Seasonal Variation in Aflatoxin Levels in Edible Seeds, Estimation of Its Dietary Intake and Vitamin E Levels in Southern Areas of Punjab, Pakistan
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemicals and Reagents
2.3. Aflatoxins Extraction
2.4. Extraction of Oil
2.5. HPLC Conditions
2.6. Dietary Intake Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. HPLC Method Validation
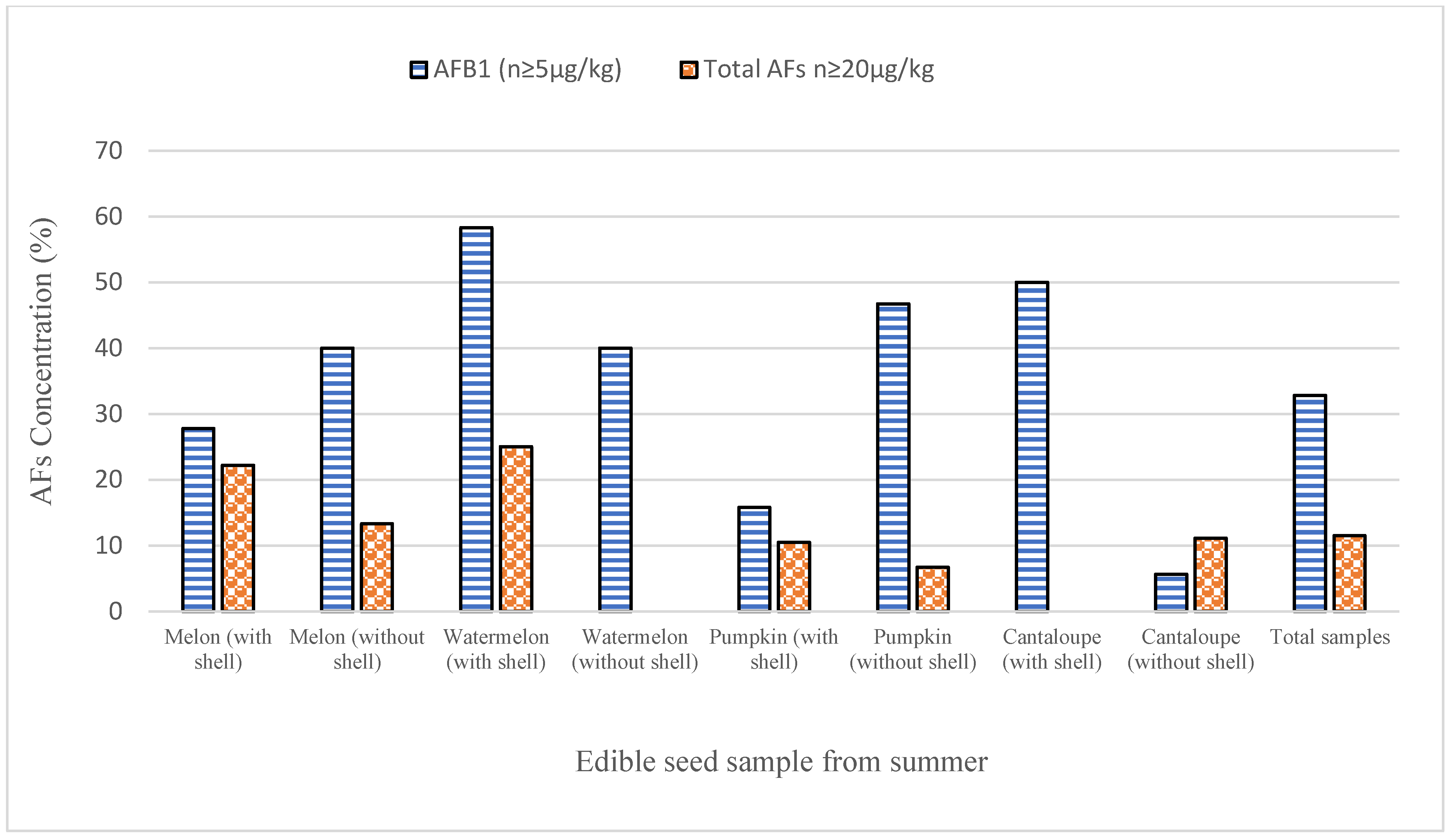
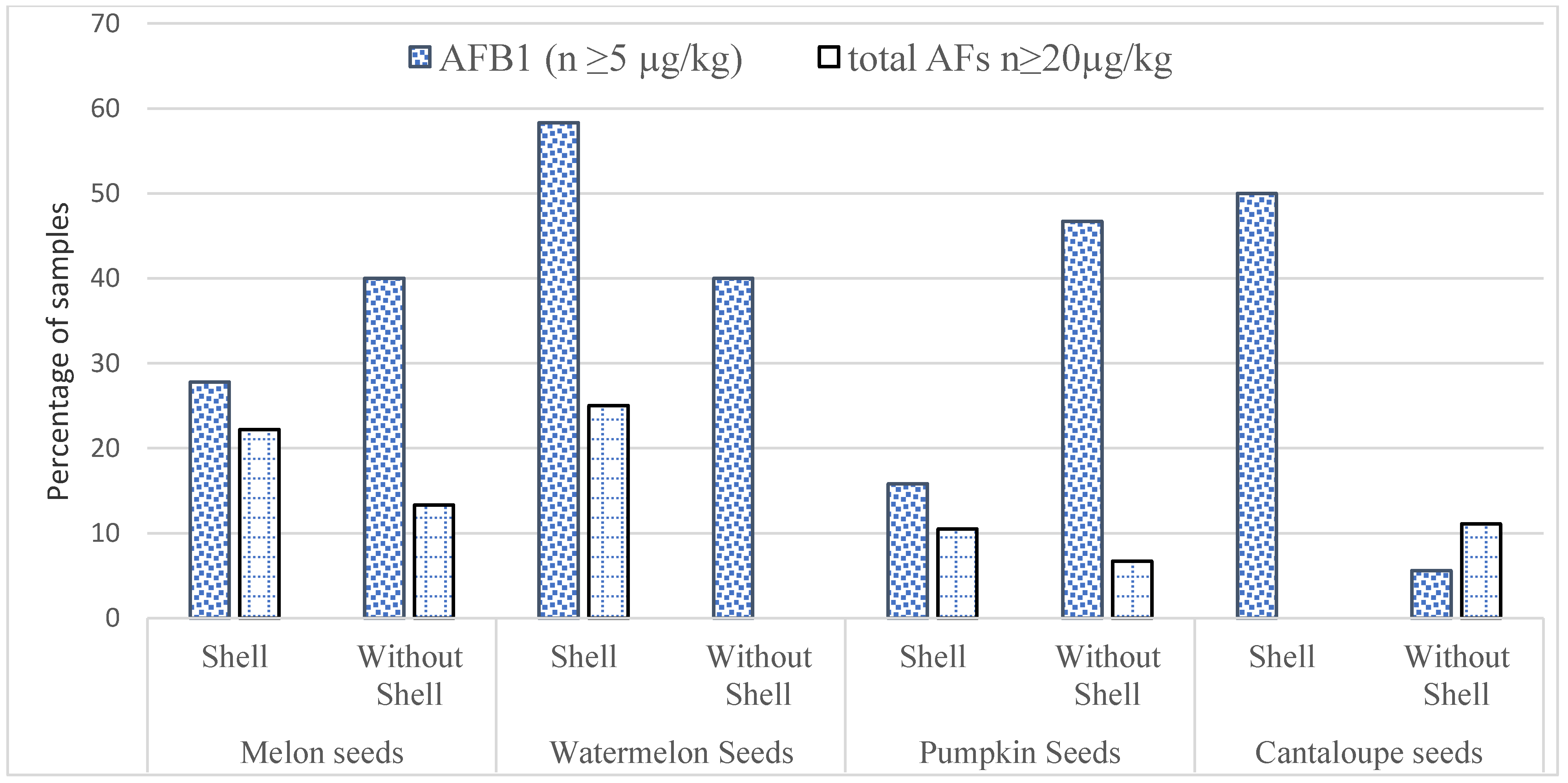
3.2. Occurrence of AFs in Edible Seeds
3.3. Dietary Intake Estimation
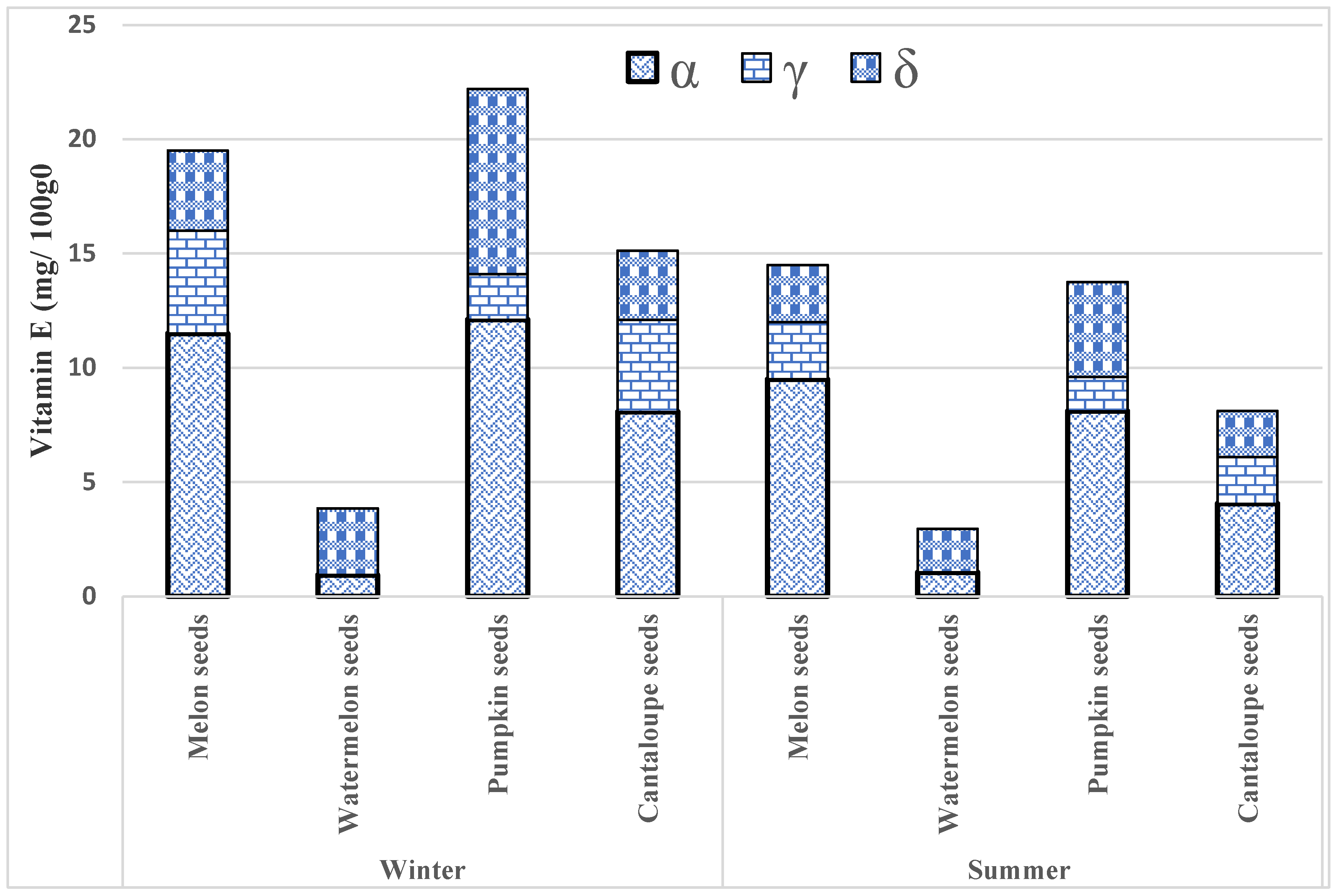
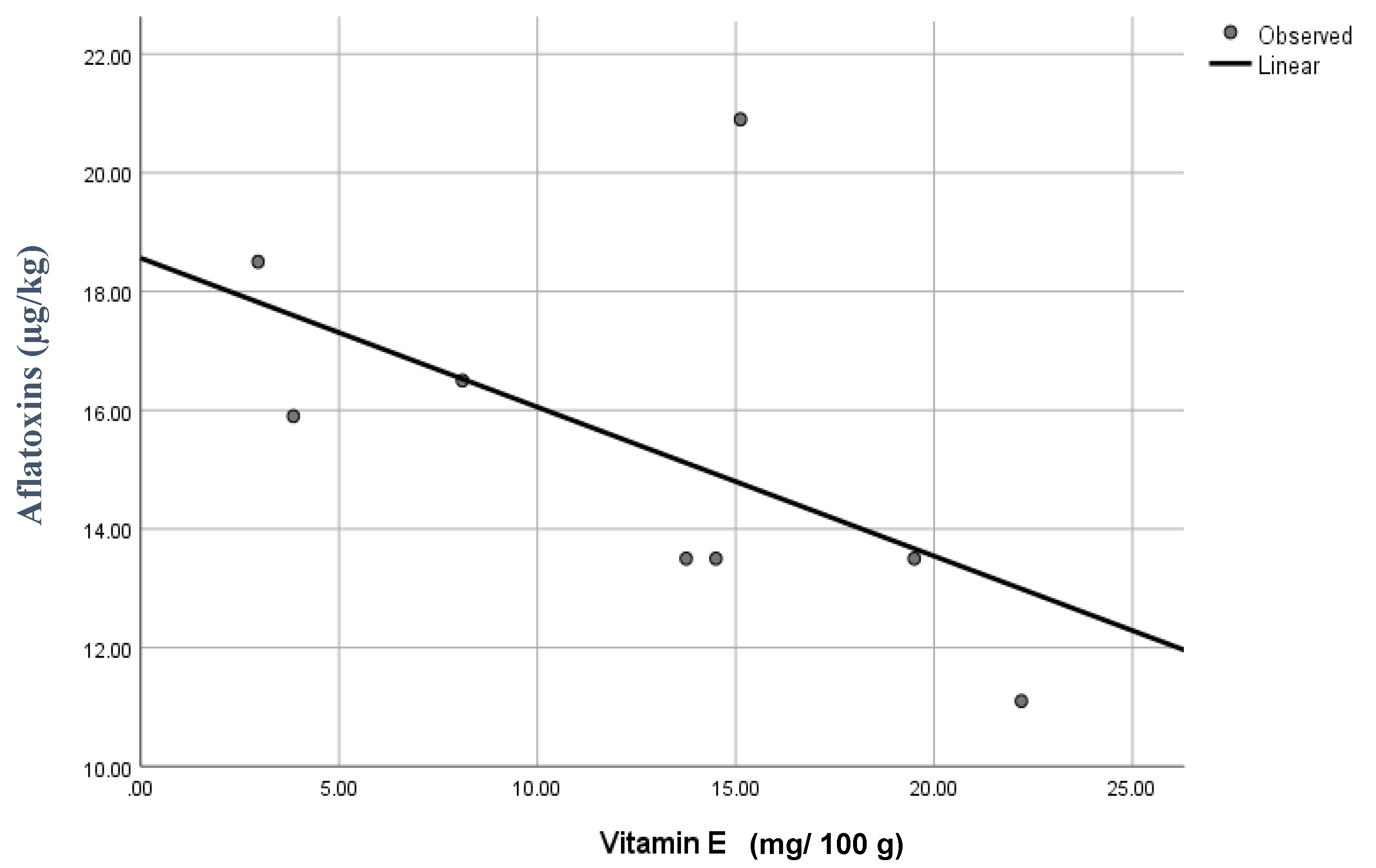
3.4. The Vitamin E Levels in Edible Seeds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugizaki, C.S.A.; Naves, M.M.V. Potential prebiotic properties of nuts and edible seeds and their relationship to obesity. Nutrients 2018, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Mora-Escobedo, R.; Berrios, J.D.J.; Gutiérrez-Lopez, G.F. Seeds as functional foods and nutraceuticals. New Front. Food Sci. 2014, 91, 220–225. [Google Scholar]
- Patel, S.; Rauf, A. Edible seeds from Cucurbitaceae family as potential functional foods: Immense promises, few concerns. Biomed. Pharmacother. 2017, 91, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Grancieri, M.; Martino, H.S.D.; De Mejia, E.G. Chia seed (Salvia hispanica L.) as a source of proteins and bioactive peptides with health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 480–499. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; García-Campaña, A.M. Determination of sulfonylurea pesticide residues in edible seeds used as nutraceuticals by QuEChERS in combination with ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1617, 460831. [Google Scholar] [CrossRef] [PubMed]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Set, E.; Erkmen, O. The aflatoxin contamination of ground red pepper and pistachio nuts sold in Turkey. Food Chem. Toxicol. 