Neonatal Mortality and Temperature in Two Northern Swedish Rural Parishes, 1860–1899—The Significance of Ethnicity and Gender
Abstract
1. Introduction
1.1. Seasonality, Temperature and Infant Mortality
1.2. Seasonality, Temperature and Neonatal Mortality in Preindustrial Sweden
2. Materials and Methods
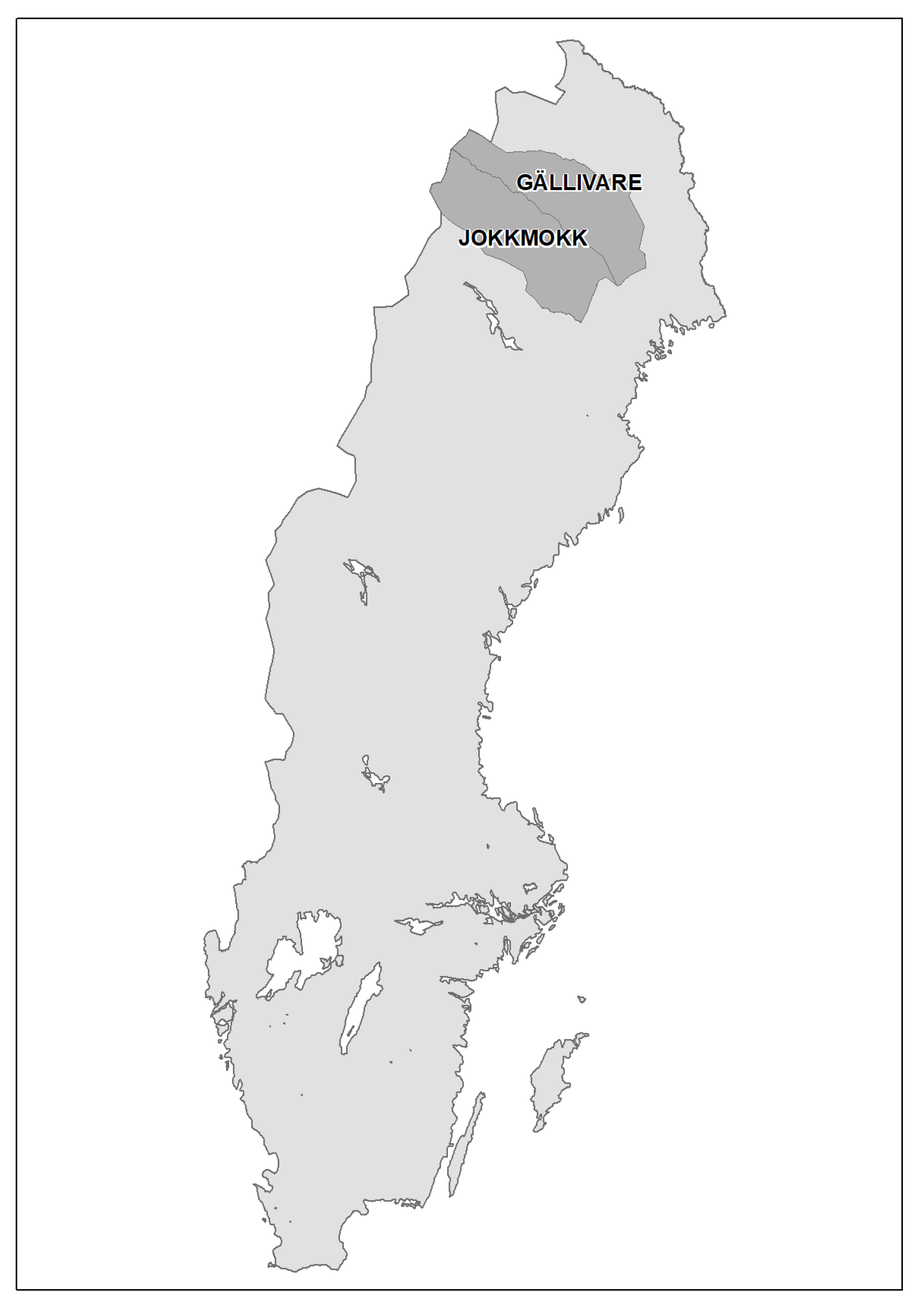
2.1. Population Data
2.2. Temperature Data
2.3. Longitudinal Dataset
2.4. Data Analysis
3. Results
3.1. Descriptives
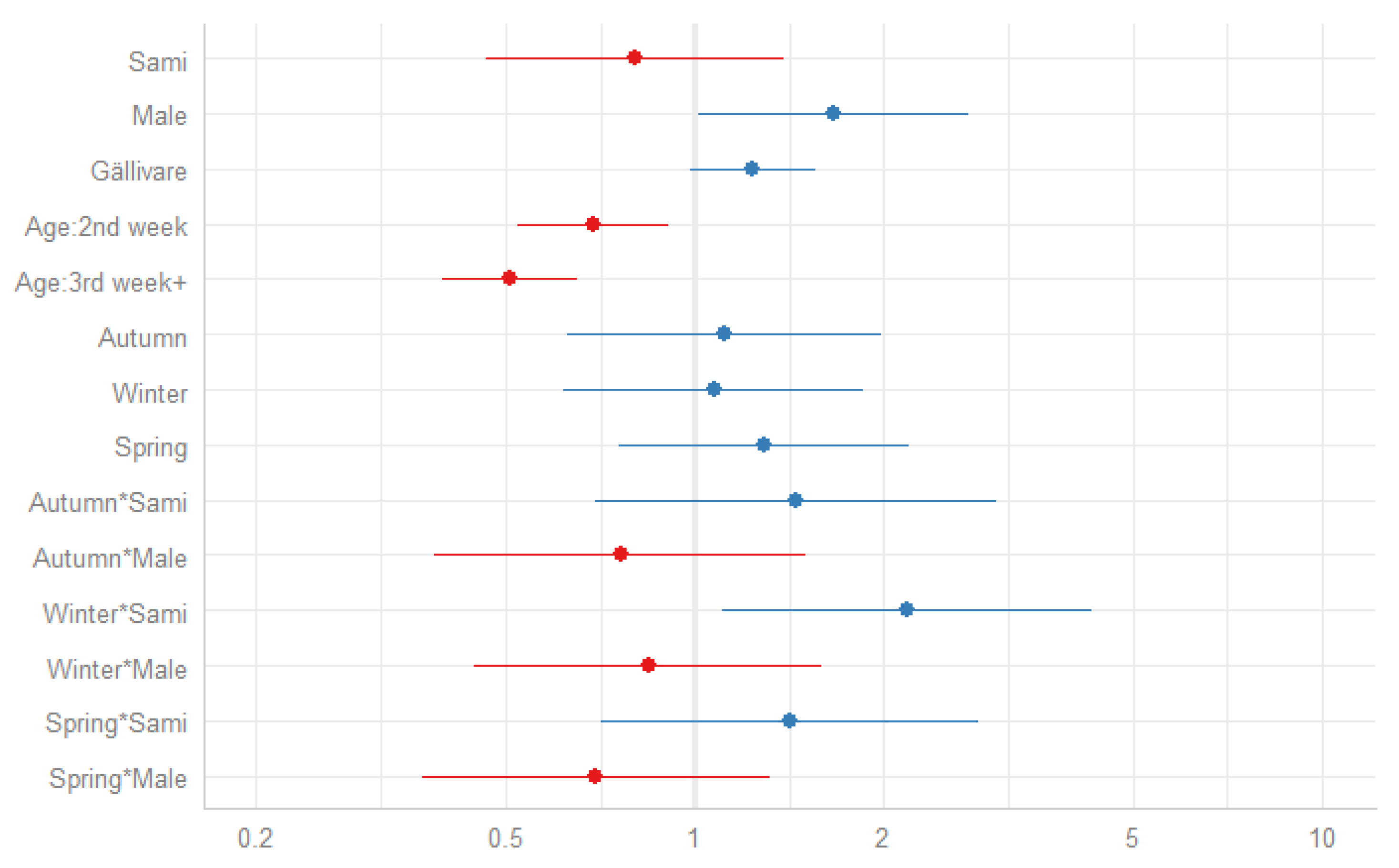
3.2. Season and Neonatal Mortality
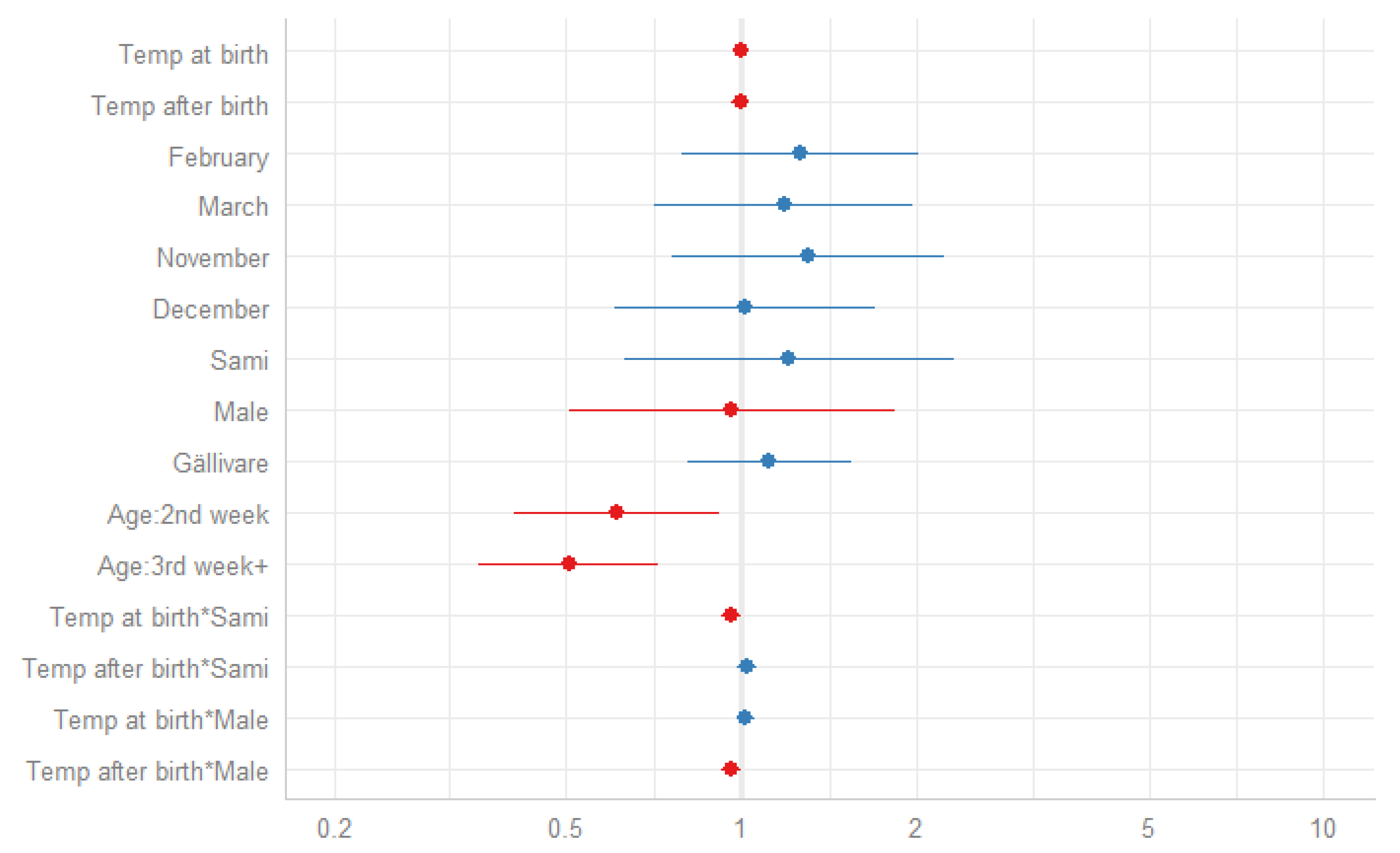
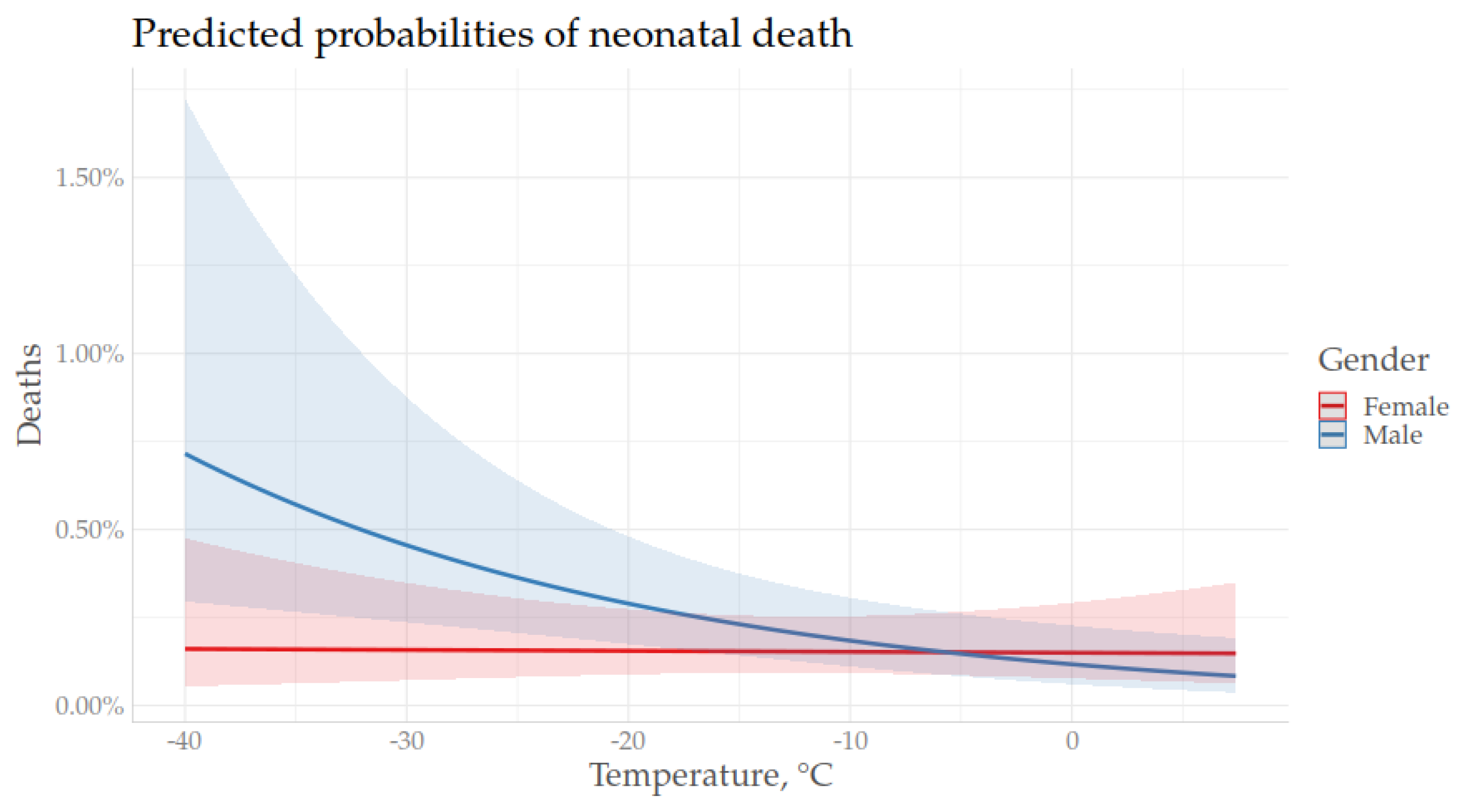
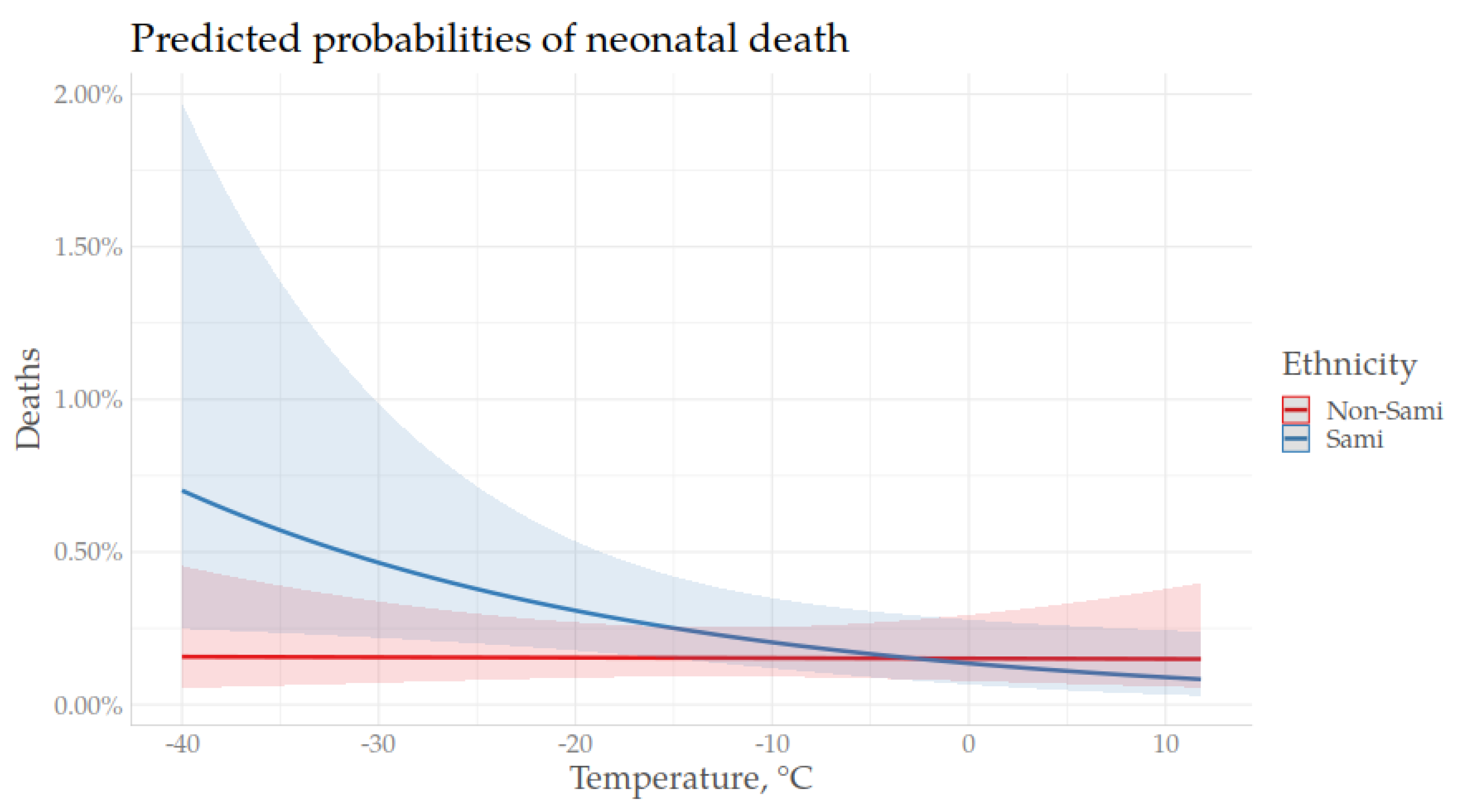
3.3. Temperature and Neonatal Mortality During an Extended Winter Season
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Predictors | Model I | Model II | Model III | Model IV | Model V | Model VI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | CI | p | HR | CI | p | HR | CI | p | HR | CI | p | HR | CI | p | HR | CI | p | |
| Autumn | 1.06 | 0.76–1.49 | 0.714 | 1.06 | 0.76–1.48 | 0.725 | 1.06 | 0.76–1.48 | 0.739 | 1.05 | 0.76–1.47 | 0.755 | 1.05 | 0.75–1.47 | 0.765 | 1.12 | 0.63–1.98 | 0.710 |
| Spring | 1.16 | 0.85–1.59 | 0.347 | 1.15 | 0.84–1.57 | 0.372 | 1.16 | 0.85–1.58 | 0.363 | 1.15 | 0.84–1.58 | 0.367 | 1.15 | 0.84–1.57 | 0.376 | 1.29 | 0.76–2.20 | 0.346 |
| Winter | 1.30 | 0.95–1.77 | 0.099 | 1.29 | 0.94–1.75 | 0.111 | 1.29 | 0.95–1.76 | 0.107 | 1.28 | 0.94–1.75 | 0.113 | 1.27 | 0.93–1.73 | 0.128 | 1.07 | 0.62–1.86 | 0.802 |
| Sami | 1.24 | 0.99–1.55 | 0.061 | 1.24 | 0.99–1.55 | 0.059 | 1.23 | 0.98–1.54 | 0.074 | 1.23 | 0.98–1.53 | 0.075 | 0.80 | 0.46–1.39 | 0.435 | |||
| Male | 1.36 | 1.09–1.69 | 0.006 | 1.35 | 1.09–1.68 | 0.007 | 1.35 | 1.08–1.68 | 0.007 | 1.67 | 1.02–2.73 | 0.042 | ||||||
| Gällivare | 1.24 | 0.99–1.56 | 0.064 | 1.24 | 0.99–1.55 | 0.064 | 1.24 | 0.99–1.55 | 0.064 | |||||||||
