Effects of Copper Oxide Nanoparticles on the Growth of Rice (Oryza Sativa L.) Seedlings and the Relevant Physiological Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. CuO NP Characterization
2.2. Culture of Rice Plants
2.3. Integrity of the Cell Membrane System of Rice
2.4. Antioxidant Enzyme Activity of the Rice Seedlings
2.5. Chlorophyll and Carotenoid Content
2.6. Quantitative Real-Time PCR (qRT-PCR) of the Chlorophyll Synthesis Genes
2.7. Statistical Analysis
3. Results and Discussion
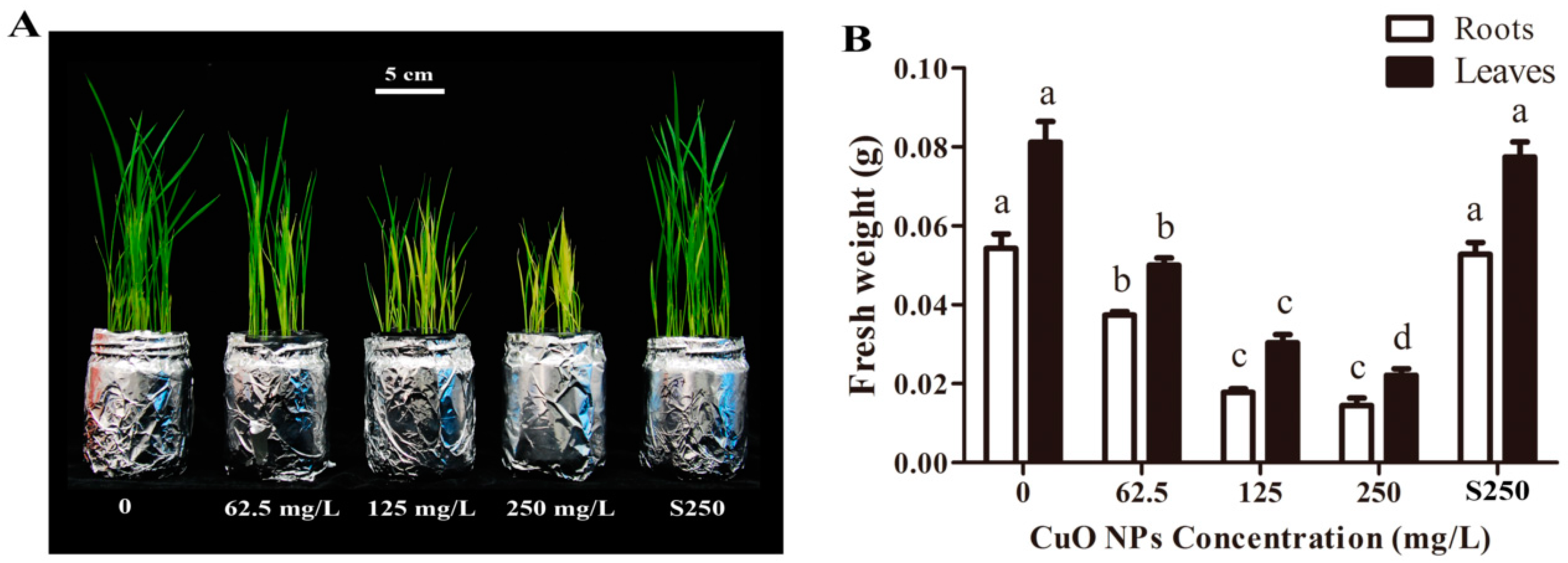
3.1. Biomass Accumulation of Rice Seedlings
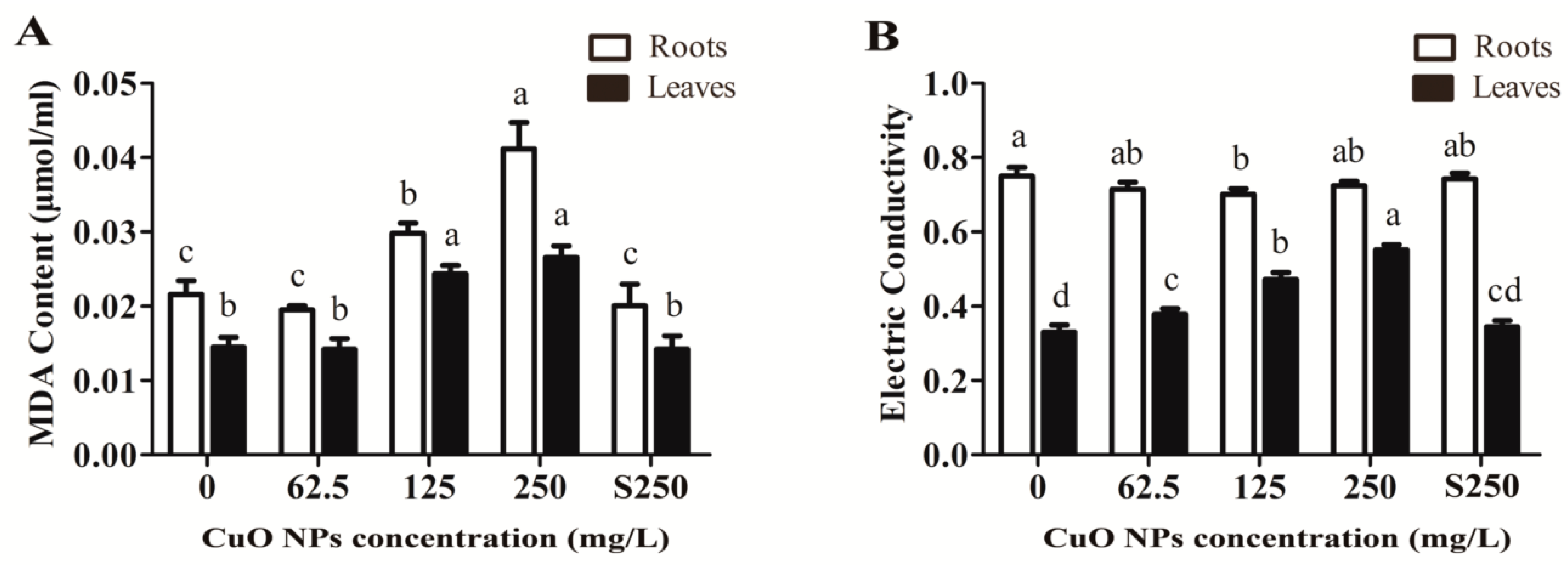
3.2. Integrity of the Cell Membrane System of Rice
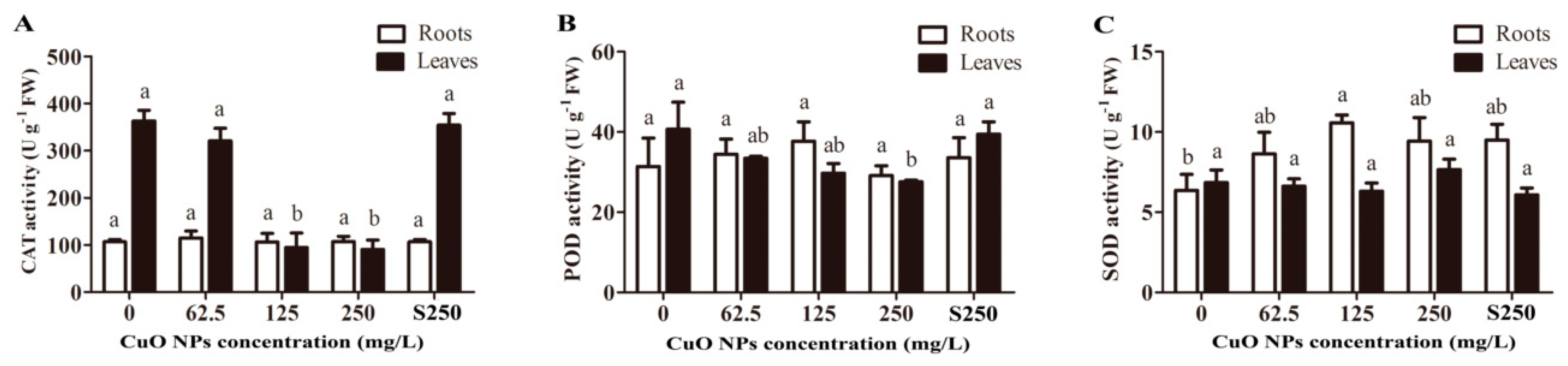
3.3. Antioxidant Enzyme Activity of Rice Seedlings
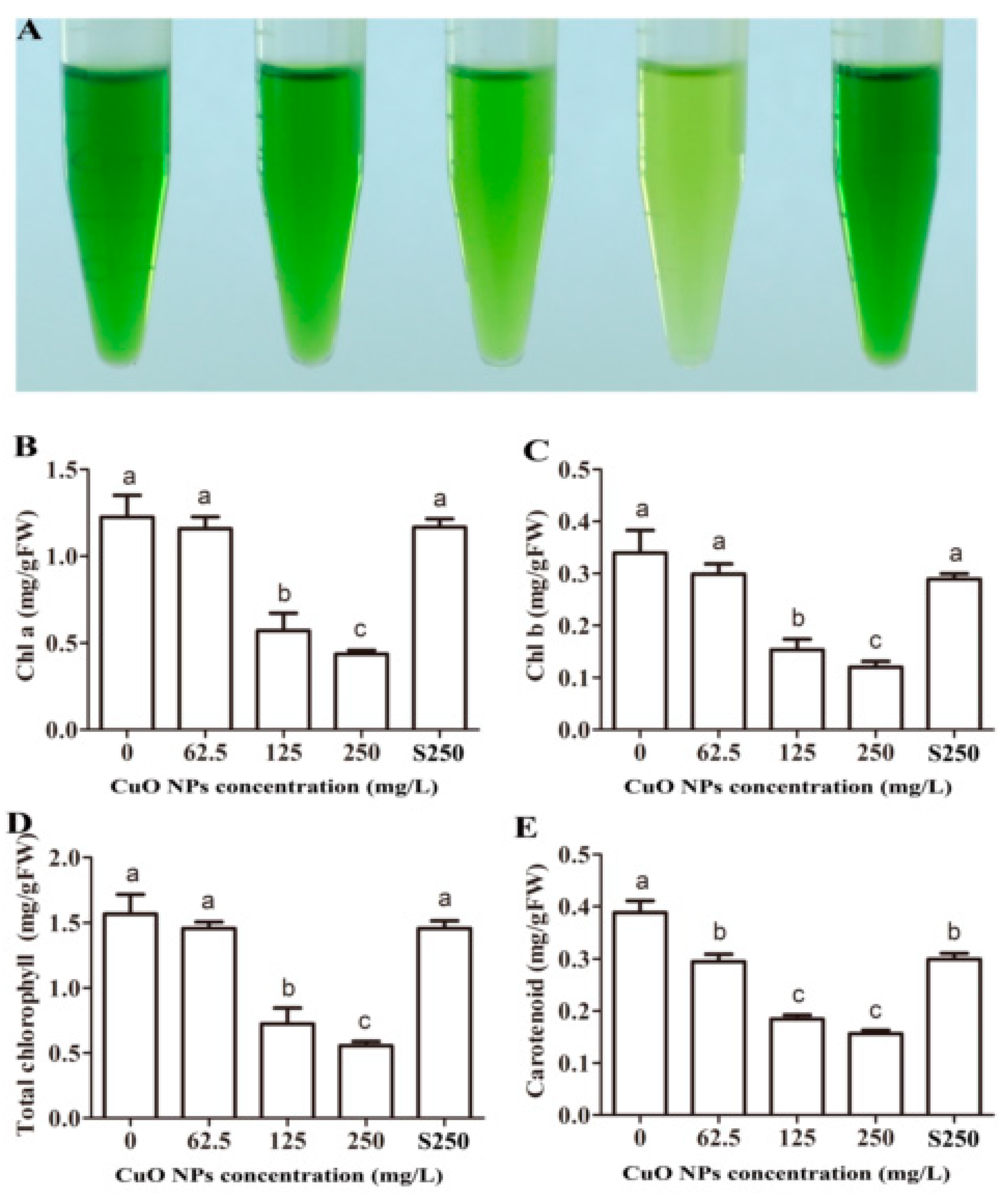
3.4. Chlorophyll and Carotenoid Content of Rice Leaves
3.5. Synthesis of Chlorophyll and Carotenoid in Rice Leaves
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.; Yang, X.; Chen, S.; Li, Q.; Wei, W.; Hou, C.; Xiao, G.; Li, W.; Wang, S. Zinc Oxide Nanoparticles Affect Biomass Accumulation and Photosynthesis in Arabidopsis. Front. Plant Sci. 2015, 6, 559. [Google Scholar]