2010, 48, 2532–2537. [Google Scholar] [CrossRef]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in food and feed: Present status and future concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Abbas, H.K.; Shier, W.T.; Plasencia, J.; Weaver, M.; Bellaloui, N.; Kotowicz, J.K.; Butler, A.M.; Accinelli, C.; De La Torre-Hernandez, M.E.; Zablotowicz, R.M. Mycotoxin contamination in corn smut (Ustilago maydis) galls in the field and in the commercial food products. Food Control. 2017, 71, 57–63. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef]
- Alberts, J.; Lilly, M.; Rheeder, J.; Burger, H.-M.; Shephard, G.; Gelderblom, W. Technological and community-based methods to reduce mycotoxin exposure. Food Control. 2017, 73, 101–109. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; HajšLová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Pleadin, J.; Vahčić, N.; Perši, N.; Ševelj, D.; Markov, K.; Frece, J. Fusarium mycotoxins’ occurrence in cereals harvested from Croatian fields. Food Control. 2013, 32, 49–54. [Google Scholar] [CrossRef]
- Zinedine, A.; Fernández-Franzón, M.; Mañes, J.; Manyes, L. Multi-mycotoxin contamination of couscous semolina commercialized in Morocco. Food Chem. 2017, 214, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.G., Jr.; Potter, M. Foodborne Infections and Intoxications; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Asghar, M.A.; Ahmed, A.; Zahir, E.; Iqbal, J.; Walker, G. Incidence of aflatoxins contamination in dry fruits and edible nuts collected from Pakistan. Food Control. 2017, 78, 169–175. [Google Scholar] [CrossRef]
- Ismail, A.; Gonçalves, B.L.; De Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; Oliveira, C.A. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef]