| Age: 2nd week | 0.69 | 0.52–0.91 | 0.008 | 0.69 | 0.52–0.91 | 0.008 | ||||||||||||
| Age: 3rd week+ | 0.51 | 0.40–0.65 | <0.001 | 0.51 | 0.40–0.65 | <0.001 | ||||||||||||
| Autumn*Sami | 1.45 | 0.69–3.03 | 0.326 | |||||||||||||||
| Spring*Sami | 1.42 | 0.71–2.83 | 0.324 | |||||||||||||||
| Winter*Sami | 2.18 | 1.11–4.29 | 0.024 | |||||||||||||||
| Autumn*Male | 0.76 | 0.38–1.51 | 0.434 | |||||||||||||||
| Spring*Male | 0.70 | 0.37–1.32 | 0.265 | |||||||||||||||
| Winter*Male | 0.84 | 0.45–1.59 | 0.597 | |||||||||||||||
| Observations | 218897 | 218897 | 218897 | 218897 | 218897 | 218897 | ||||||||||||
| R2 Nagelkerke | 0.001 | 0.001 | 0.003 | 0.004 | 0.009 | 0.011 | ||||||||||||
| Model I | Model II | Model III | Model IV | Model V | Model VI | Model VII | Model VIII | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | HR | CI | p | HR | CI | p | HR | CI | p | HR | CI | p | HR | CI | p | HR | CI | p | HR | CI | p | HR | CI | p |
| Temp at birth | 0.99 | 0.97–1.01 | 0.163 | 0.99 | 0.97–1.01 | 0.373 | 0.99 | 0.97–1.01 | 0.309 | 0.99 | 0.97–1.01 | 0.321 | 0.99 | 0.97–1.01 | 0.341 | 0.99 | 0.97–1.01 | 0.347 | 0.99 | 0.97–1.01 | 0.408 | 1.00 | 0.97–1.03 | 0.957 |
| Temp after birth | 0.99 | 0.97–1.00 | 0.132 | 0.98 | 0.96–1.00 | 0.107 | 0.98 | 0.96–1.00 | 0.107 | 0.98 | 0.96–1.00 | 0.108 | 0.98 | 0.96–1.00 | 0.108 | 0.98 | 0.96–1.00 | 0.120 | 1.00 | 0.96–1.03 | 0.922 | |||
| February | 1.24 | 0.78–1.98 | 0.365 | 1.25 | 0.78–2.00 | 0.347 | 1.26 | 0.79–2.00 | 0.340 | 1.25 | 0.79–2.00 | 0.344 | 1.26 | 0.79–2.01 | 0.329 | 1.26 | 0.79–2.02 | 0.325 | ||||||
| March | 1.15 | 0.69–1.91 | 0.587 | 1.16 | 0.70–1.93 | 0.561 | 1.16 | 0.70–1.92 | 0.568 | 1.16 | 0.70–1.92 | 0.569 | 1.17 | 0.70–1.94 | 0.548 | 1.18 | 0.71–1.97 | 0.520 | ||||||
| November | 1.32 | 0.77–2.26 | 0.316 | 1.33 | 0.77–2.27 | 0.304 | 1.32 | 0.77–2.26 | 0.309 | 1.32 | 0.77–2.26 | 0.312 | 1.31 | 0.77–2.24 | 0.318 | 1.30 | 0.76–2.22 | 0.338 | ||||||
