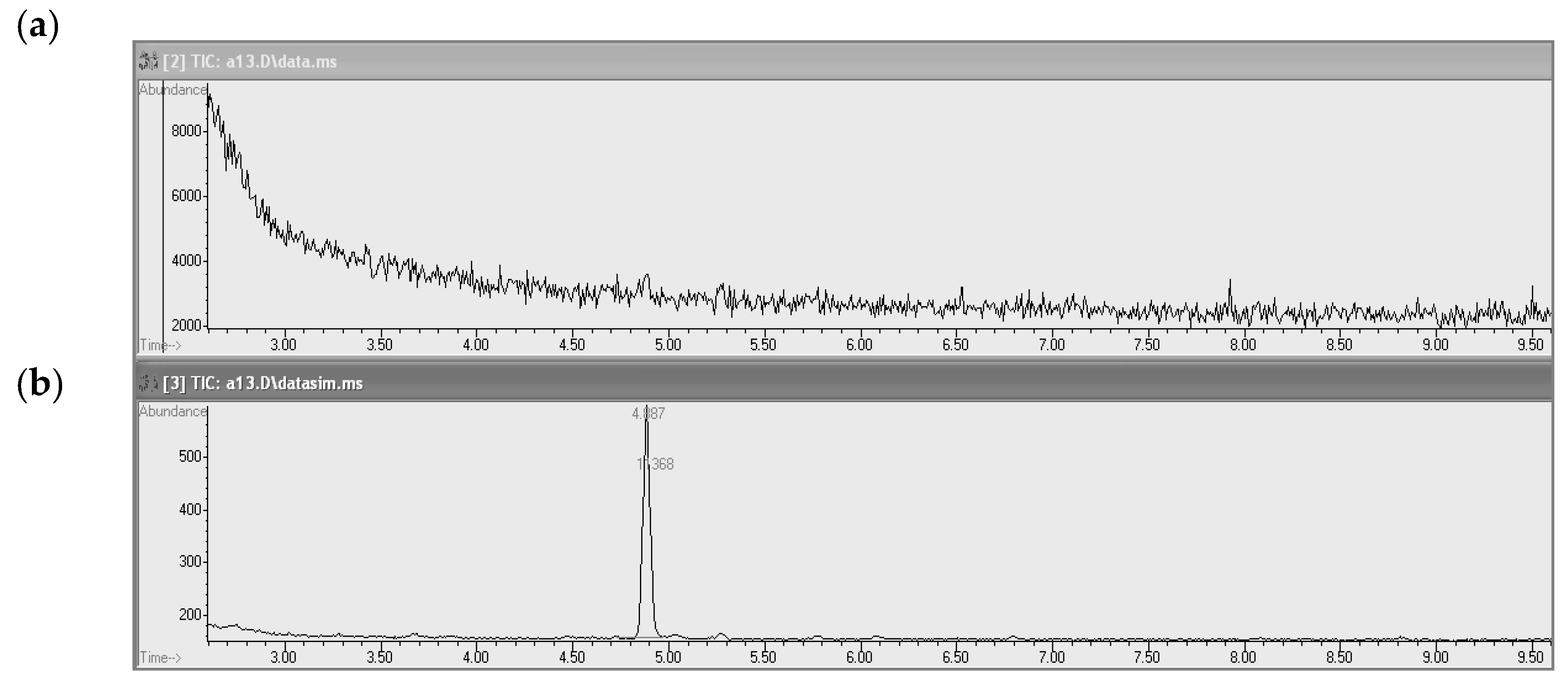
4.1. Testing of Air Sampling Conditions
4.2. Determination of Extraction Rate
4.3. Calibration and Precision
4.4. Sample Storage
4.5. Validation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GESTIS. Substance Database. BG Institute for Occupational Safety and Health. Sankt Augustin, Germany. Available online: http://gestis-en.itrust.de/nxt/gateway.dll/gestis_en/000000.xml?f=templates$fn=default.htm$vid=gestiseng:sdbeng$3.0 (accessed on 16 May 2019).