- De Roma, A.; Rossini, C.; Ritieni, A.; Gallo, P.; Esposito, M. A survey on the Aflatoxin M1 occurrence and seasonal variation in buffalo and cow milk from Southern Italy. Food Control. 2017, 81, 30–33. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Mustafa, H.G.; Asi, M.R.; Selamat, J. Variation in vitamin E level and aflatoxins contamination in different rice varieties. J. Cereal Sci. 2014, 60, 352–355. [Google Scholar] [CrossRef]
- Ruadrew, S.; Craft, J.; Aidoo, K. Occurrence of toxigenic Aspergillus spp. and aflatoxins in selected food commodities of Asian origin sourced in the West of Scotland. Food Chem. Toxicol. 2013, 55, 653–658. [Google Scholar] [CrossRef]
- Blankson, G.; Mill-Robertson, F. Aflatoxin contamination and exposure in processed cereal-based complementary foods for infants and young children in greater Accra, Ghana. Food Control. 2016, 64, 212–217. [Google Scholar] [CrossRef]
- World Health Organization & International Agency for Research on Cancer. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; IARC Pubication: Lyon, France, 1993. [Google Scholar]
- UN Food and agriculture organization of the United Nations (FAO). Yearbook of the United Nations 2004; United Nations Publications: New York, NY, USA, 2004; Volume 56, pp. 1470–1471. [Google Scholar]
- European Commission (EC). Commission Regulation (EC) No 165/2010 of 26 February 2010, amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Union 2010, 50, 8–12. [Google Scholar]
- Nesci, A.; Gsponer, N.; Etcheverry, M. Natural maize phenolic acids for control of aflatoxigenic fungi on maize. J. Food Sci. 2007, 72, M180–M185. [Google Scholar] [CrossRef] [PubMed]
- Samapundo, S.; De Meulenaer, B.; Osei-Nimoh, D.; Lamboni, Y.; Debevere, J.; Devlieghere, F. Can phenolic compounds be used for the protection of corn from fungal invasion and mycotoxin contamination during storage? Food Microbiol. 