| December | 1.03 | 0.62–1.71 | 0.917 | 1.03 | 0.61–1.71 | 0.922 | 1.03 | 0.62–1.71 | 0.918 | 1.03 | 0.61–1.71 | 0.922 | 1.02 | 0.61–1.70 | 0.946 | 1.01 | 0.61–1.69 | 0.959 | ||||||
| Sami | 1.47 | 1.07–2.02 | 0.016 | 1.48 | 1.08–2.03 | 0.015 | 1.47 | 1.07–2.02 | 0.017 | 1.46 | 1.07–2.01 | 0.018 | 1.21 | 0.63–2.32 | 0.569 | |||||||||
| Male | 1.34 | 0.98–1.83 | 0.070 | 1.33 | 0.97–1.83 | 0.073 | 1.33 | 0.97–1.83 | 0.072 | 0.96 | 0.51–1.83 | 0.910 | ||||||||||||
| Gällivare | 1.11 | 0.80–1.53 | 0.531 | 1.11 | 0.80–1.53 | 0.530 | 1.12 | 0.81–1.55 | 0.498 | |||||||||||||||
| Age: 2nd week | 0.61 | 0.41–0.92 | 0.019 | 0.61 | 0.41–0.92 | 0.018 | ||||||||||||||||||
| Age: 3rd week+ | 0.51 | 0.36–0.73 | <0.001 | 0.51 | 0.35–0.72 | <0.001 | ||||||||||||||||||
| Temp at birth*Sami | 0.96 | 0.93–1.00 | 0.036 | |||||||||||||||||||||
| Temp after birth*Sami | 1.03 | 0.99–1.07 | 0.193 | |||||||||||||||||||||
| Temp at birth*Male | 1.02 | 0.98–1.06 | 0.350 | |||||||||||||||||||||
| Temp after birth*Male | 0.96 | 0.92–1.00 | 0.028 | |||||||||||||||||||||
| Observations | 93368 | 93368 | 93368 | 93368 | 93368 | 93368 | 93368 | 93368 | ||||||||||||||||
| R2 Nagelkerke | 0.001 | 0.002 | 0.002 | 0.005 | 0.006 | 0.007 | 0.012 | 0.017 | ||||||||||||||||
References
- Sundin, J. Svenska Folkets Hälsa I Historiskt Perspektiv; Sundin, J., Statens, F., Eds.; Statens folkhälsoinstitut: Stockholm, Sweden, 2005. [Google Scholar]
- Bengtsson, T. The vulnerable child. Economic insecurity and child mortality in pre-industrial Sweden: A case study of Vastanfors, 1757–1850. Eur. J. Popul. 1999, 15, 117–151. [Google Scholar] [CrossRef]
- Edvinsson, S. Den Osunda Staden Sociala Skillnader I Dödlighet I 1800-Talets Sundsvall; Almqvist & Wiksell International: Stockholm, Sweden, 1992. [Google Scholar]
