Microplastics Pollution as an Invisible Potential Threat to Food Safety and Security, Policy Challenges and the Way Forward
Abstract
1. Introduction
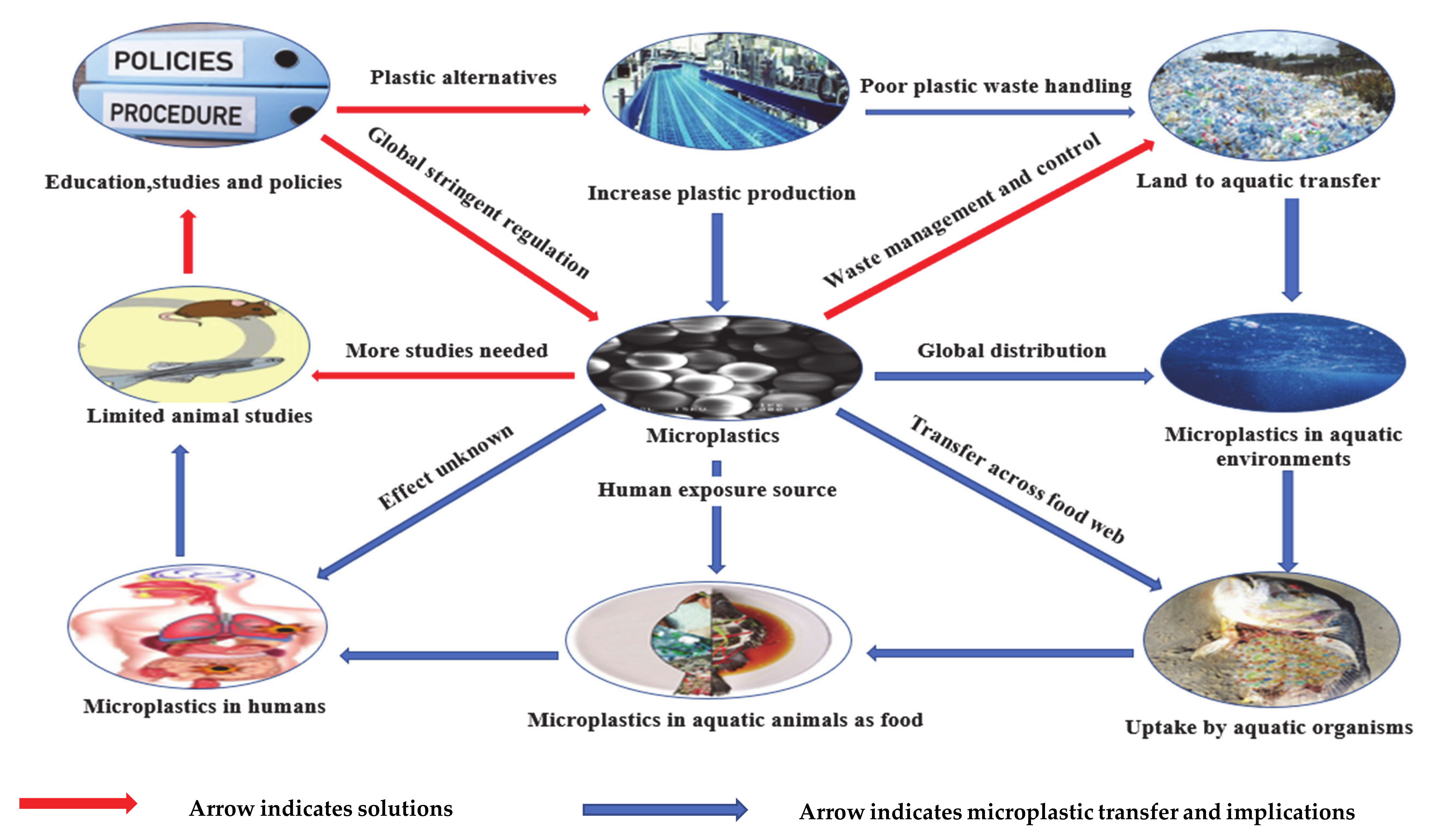
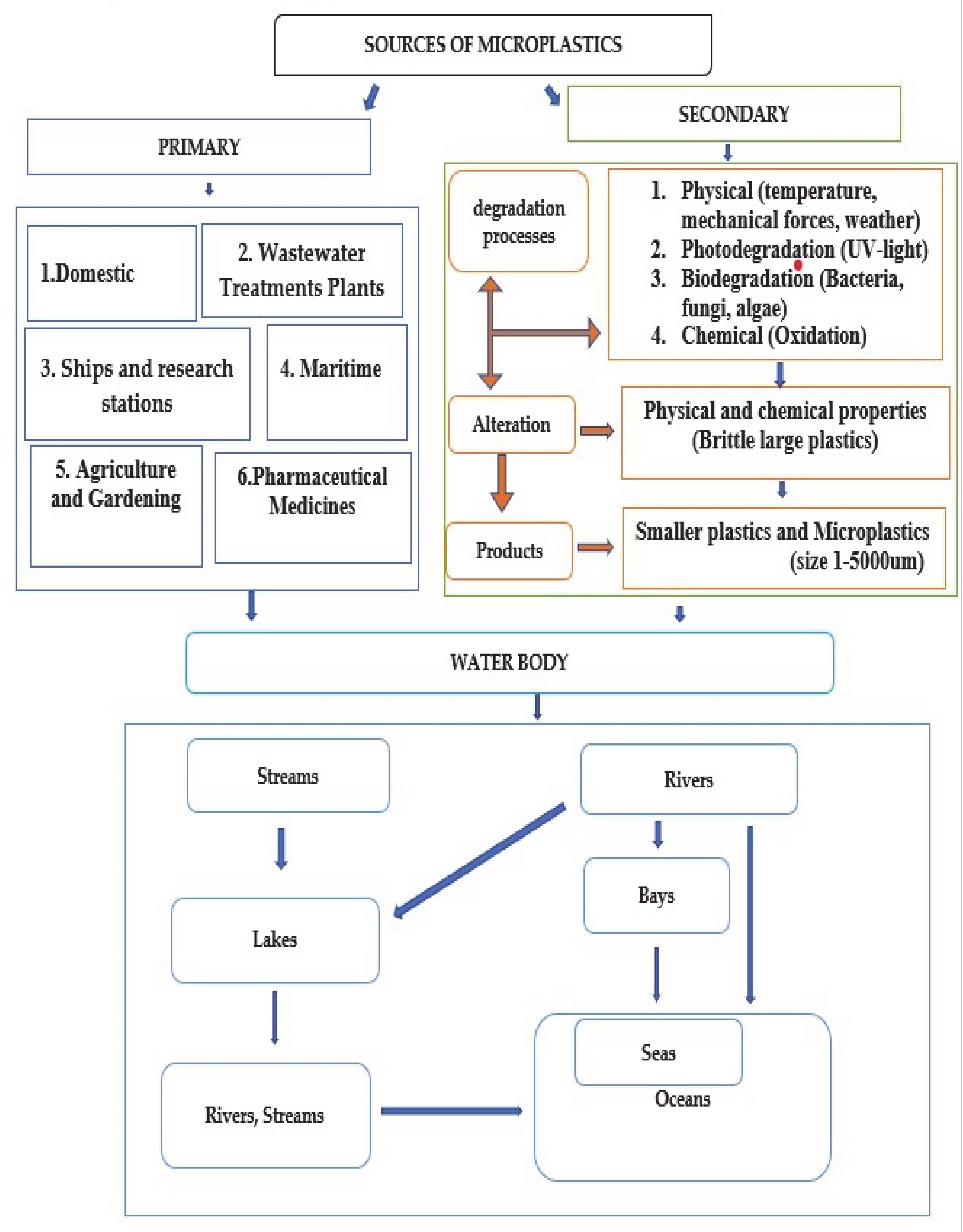