- White, J.C.; Gardeatorresdey, J. Achieving food security through the very small. Nat. Nanotechnol. 2018, 13, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Videa, J.R.; Zhao, L.; Lopez-Moreno, M.L.; de la Rosa, G.; Hong, J.; Gardea-Torresdey, J.L. Nanomaterials and the environment: A review for the biennium 2008–2010. J. Hazard. Mater. 2011, 186, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Meesters, J.A.; Ter Braak, C.J.; Van, D.M.D.; Van, D.V.H. Combining exposure and effect modelling into an integrated probabilistic environmental risk assessment for nanoparticles. Environ. Toxicol. Chem. 2016, 35, 2958–2967. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Srivastava, A.K.; Karmakar, S. Nanomaterial toxicity for plants. Environ. Chem. Lett. 2017, 16, 1–16. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef]
- Mckee, M.S.; Filser, J. Impacts of metal-based engineered nanomaterials on soil communities. Environ. Sci. Nano 2016, 3, 506–533. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, L.; Zhao, X.; Zhao, S.; Gu, X.; Du, W.; Wei, H.; Ji, R.; Zhao, L. Metabolomics Reveals the “Invisible” Responses of Spinach Plants Exposed to CeO2 Nanoparticles. Environ. Sci. Technol. 2019, 53, 6007–6017. [Google Scholar] [CrossRef]
- Li, J.; Tappero, R.V.; Acerbo, A.S.; Yan, H.; Unrine, J.M. Effect of CeO2 nanomaterial surface functional groups on tissue and subcellular distribution of Ce in tomato (Solanum lycopersicum). Environ. Sci. Nano 2019, 6, 273–285. [Google Scholar] [CrossRef]
- Štefaniæ, P.P.; Jarnević, M.; Cvjetko, P.; Biba, R.; Šikić, S.; Tkalec, M.; Cindrić, M.; Letofsky-Papst, I.; Balen, B. Comparative proteomic study of phytotoxic effects of silver nanoparticles and silver ions on tobacco plants. Environ. Sci. Pollut. Res. 2019, 26, 22529–22550. [Google Scholar]
- Dar, M.A.; Kim, Y.S.; Kim, W.B.; Sohn, J.M.; Shin, H.S. Structural and magnetic properties of CuO nanoneedles synthesized by hydrothermal method. Appl. Surf. Sci. 2008, 254, 7477–7481. [Google Scholar] [CrossRef]
- Da-Wei, Z.; Tang-Hong, Y.; Chun-Hua, C. Cu nanoparticles derived from CuO electrodes in lithium cells. Nanotechnology 2005, 16, 2338–2341. [Google Scholar]