- Sköld, P.; Axelsson, P.; Karlsson, L.; Smith, L. Infant mortality of Sami and settlers in Northern Sweden: The era of colonization 1750–1900. Glob. Health Action 2011, 4. [Google Scholar] [CrossRef]
- Edvinsson, S.; Brändström, A.; Rogers, J. Regional variations in infant mortality in Sweden during the first half of the 19th century. In Nordic Demography in History and Present-Day Society; Lars-Göran, T., Peter, S., Eds.; Demografiska databasen; Umeå Universitet: Umeå, Sweden, 2001; pp. 145–164. [Google Scholar]
- Karlsson, L.; Lundevaller, E.; Schumann, B. The association between cold extremes and neonatal mortality in Swedish Sápmi from 1800 to 1895. Glob. Health Action 2019, 12, 1623609. [Google Scholar] [CrossRef]
- Karlsson, L.; Lundevaller, E.H.; Schumann, B. Season of birth, stillbirths, and neonatal mortality in Sweden: The Sami and non-Sami population, 1800–1899. Int. J. Circumpolar Health 2019, 78, 1629784. [Google Scholar] [CrossRef]
- Schumann, B.; Haggstrom Lundevaller, E.; Karlsson, L. Weather extremes and perinatal mortality - Seasonal and ethnic differences in northern Sweden, 1800–1895. PLoS ONE 2019, 14, e0223538. [Google Scholar] [CrossRef]
- Coory, M. Can a mortality excess in remote areas of Australia be explained by Indigenous status? A case study using neonatal mortality in Queensland. Aust. N. Z. J. Public Health 2003, 27, 425–427. [Google Scholar] [CrossRef]
- Luo, Z.-C.; Wilkins, R.; Heaman, M.; Smylie, J.; Martens, P.J.; McHugh, N.G.L.; Labranche, E.; Simonet, F.; Wassimi, S.; Minich, K.; et al. Birth outcomes and infant mortality among First Nations Inuit, and non-Indigenous women by northern versus southern residence, Quebec. J. Epidemiol. Commun. H 2012, 66, 328–333. [Google Scholar] [CrossRef]
- Smylie, J.; Crengle, S.; Freemantle, J.; Taualii, M. Indigenous Birth Outcomes in Australia, Canada, New Zealand and the United States—An Overview. Open Womens Health J. 2010, 7–17. [Google Scholar] [CrossRef]