- Szymańska, J.; Frydrych, B. Propane-1,3-sultone. Documentation of suggested occupational exposure limits. Princ. Methods Assess. Work. Environ. 2014, 3, 39–56. [Google Scholar]
- Scientific Committee on Occupational Exposure Limits. Recommendation from the Scientific Committee on Occupational Exposure Limits for 1,3-Propane Sultone; (SCOEL/SUM/189, June 2013); European Commission Employment, Social Affairs & Inclusion: Brussels, Belgium, 2013. [Google Scholar]
- Soile, O.O.B. Commercial potential of sultones. Int. J. Adv. Res. Technol. 2014, 10, 81–98. [Google Scholar]
- Xia, J.; Harlow, J.E.; Petibon, R.; Burns, J.C.; Chen, L.P.; Dahn, J.R. Comparative study on methylene methyl disulfonate (MMDS) and 1,3-propane sultone (PS) as electrolyte additives for Li-ion batteries. J. Electrochem. Soc. 2014, 161, a547–a553. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). 1,3-Propane sultone. In Some Aromatic Amines, Hydrazine and Related Substances, N-Nitroso Compounds and Miscellaneous Alkylating Agents; IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans; International Agency for Research on Cancer: Lyon, France, 1974; Volume 4, pp. 253–258. [Google Scholar]
- IARC (International Agency for Research on Cancer). 1,3-Propane sultone. In Re-Evaluation of Some Organic Chemicals, Hydrazine, And Hydrogen Peroxide; IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans; International Agency for Research on Cancer: Lyon, France, 1999; Volume 71, pp. 1095–1102. [Google Scholar]
- IARC (International Agency for Research on Cancer). 1,3-Propane sultone. In Some Chemicals Used as Solvents and in Polymer Manufacture; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2017; Volume 110, pp. 257–273. [Google Scholar]
- Ulland, B.; Finkelstein, M.; Weisburger, E.K.; Rice, J.M.; Weisburger, J.H. Carcinogenicity of industrial chemicals propylene imine and propane sultone. Nature 1971, 230, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Program, N.T. 1,3-Propane sultone. Rep. Carcinog. 2002, 10, 207. [Google Scholar]
- Program, N.T. 1,3-Propane sultone. Rep. Carcinog. 2011, 12, 364–366. [Google Scholar]
- Bolt, H.; Golka, K. 1,3-Propane sultone, an extremely potent experimental carcinogen: What should be expected in humans? Toxicol. Lett. 2004, 151, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Bolt, H.; Golka, K. 1,3-Propane sultone as an extremely potent human carcinogen: Description of an exposed cohort in Germany. J. Toxicol. Environ. Health Part A 2012, 75, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mehrotra, T.; Srivastava, U. Carcinogenic effect of 1,3-propane sultone. Int. Surg. 1981, 66, 161–163. [Google Scholar] [PubMed]
- Weisburger, E.; Ulland, B.; Nam, J.; Gart, J.; Weisburger, J. Carcinogenicity tests of certain environmental and industrial-chemicals. J. Natl. Cancer Inst. 1981, 67, 75–88. [Google Scholar] [PubMed]
- NIOSH (National Institute for Occupational Safety and Health). Propane sultone. Centres for Disease Control and Prevention. The National Institute for Occupational Safety and Health. Available online: https://www.cdc.gov/niosh/npg/npgd0525.html (accessed on 20 August 2019).
- Regulation of the Minister of Family, Labour and Social Policy of 12 June 2018 on Maximum Permissible Concentration and Intensity of Agents Harmful to Health in the Working Environment; Polish Legal Act: Warsaw, Poland, 2018.
- Oldeweme, J.; Klockow, D. Chromatographic procedures for the determination of 1,3-propanesultone (1,2-oxathiolane-2,2-dioxide) in workplace atmosphere. Fresenius Z. Anal. Chem. 1986, 325, 57–63. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Determination of Volatile Organic Compounds (VOCs) in Air Collected in Specially-Prepared Canisters and Analyzed by Gas Chromatography/Mass Spectrometry (GC/MS); Compendium Method TO-15 (EPA/625/R-96/010b); Environmental Protection Agency: Cincinnati, OH, USA, 1999.
- PN-Z-04188-2:1988 Air Purity Protection—Tests for 1,3-Propane Sultone Content—Determination of 1,3-Propane Sultone in Workplace Air by Gas Chromatography with Sample Enrichment; Polish Committee for Standardization: Warsaw, Poland, 1988.
- EN 482:2012+A1:2015 Workplace Exposure—General Requirements for the Performance of Procedures for the Measurement of Chemical Agents; European Committee for Standardization: Brussels, Belgium, 2015.