- Chowdhuri, A.; Gupta, V.; Sreenivas, K.; Kumar, R.; Patanjali, P.K. Response speed of SnO2-based H2S gas sensors with CuO nanoparticles. Appl. Phys. Lett. 2004, 84, 1180–1182. [Google Scholar] [CrossRef]
- Jatti, V.S.; Singh, T.P. Copper oxide nano-particles as friction-reduction and anti-wear additives in lubricating oil. J. Mech. Sci. Technol. 2015, 29, 793–798. [Google Scholar] [CrossRef]
- Reddy, K.J.; McDonald, K.J.; King, H. A novel arsenic removal process for water using cupric oxide nanoparticles. J. Colloid Interf. Sci. 2013, 397, 96–102. [Google Scholar] [CrossRef]
- Suribabu, J.; Sekarpandi, S.; Laxmidhar, R.; Tathagata, M.; Santu, M.; Raja, M.; Prasenjit, S.; Tharmalingam, P. CuO nanoparticles catalyzed C-N, C-O, and C-S cross-coupling reactions: Scope and mechanism. J. Org. Chem. 2010, 40, 1971–1976. [Google Scholar]
- The Global Market Forecast from 2010 to 2025: Production Volumes, Prices, Future Projections and End User Markets. Available online: https://www.futuremarketsinc.com/ (accessed on 15 February 2020).