- Brändström, A. Från förebild till motbild. Spädbarnsvård och spädbarnsdödlighet i Jokkmokk. In Älvdal I Norr Människor Och Resurser I Luledalen 1300–1800; Åkerman, S., Lundholm, K., Eds.; Almqvist & Wiksell: Stockholm, Sweden, 1990; pp. 307–351. [Google Scholar]
- Karlsson, L. Indigenous Infant Mortality by Age and Season of Birth, 1800–1899: Did Season of Birth Affect Children’s Chances for Survival? Int. J. Environ. Res. Public Health 2017, 15, 18. [Google Scholar] [CrossRef]
- Naeye, R.L.; Burt, L.S.; Wright, D.L.; Blanc, W.A.; Tatter, D. Neonatal mortality, the male disadvantage. Pediatrics 1971, 48, 902. [Google Scholar]
- Yerushalmy, J. Neonatal mortality by order of birth and age of parents. Am. J. Hyg. 1938, 28, 244–270. [Google Scholar] [PubMed]
- Zhao, D.; Zou, L.; Lei, X.; Zhang, Y. Gender Differences in Infant Mortality and Neonatal Morbidity in Mixed-Gender Twins. Sci. Rep. 2017, 7, 8736. [Google Scholar] [CrossRef] [PubMed]
- Yaya, S.; Diarra, S.; Mabeu, M.C.; Pongou, R. The sex gap in neonatal mortality and the AIDS epidemic in sub-Saharan Africa. BMJ Glob. Health 2018, 3, e000940. [Google Scholar] [CrossRef]
- Mizuno, R. The male/female ratio of fetal deaths and births in Japan. Lancet 2000, 356, 738–739. [Google Scholar] [CrossRef]
- Ekamper, P.; van Poppel, F. Infant mortality in mid-19th century Amsterdam: Religion, social class, and space. Popul. Space Place 2019, 25. [Google Scholar] [CrossRef]
- Scalone, F.; Samoggia, A. Neonatal mortality, cold weather, and socioeconomic status in two northern Italian rural parishes, 1820–1900. Demogr. Res. 2018, 39, 525–560. [Google Scholar] [CrossRef]
- Sköld, P.; Axelsson, P. The northern population development; Colonization and mortality in Swedish Sapmi, 1776–1895. Int. J. Circumpolar Health 2008, 67, 27–42. [Google Scholar] [CrossRef]