2. Microplastic Sources
3. Microplastic Distribution in Aquatic Ecosystems
4. Detection and Quantification of Microplastics
5. Sampling and Separation Methods of Microplastics
6. Characterization Methods of Microplastics
7. Bioavailability and Uptake of Microplastics by Aquatic Organisms in Natural Environments
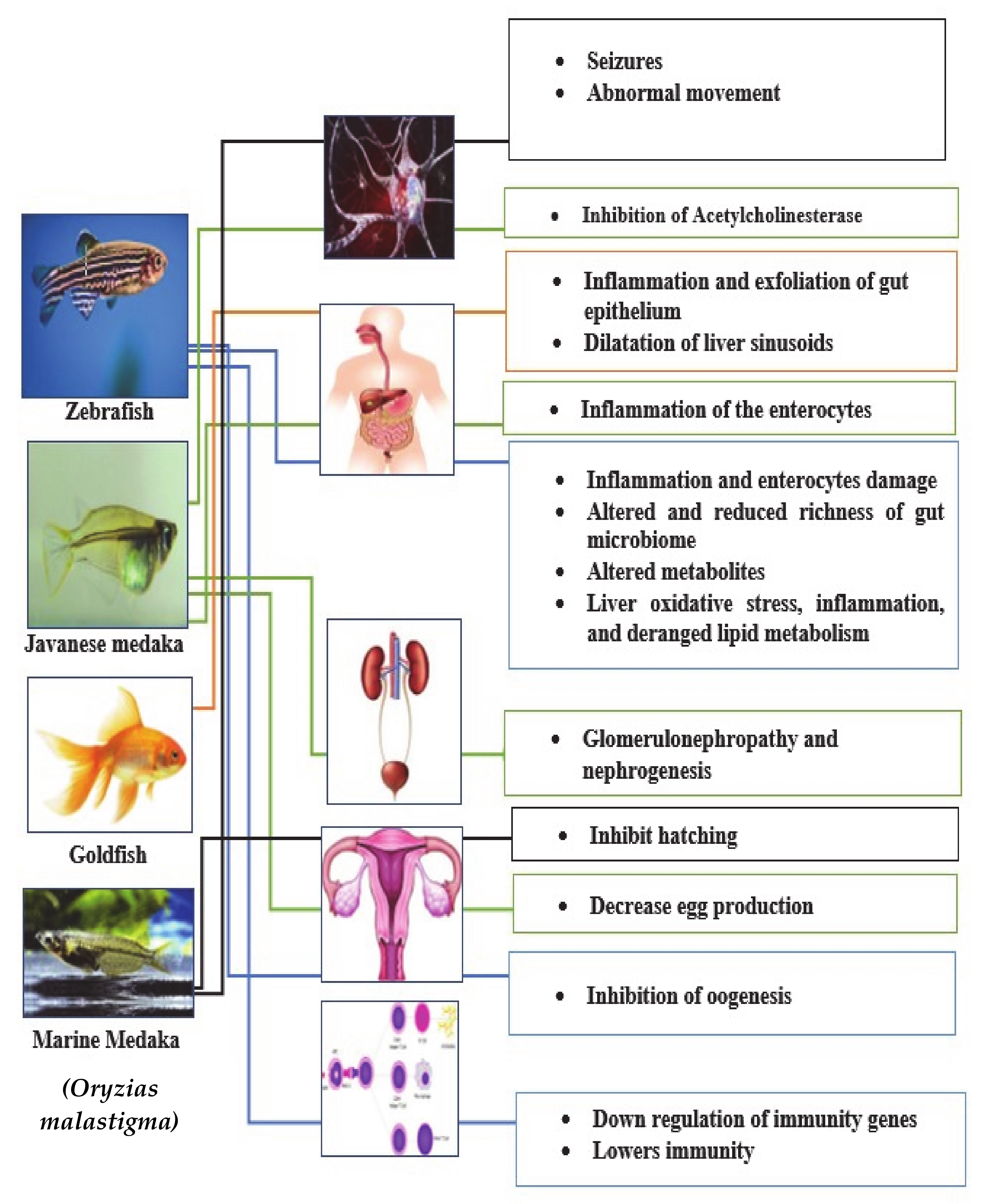
8. Toxic Effect of Microplastics on Aquatic Organisms and Mammals
9. Microplastics Exposure Effect on Fish Species