2007, 24, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Sýs, M.; Švecová, B.; Švancara, I.; Metelka, R. Determination of vitamin E in margarines and edible oils using square wave anodic stripping voltammetry with a glassy carbon paste electrode. Food Chem. 2017, 229, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z.; Mumtaz, A.; Mahmood, Z.; Waqas, M.; Ghaffar, A.; Ismail, A.; Pervaiz, W. Assessment of aflatoxins and ochratoxin a in chili sauce samples and estimation of dietary intake. Food Control 2021, 121, 107621. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Mehmood, Z.; Asi, M.R.; Shahid, M.; Sehar, M.; Malik, N. Co-occurrence of aflatoxins and ochratoxin A in nuts, dry fruits, and nuty products. J. Food Saf. 2018, 38, e12462. [Google Scholar] [CrossRef]
- Masood, M.; Iqbal, S.Z.; Asi, M.R.; Malik, N. Natural occurrence of aflatoxins in dry fruits and edible nuts. Food Control. 2015, 55, 62–65. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Zuber, M.; Akram, N.; Batool, N. Aflatoxins contamination in peanut and peanut products commercially available in retail markets of Punjab, Pakistan. Food Control. 2013, 32, 83–86. [Google Scholar] [CrossRef]
- Bankole, S.A.; Adenusi, A.A.; Lawal, O.; Adesanya, O. Occurrence of aflatoxin B1 in food products derivable from ‘egusi’ melon seeds consumed in southwestern Nigeria. Food Control. 2010, 21, 974–976. [Google Scholar] [CrossRef]
- Gliszczynska-Swigło, A.; Sikorska, E. Simple reversed-phase liquid chromatography method for determination of tocopherols in edible plant oils. J. Chromatogr. A. 2004, 1048, 195–198. [Google Scholar] [CrossRef]
- McGuire, S. WHO, World Food Programme, and International Fund for Agricultural Development. 2012; The State of Food Insecurity in the World 2012: Economic Growth is Necessary but not Sufficient to Accelerate the Reduction of Hunger and Malnutrition; FAO: Rome, Italy, 2014. [Google Scholar]
- Huang, B.; Han, Z.; Cai, Z.; Wu, Y.; Ren, Y. Simultaneous determination of aflatoxins B1, B2, G1, G2, M1 and M2 in peanuts and their derivative products by ultra-high-performance liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2010, 662, 62–68. [Google Scholar] [CrossRef]