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Liu, J.; Dhungana, B.; Cobb, G.P. Environmental Behavior, Potential Phytotoxicity, and Accumulation of Copper Oxide Nanoparticles and Arsenic in Rice Plants. Environ. Toxicol. Chem. 2018, 37, 11–20. [Google Scholar] [CrossRef]
- Farzad, A.; Samira, B.; Nurhidayatullaili, M.J.; Shukor, J.A.; Golestan, H.F.S.; Ali, B. Effects of Engineered Nanomaterials on Plants Growth: An Overview. Sci. World J. 2014, 2014, 641759. [Google Scholar]
- Nair, P.M.G.; Chung, I.M. Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignification in Indian mustard (Brassica juncea L.). Ecotoxicol. Environ. Saf. 2015, 113, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.J.; Zhang, J.Z.; Wang, Z.Y. Toxicity of Copper Oxide Engineered Nanoparticles to Maize (Zea mays L.) at Different Aging Times. Adv. Mater. Res. 2014, 881, 972–975. [Google Scholar] [CrossRef]
- Van, N.; Ma, C.; Shang, J.; Rui, Y.; Liu, S.; Xing, B. Effects of CuO nanoparticles on insecticidal activity and phytotoxicity in conventional and transgenic cotton. Chemosphere 2016, 144, 661–670. [Google Scholar] [CrossRef]
- Ochoa, L.; Medina-Velo, I.A.; Barrios, A.C.; Bonilla-Bird, N.J.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Modulation of CuO nanoparticles toxicity to green pea (Pisum sativum Fabaceae) by the phytohormone indole-3-acetic acid. Sci. Total. Environ. 2017, 598, 513–524. [Google Scholar] [CrossRef]
- Zhang, D.; Tao, H.; Fei, X.; Chen, C.; Gersberg, R.M.; Yu, L.; Ng, W.J.; Tan, S.K. Uptake and accumulation of CuO nanoparticles and CdS/ZnS quantum dot nanoparticles by Schoenoplectus tabernaemontani in hydroponic mesocosms. Ecol. Eng. 2014, 70, 114–123. [Google Scholar] [CrossRef]
- Shaw, A.K.; Ghosh, S.; Kalaji, H.M.; Bosa, K.; Brestic, M.; Zivcak, M.; Hossain, Z. Nano-CuO stress induced modulation of antioxidative defense and photosynthetic performance of Syrian barley (Hordeum vulgare L.). Environ. Exp. Bot. 2014, 102, 37–47. [Google Scholar] [CrossRef]
- Jiyan, S.; Abid, A.D.; Kennedy, I.M.; Hristova, K.R.; Silk, W.K. To duckweeds (Landoltia punctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ. Pollut. 2011, 159, 1277–1282. [Google Scholar]
- Shaw, A.K.; Hossain, Z. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 2013, 93, 906–915. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the Phytotoxicity of Metal Oxide Nanoparticles on Two Crop Plants, Maize (Zea mays L.) and Rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 15100–15109. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dou, R.; Yang, Z.; You, T.; Gao, X.; Wang, L. Phytotoxicity and bioaccumulation of zinc oxide nanoparticles in rice (Oryza sativa L.). Plant Physiol. Biochem. 2018, 130, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, M.R.; Giorgetti, L.; Geri, C.; Cremonini, R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J. Nanopart. Res. 2011, 13, 2443–2449. [Google Scholar] [CrossRef]
- Shouichi, Y. Laboratory manual for physiological studies of rice. Int. Rice Res. Inst. 1976. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Zhao, L.; Peng, B.; Hernandez-Viezcas, J.A.; Rico, C.; Sun, Y.; Peralta-Videa, J.R.; Tang, X.; Niu, G.; Jin, L.; Varela-Ramirez, A.; et al. Stress response and tolerance of Zea mays to CeO2 nanoparticles: Cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano. 2012, 6, 9615–9622. [Google Scholar] [CrossRef]