- Axelsson, P.; Sköld, P. Indigenous Populations and Vulnerability. Characterizing Vulnerability in a Sami Context. Ann. Démographie Hist. 2006, 111, 115–132. [Google Scholar] [CrossRef]
- Nordin, G.; Sköld, P. True or false? Nineteenth-century Sápmi fertility in qualitative vs. demographic sources. Hist. Fam. 2012, 17, 157–177. [Google Scholar] [CrossRef]
- Breschi, M.; Derosas, R.; Manfredini, M. Mortality and Environment in Three Emilian, Tuscan, and Venetian Communities, 1800–1883. In Life under Pressure: Morality and Living Standards in Europe and Asia, 1700–1900; Bengtsson, T., Campbell, C., Lee, J., Eds.; MIT Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Breschi, M.; Livi-Bacci, M. Month of Birth as a Factor in Children’s Survival. In Infant and Child Mortality in the Past Oxford; Desjardins, B., Ed.; Clarendon Press: Oxford, UK, 1997. [Google Scholar]
- Dalla-Zuanna, G.; Rosina, A. An Analysis of Extremely High Nineteenth-Century Winter Neonatal Mortality in a Local Context of Northeastern Italy. Eur. J. Popul. 2011, 27, 33–55. [Google Scholar] [CrossRef]
- Derosas, R. The joint effect of maternal malnutrition and cold weather on neonatal mortality in nineteenth-century Venice: An assessment of the hypothermia hypothesis. Pop. Stud. J. Demog. 2009, 63, 233–251. [Google Scholar] [CrossRef]
- Reher, D.S.; Gimeno, A.S. Marked from the outset: Season of birth and health during early life in Spain during the demographic transition. Contin. Chang. 2006, 21, 107–129. [Google Scholar] [CrossRef]
- Breschi, M.; Pozzi, L. The Determinants of Infant And Child Mortality in Past European Populations; Udine Forum: Udine, Italy, 2004. [Google Scholar]
- Astolfi, P.; Zonta, L.A. Risks of preterm delivery and association with maternal age, birth order, and fetal gender. Hum. Reprod. 1999, 14, 2891–2894. [Google Scholar] [CrossRef]