- Kabak, B. Aflatoxins in hazelnuts and dried figs: Occurrence and exposure assessment. Food Chem. 2016, 211, 8–16. [Google Scholar] [CrossRef]
- Bankole, S.A.; Ogunsanwo, B.; Osho, A.; Adewuyi, G. Fungal contamination and aflatoxin B1 of ‘egusi’ melon seeds in Nigeria. Food Control. 2006, 17, 814–818. [Google Scholar] [CrossRef]
- Juan, C.; Zinedine, A.; Moltó, J.; Idrissi, L.; Mañes, J. Aflatoxins levels in dried fruits and nuts from Rabat-Salé area, Morocco. Food Control. 2008, 19, 849–853. [Google Scholar] [CrossRef]
- Pakistan Meteorological Department. Pakistan Meteorological Department Pakistan Government. 2016. Available online: https://www.pmd.gov.pk/en/ (accessed on 5 November 2020).
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009. [Google Scholar]
- Heshmati, A.; Zohrevand, T.; Khaneghah, A.M.; Nejad, A.S.M.; Sant’Ana, A.S. Co-occurrence of aflatoxins and ochratoxin A in dried fruits in Iran: Dietary exposure risk assessment. Food Chem. Toxicol. 2017, 106, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Sugita-Konishi, Y.; Sato, T.; Saito, S.; Nakajima, M.; Tabata, S.; Tanaka, T.; Norizuki, H.; Itoh, Y.; Kai, S.; Sugiyama, K.; et al. Exposure to aflatoxins in Japan: Risk assessment for aflatoxin B1. Food Addit. Contam. Part. A 2010, 27, 365–372. [Google Scholar] [CrossRef]
- Da Silva, E.; Bracarense, A.P.F.; Oswald, I. Mycotoxins and oxidative stress: Where are we? World Mycotoxin J. 2018, 11, 113–134. [Google Scholar] [CrossRef]
- Mohajeri, M.; Behnam, B.; Cicero, A.F.G.; Sahebkar, A. Protective effects of curcumin against aflatoxicosis: A comprehensive review. J. Cell. Physiol. 2017, 233, 3552–3577. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Mehri, S.; Behbahani, F.S.; Hosseinzadeh, H. Protective effects of Vitis vinifera (grapes) and one of its biologically active constituents, resveratrol, against natural and chemical toxicities: A comprehensive review. Phytother. Res. 2018, 32, 2164–2190. [Google Scholar] [CrossRef]