- Gallego, S.M.; Benavídes, M.P.; Tomaro, M.L. Effect of heavy metal ion excess on sunflower leaves: Evidence for involvement of oxidative stress. Plant Sci. 1996, 121, 151–159. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Egley, G.H.; Paul, R.N., Jr.; Vaughn, K.C.; Duke, S.O. Role of peroxidase in the development of water-impermeable seed coats in Sida spinosa L. Planta 1983, 157, 224–232. [Google Scholar] [CrossRef]
- Nair, P.M.; Chung, I.M. Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ. Sci. Pollut. Res. Int. 2014, 21, 8858–8869. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Mclean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1–15. [Google Scholar] [CrossRef]
- Mocquot, B.; Vangronsveld, J.; Clijsters, H.; Mench, M. Copper toxicity in young maize (Zea mays L.) plants: Effects on growth, mineral and chlorophyll contents, and enzyme activities. Plant Soil 1996, 182, 287–300. [Google Scholar] [CrossRef]
- Khatun, S.; Ali, M.B.; Hahn, E.-J.; Paek, K.-Y. Copper toxicity in Withania somnifera: Growth and antioxidant enzymes responses of in vitro grown plants. Environ. Exp. Bot. 2008, 64, 279–285. [Google Scholar] [CrossRef]
- Wang, Z.; von dem Bussche, A.; Kabadi, P.K.; Kane, A.B.; Hurt, R.H. Biological and Environmental Transformations of Copper-Based Nanomaterials. ACS Nano 2013, 7, 8715–8727. [Google Scholar] [CrossRef]
- Gunawan, C.; Teoh, W.Y.; Marquis, C.P.; Amal, R. Cytotoxic Origin of Copper(II) Oxide Nanoparticles: Comparative Studies with Micron-Sized Particles, Leachate, and Metal Salts. Acs Nano 2011, 5, 7214–7225. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ. Sci. Pollut. Res. 2004, 21, 12709–12722. [Google Scholar] [CrossRef]
- Takahashi, M.A.; Asada, K. Superoxide anion permeability of phospholipid membranes and chloroplast thylakoids. Arch. Biochem. Biophys. 1983, 226, 558. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef]
- Navarro, E.; Wagner, B.; Odzak, N.; Sigg, L.; Behra, R. Effects of Differently Coated Silver Nanoparticles on the Photosynthesis of Chlamydomonas reinhardtii. Environ. Sci. Technol. 2015, 49, 8041–8047. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Xiao, Y.; Jiao, T.; Zhang, Y.; Chen, J.; Gao, Y. Effects of Copper Oxide Nanoparticles on the Growth of Rice (Oryza Sativa L.) Seedlings and the Relevant Physiological Responses. Int. J. Environ. Res. Public Health 2020, 17, 1260. https://doi.org/10.3390/ijerph17041260
Yang Z, Xiao Y, Jiao T, Zhang Y, Chen J, Gao Y. Effects of Copper Oxide Nanoparticles on the Growth of Rice (Oryza Sativa L.) Seedlings and the Relevant Physiological Responses. International Journal of Environmental Research and Public Health. 2020; 17(4):1260. https://doi.org/10.3390/ijerph17041260
Chicago/Turabian StyleYang, Zhongzhou, Yifan Xiao, Tongtong Jiao, Yang Zhang, Jing Chen, and Ying Gao. 2020. "Effects of Copper Oxide Nanoparticles on the Growth of Rice (Oryza Sativa L.) Seedlings and the Relevant Physiological Responses" International Journal of Environmental Research and Public Health 17, no. 4: 1260. https://doi.org/10.3390/ijerph17041260
APA StyleYang, Z., Xiao, Y., Jiao, T., Zhang, Y., Chen, J., & Gao, Y. (2020). Effects of Copper Oxide Nanoparticles on the Growth of Rice (Oryza Sativa L.) Seedlings and the Relevant Physiological Responses. International Journal of Environmental Research and Public Health, 17(4), 1260. https://doi.org/10.3390/ijerph17041260