- PN-Z-04008-7:2002 Air Purity Protection—Sampling Methods—Principles of Air Sampling in Work Place and Interpretation of Results; Polish Committee for Standardization: Warsaw, Poland, 2002.
| Air Flow Rate [L/min] | Sampling Time [min] | Approximate Substance Concentration in the Air [mg/m3] | Area of PS Peaks in Solutions after Desorption | Quantity of the Substance in Layer II of XAD-2 (in % of the Quantity Determined in Layer I) | ||
|---|---|---|---|---|---|---|
| Glass Fiber | Layer I | Layer II | ||||
| 2 | 360 | pure substance | no peaks | 4,346,325 | 490,610 | 11 |
| 1 | 360 | pure substance | no peaks | 11,124,567 | 1,333,226 | 12 |
| 2 | 360 | 0.08 | no peaks | 1,292,867 | 211,425 | 16.4 |
| 1 | 360 | 0.16 | no peaks | 1,035,235 | 36,662 | 3.5 |
| 1 | 360 | 0.08 | no peaks | 538,958 | 14,463 | 2.7 |
| 1 | 360 | 0.04 | no peaks | 397,602 | 13,531 | 3.4 |
| Air Flow Rate [L/min] | Sampling Time [min] | Approximate Substance Concentration in the Air [mg/m3] | Area of PS Peaks in Solutions after Extraction with Acetonitrile (1 mL) | |||
|---|---|---|---|---|---|---|
| Glass Fiber | Glass Fiber Filter | Gel Layer I | Gel Layer II | |||
| 1 | 60 | pure substance | no peaks | 47,141 | 210,340 | no peaks |
| 1 | 360 | 0.08 | no peaks | 3629 | 530,567 | no peaks |
| 1 | 360 | 0.04 | no peaks | 3356 | 231,738 | no peaks |
| Mass of PS Applied to the Adsorption Tube [µg] | Average Peak Area of Extracted Solutions | Average Peak Area of the Comparative Solutions | Average Extraction Coefficient |
|---|---|---|---|
| 0.252 | 11,860.3 | 12,022 | 0.99 |
| 2.22 | 107,519.5 | 108,996 | 0.99 |
| 5.55 | 247,082.6 | 249,414 | 0.99 |
| Tube No. | Storage Place | Storage Time [Number of Days] | Average Peak Area of the PS | Two-Tube Average | Standard Deviation |
|---|---|---|---|---|---|
| 1 | desiccator | 1 | 247,687.0 | 249,692.00 | 2835.5 |
| 2 | 251,697.0 | ||||
| 1 | desiccator | 2 | 263,444.0 | 253,379.50 | 14,233.4 |
| 2 | 243,315.0 | ||||
| 1 | refrigerator | 2 | 237,593.0 | 242,193.00 | 6506.1 |
| 2 | 246,794.0 | ||||
| 1 | desiccator | 5 | 246,684.0 | 249,647.00 | 4190.3 |
| 2 | 252,610.0 | ||||
| 1 | refrigerator | 5 | 246,472.5 | 247,481.75 | 1427.3 |
| 2 | 248,491.0 | ||||
| 1 | desiccator | 7 | 253,114.5 | 254,277.00 | 1644.0 |
| 2 | 255,439.5 | ||||
| 1 | refrigerator | 7 | 257,130.0 | 256,742.50 | 548.0 |
| 2 | 256,355.0 |
| Parameter | Value |
|---|---|
| Measurement range | 0.7 ÷ 14 µg/m3 |
| Sampled air volume | 360 L |
| Range of calibration curve | 0.252 ÷ 5.04 µg/mL |
| Limit of detection (LOD) | 4.82 ng/mL (13 ng/m3) |
| Limit of quantitation (LOQ) | 14.46 ng/mL (40 ng/m3) |
| Overall precision of examination | 5.99% |
| Relative total uncertainty | 13% |
| Relative expanded uncertainty | 26% |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeżewska, A. Determination of Propane-1,3-sultone in Workplace Air for Occupational Exposure Assessment. Int. J. Environ. Res. Public Health 2020, 17, 1414. https://doi.org/10.3390/ijerph17041414
Jeżewska A. Determination of Propane-1,3-sultone in Workplace Air for Occupational Exposure Assessment. International Journal of Environmental Research and Public Health. 2020; 17(4):1414. https://doi.org/10.3390/ijerph17041414
Chicago/Turabian StyleJeżewska, Anna. 2020. "Determination of Propane-1,3-sultone in Workplace Air for Occupational Exposure Assessment" International Journal of Environmental Research and Public Health 17, no. 4: 1414. https://doi.org/10.3390/ijerph17041414
APA StyleJeżewska, A. (2020). Determination of Propane-1,3-sultone in Workplace Air for Occupational Exposure Assessment. International Journal of Environmental Research and Public Health, 17(4), 1414. https://doi.org/10.3390/ijerph17041414