- Hedayati, N.; Naeini, M.B.; Nezami, A.; Hosseinzadeh, H.; Wallace Hayes, A.; Hosseini, S.; Imenshahidi, M.; Karimi, G. Protective effect of lycopene against chemical and natural toxins: A review: Lycopene against chemical and natural toxins. BioFactors 2019, 45, 5–23. [Google Scholar] [CrossRef] [PubMed]

| Aflatoxins | Linearity μg/mL | LOD μg/kg | LOQ μg/kg | R2 | Precision (%RSD) | |
|---|---|---|---|---|---|---|
| Reproducibility | Repeatability | |||||
| AFB1 | 1–120 | 0.05 | 0.15 | 0.9981 | 17 | 15 |
| AFB2 | 0.5–25 | 0.08 | 0.24 | 0.9947 | 14 | 16 |
| AFG1 | 1–120 | 0.05 | 0.15 | 0.9972 | 12 | 17 |
| AFG2 | 0.5–25 | 0.08 | 0.24 | 0.9972 | 10 | 16 |
| α | 0.5–60 | 0.04 | 0.12 | 0.9986 | 11 | 19 |
| γ | 0.1–30 | 0.07 | 0.21 | 0.9883 | 16 | 18 |
| δ | 0.1–30 | 0.07 | 0.21 | 0.9891 | 14 | 18 |
| Seasons | Type | Melon Seeds | Watermelon Seeds | Pumpkin Seeds | Cantaloupe Seeds | Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shelled | Without Shell | Shelled | Without Shell | Shelled | Without Shell | Shelled | Without Shell | |||
| Winter Season | Total sample (n) | 60 | 65 | 42 | 32 | 75 | 60 | 40 | 40 | 414 |
| Positive Sample n (%) | 23 (38.33) | 20 (30.76) | 18 (42.85) | 20 (62.5) | 23 (30.66) | 28 (46.66) | 23 (57.5) | 25 (62.5) | 180 (43.4) | |
| AFB1 (μg/kg) ± SD | 10.5 ± 2.10 | 12.6 ± 2.50 | 8.90 ±2.80 | 16.5 ± 2.45 | 12.9 ± 2.60 | 12.6 ± 3.40 | 7.9 ± 2.60 | 11.8 ± 3.20 | ||
| Total AFs (μg/kg) ± SD | 13.5 ± 3.40 * | 15.9 ± 3.60 * | 11.1 ± 2.10 ** | 20.9 ± 3.10 ** | 13.5 ± 2.90 ** | 18.5 ± 2.90 ** | 13.5 ± 3.10 * | 16.5 ± 3.50 * | ||
| Range (μg/kg) | 0.05–25.40 | 0.05–35.5 | 0.05–20.5 | 0.05–35.5 | 0.05–28.5 | 0.05–38.9 | 0.05–50.5 | |||
| Summer Season | Total sample (n) | 50 | 60 | 40 | 40 | 60 | 50 | 30 | 35 | 365 |