- Ingemarsson, I. Gender aspects of preterm birth. BJOG 2003, 110, 34–38. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Aly, H. Would fetal sex affect the odds for premature delivery? Pediatrics 2018, 142, 199. [Google Scholar] [CrossRef]
- Miller, S.S.; Lee, H.C.; Gould, J.B. Hypothermia in very low birth weight infants: Distribution, risk factors and outcomes. J. Perinatol. 2011, 31, S49–S56. [Google Scholar] [CrossRef]
- Khoury, M.J.; Marks, J.S.; McCarthy, B.J.; Zaro, S.M. Factors affecting the sex differential in neonatal mortality: The role of respiratory distress syndrome. Am. J. Obs. Gynecol. 1985, 151, 777–782. [Google Scholar] [CrossRef]
- Babalola, O.; Razzaque, A.; Bishai, D. Temperature extremes and infant mortality in Bangladesh: Hotter months, lower mortality. PLoS ONE 2018, 13, e0189252. [Google Scholar] [CrossRef]
- DDB. Demographic Data Base; Umeå Univeristy: Umeå, Sweden, 2019. [Google Scholar]
- Westberg, A.; Engberg, E.; Edvinsson, S. A Unique Source for Innovative Longitudinal Research: The POPLINK Database. Hist. Life Course Stud. 2016, 3, 20–31. [Google Scholar]
- Woods, R. Death before Birth: Fetal Health and Mortality in Historical Perspective; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Nordin, G. Äktenskap i Sápmi: Giftermålsmönster Och Etnisk Komplexitet I Kolonisationens Tidevarv, 1722–1895; Institutionen för Idé-Och Samhällsstudier, Umeå Universitet: Umeå, Sweden, 2009. [Google Scholar]
- Karlsson, L. Indigenous life expectancy in Sweden 1850–1899: Towards a long and healthy life? Demogr. Res. 2013, 28, 433–456. [Google Scholar] [CrossRef]
- Brännlund, I. Diverse Sami Livelihoods: A Comparative Study of Livelihoods in Mountain-Reindeer Husbandry Communities in Swedish Sápmi 1860–1920. J. North. Stud. 2018, 12, 37–62. [Google Scholar]
- Swedish Meteorological and Hydrological Institute. 2019. Available online: www.smhi.se (accessed on 25 March 2019).