| Positive Sample (%) | 18 (36) | 15 (25) | 12 (30) | 15 (37.5) | 19 (31.7) | 15 (30) | 10 (33.3) | 18 (51.4) | 122 (33.4) | |
| AFB1 (μg/kg) ± SD | 8.20 ± 2.50 | 10.9 ± 2.50 | 6.70 ±1.90 | 10.1 ± 1.90 | 9.8 ± 2.50 | 14.4 ± 1.90 | 6.3 ± 1.90 | 10.2 ± 2.80 | ||
| Total AFs (μg/kg) ± SD | 11.80 ± 2.10 * | 13.2 ± 2.80 * | 8.40 ±1.95 * | 11.6 ± 1.80 * | 15.0 ± 2.50 * | 17.3 ± 1.50 * | 10.5 ± 1.95 * | 13.9 ± 2.10 * | ||
| Range (μg/kg) | 0.05–25.5 | 0.05–39.8 | 0.05–23.6 | 0.05–45.6 | 0.05–29.8 | 0.05–39.7 | 0.05–20.5 | 0.05–33.2 | ||
| Seasons | Type | Melon Seeds | Watermelon Seeds | Pumpkin Seeds | Cantaloupe Seeds | ||||
|---|---|---|---|---|---|---|---|---|---|
| Shelled | Without Shell | Shelled | Without Shell | Shelled | Without Shell | Shelled | Without Shell | ||
| Consumption g/day | 20 | 5 | 10 | 20 | 15 | 10 | 15 | 10 | |
| Winter | AFs mean level (μg/kg) | 13.5 | 14.9 | 11.1 | 13.5 | 20.9 | 18.5 | 13.5 | 14.5 |
| Dietary Intake (male) μg/kg/day | 3.78 | 1.04 | 1.55 | 3.78 | 2.59 | 4.38 | 2.83 | 2.03 | |
| Dietary Intake (female) μg/kg/day | 5.4 | 1.50 | 2.20 | 5.40 | 3.70 | 6.30 | 4.10 | 2.90 | |
| Summer | AFs mean level μg/kg | 11.8 | 12.20 | 8.40 | 11.60 | 17.30 | 15 | 10.50 | 11.90 |
| Dietary Intake (male) μg/kg/day | 1.65 | 0.85 | 0.59 | 1.62 | 1.21 | 2.10 | 1.47 | 0.83 | |
| Dietary Intake (female) μg/kg/day | 2.40 | 1.20 | 0.80 | 2.30 | 1.70 | 3.00 | 2.10 | 1.20 | |
| Seasons | Seeds Type | Samples | α Tocopherol (mg/100 g) | γ Tocopherol (mg/100 g) | δ Tocopherol (mg/100 g) | Total Tocopherol Content (mg/100 g) |
|---|---|---|---|---|---|---|
| Winter Season | Melon seeds | 125 | 11.5 ± 2.70 | 4.5 ± 2.30 | 3.5 ± 1.90 | 19.5 ± 4.90 * |
| Watermelon seeds | 74 | 0.93 ± 1.40 | 0.01 ± 4.15 | 2.91 ± 1.05 | 3.85 ± 3.60 N.S | |
| Pumpkin seeds | 135 | 12.1 ± 2.80 | 2.0 ± 2.50 | 8.1 ± 2.10 | 22.2 ± 7.70 * | |
| Cantaloupe seeds | 80 | 8.07 ± 1.30 | 4.03 ± 1.15 | 3.02 ± 1.10 | 15.12 ± 4.65 * | |
| Summer Season | Melon seeds | 110 | 9.5 ± 3.75 | 2.5 ± 2.80 | 2.5 ± 2.94 | 14.5 ± 5.50 * |
| Watermelon seeds | 80 | 1.04 ± 2.45 | 0.01 ± 1.15 | 1.91 ± 1.45 | 2.96 ± 5.60 NS | |
| Pumpkin seeds | 110 | 8.1 ± 3.85 | 1.50 ± 2.10 | 4.15 ± 3.15 | 13.75 ± 6.50 * | |
| Cantaloupe seeds | 65 | 4.05 ± 3.35 | 2.04 ± 3.10 | 2.02 ± 3.15 | 8.11 ± 3.40 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razis, A.F.A.; Shehzad, M.M.; Usman, S.; Ali, N.B.; Iqbal, S.Z.; Naheed, N.; Asi, M.R. Seasonal Variation in Aflatoxin Levels in Edible Seeds, Estimation of Its Dietary Intake and Vitamin E Levels in Southern Areas of Punjab, Pakistan. Int. J. Environ. Res. Public Health 2020, 17, 8964. https://doi.org/10.3390/ijerph17238964
Razis AFA, Shehzad MM, Usman S, Ali NB, Iqbal SZ, Naheed N, Asi MR. Seasonal Variation in Aflatoxin Levels in Edible Seeds, Estimation of Its Dietary Intake and Vitamin E Levels in Southern Areas of Punjab, Pakistan. International Journal of Environmental Research and Public Health. 2020; 17(23):8964. https://doi.org/10.3390/ijerph17238964
Chicago/Turabian StyleRazis, Ahmad Faizal Abdull, Muhammed Muzammel Shehzad, Sunusi Usman, Nada Basheir Ali, Shahzad Zafar Iqbal, Nadia Naheed, and Muhammad Rafique Asi. 2020. "Seasonal Variation in Aflatoxin Levels in Edible Seeds, Estimation of Its Dietary Intake and Vitamin E Levels in Southern Areas of Punjab, Pakistan" International Journal of Environmental Research and Public Health 17, no. 23: 8964. https://doi.org/10.3390/ijerph17238964
APA StyleRazis, A. F. A., Shehzad, M. M., Usman, S., Ali, N. B., Iqbal, S. Z., Naheed, N., & Asi, M. R. (2020). Seasonal Variation in Aflatoxin Levels in Edible Seeds, Estimation of Its Dietary Intake and Vitamin E Levels in Southern Areas of Punjab, Pakistan. International Journal of Environmental Research and Public Health, 17(23), 8964. https://doi.org/10.3390/ijerph17238964