- Abbott, R.D. Logistic regression in survival analysis. Am. J. Epidemiol. 1985, 121, 465–471. [Google Scholar] [CrossRef]
- Oris, M.; Derosas, R.; Breschi, M. Infant and Child Mortality Life under Pressure: Mortality and Living Standards in Europe and Asia, 1700–1900; MIT Press: Cambridge, MA, USA, 2004; pp. 359–398. [Google Scholar]
- Düben, G.V. Om Lappland Och Lapparne, Företrädesvis De Svenske: Ethnografiska Studier; Norstedt: Stockholm, Sweden, 1873. [Google Scholar]
- Serning, I. Lappbarnen, Deras Vård och Uppfostran i Spädbarnsåldern och Lekåldern. 1949 s. 55–109; Norrbottens-Kurirens Tryckeri: Luleå, Sweden, 1950. [Google Scholar]
- Thorvaldsen, G. Was there a European breastfeeding pattern? Hist. Fam. 2008, 13, 283–295. [Google Scholar] [CrossRef]
- Brändström, A. Infant Mortality in Sweden 1750–1950: Past and Present Research into Its Decline; UNICEF: Florence, Italy, 1993. [Google Scholar]
- Ellmin, J. Annual Report. In District Physician of Jämtland; National Archives: Stockholm, Sweden, 1851. [Google Scholar]
- Kohler, L. Infant-Mortality—The Swedish Experience. Annu. Rev. Publ. Health 1991, 12, 177–193. [Google Scholar] [CrossRef]
- Wallgren, A. The neonatal mortality in Sweden from a pedlatric point of view. Acta Paediatr. 1942, 28, 372–386. [Google Scholar] [CrossRef]
- Zetterström, R.; Eriksson, M. Hälsa och Social Klass: Spädbarnsdödlighet och Graviditetsutfall. Soc. Med. Tidskr. 1987, 64, 33–36. [Google Scholar]
- Strand, L.B.; Barnett, A.G.; Tong, S. The influence of season and ambient temperature on birth outcomes: A review of the epidemiological literature. Environ. Res. 2011, 111, 451–462. [Google Scholar] [CrossRef]
| Month | Min | Mean | Max | SD |
|---|---|---|---|---|
| January | −39.3 | −14.8 | 4.5 | 8.6 |
| February | −40.0 | −13.3 | 4.7 | 8.4 |
| March | −27.0 | −7.8 | 6.7 | 6.2 |
| April | −13.2 | −0.5 | 10.4 | 4.1 |
| May | −5.9 | 5.5 | 20.5 | 4.4 |
| June | 0.0 | 12.4 | 26.2 | 4.8 |
| July | 2.3 | 15.0 | 25.7 | 3.7 |
| August | 2.4 | 12.3 | 25.7 | 3.2 |
| September | −3.7 | 6.6 | 15.7 | 3.6 |
| October | −23.5 | −1.3 | 12.7 | 5.7 |
| November | −31.2 | −8.9 | 7.4 | 7.5 |
| December | −39.7 | −13.5 | 5.3 | 8.6 |
| Total (%) | Neonatal Mortality, January–December | Neonatal Mortality in Winter-Born (November–March) |
|---|---|---|
| Total number of live births | 8024 | 4007 |
| Total number of neonatal deaths | 330 (4.1%) | 159 (4.0%) |
| Neonatal deaths by: | ||
| Ethnicity | ||
| Sami | 121 (36.7%) | 66 (41.5%) |
| Non-Sami | 209 (63.3%) | 93 (58.5%) |
| Gender | ||
| Male | 190 (57.6%) | 91 (57.2%) |
| Female | 140 (42.4%) | 68 (42.8%) |
| Parish | ||
| Jokkmokk | 115 (34.8%) | 58 (36.5%) |
| Gällivare | 215 (65.2%) | 101 (63.5%) |
| Age at death | ||
| First week | 124 (37.6%) | 62 (39%) |
| Second week | 84 (25.5%) | 37 (23.3%) |
| Third week+ | 122 (37.0%) | 60 (37.7%) |
| Season | ||
| Winter (Jan–Feb) | 98 (29.7%) | 98 (61.6%) |
| Spring (March–May) | 94 (28.5%) | 33 (20.8%) |
| Summer (June–Aug) | 68 (20.6%) | |
| Autumn (Sep–Nov) | 70 (21.2%) | 28 (17.6%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlsson, L.; Lundevaller, E.H.; Schumann, B. Neonatal Mortality and Temperature in Two Northern Swedish Rural Parishes, 1860–1899—The Significance of Ethnicity and Gender. Int. J. Environ. Res. Public Health 2020, 17, 1216. https://doi.org/10.3390/ijerph17041216
Karlsson L, Lundevaller EH, Schumann B. Neonatal Mortality and Temperature in Two Northern Swedish Rural Parishes, 1860–1899—The Significance of Ethnicity and Gender. International Journal of Environmental Research and Public Health. 2020; 17(4):1216. https://doi.org/10.3390/ijerph17041216
Chicago/Turabian StyleKarlsson, Lena, Erling H. Lundevaller, and Barbara Schumann. 2020. "Neonatal Mortality and Temperature in Two Northern Swedish Rural Parishes, 1860–1899—The Significance of Ethnicity and Gender" International Journal of Environmental Research and Public Health 17, no. 4: 1216. https://doi.org/10.3390/ijerph17041216
APA StyleKarlsson, L., Lundevaller, E. H., & Schumann, B. (2020). Neonatal Mortality and Temperature in Two Northern Swedish Rural Parishes, 1860–1899—The Significance of Ethnicity and Gender. International Journal of Environmental Research and Public Health, 17(4), 1216. https://doi.org/10.3390/ijerph17041216