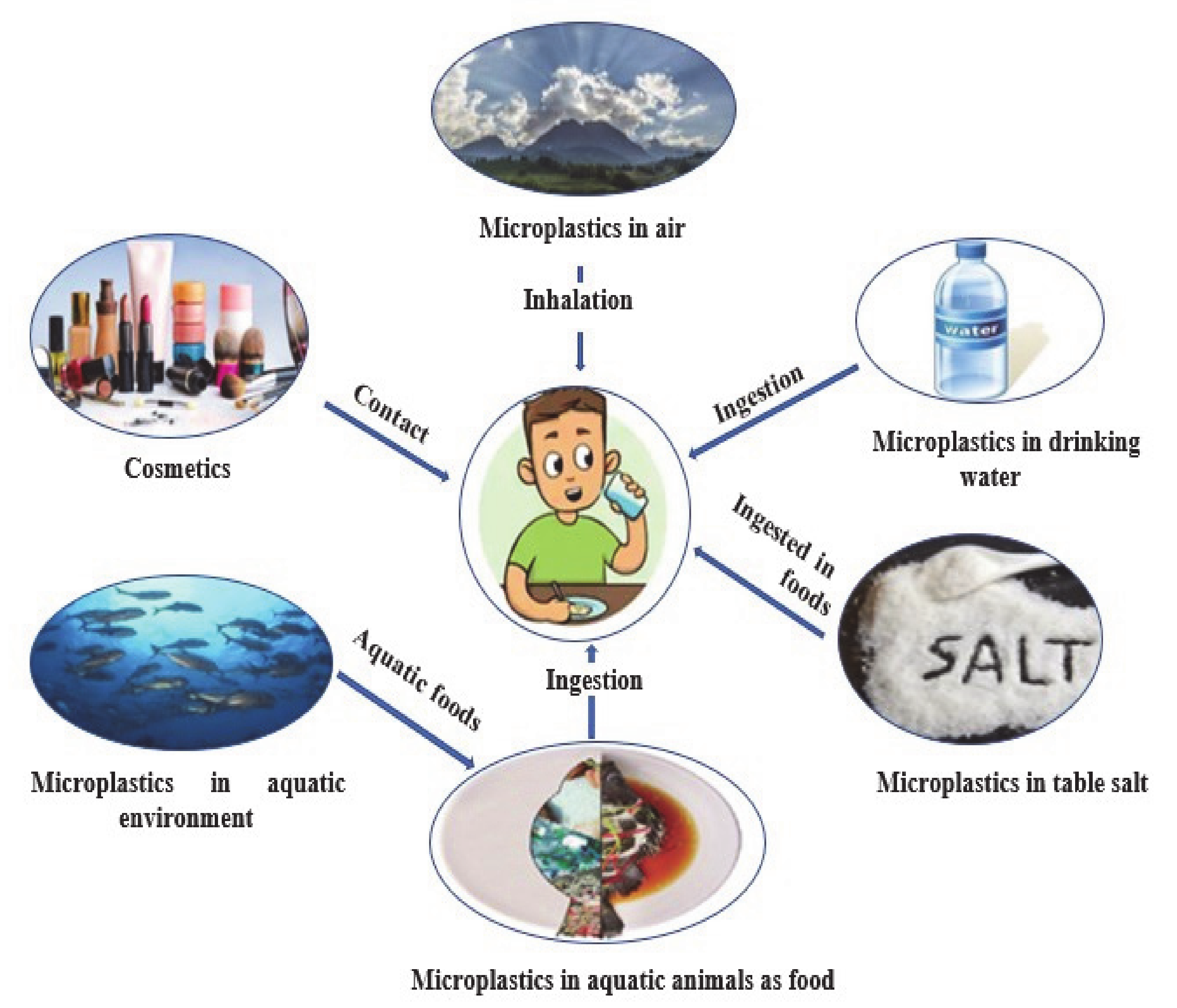
10. Human Exposure Pathways and Health Impact
11. Solution and Policy Development to Microplastic Pollution
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Urbanek, A.K.; Rymowicz, W.; Mirończuk, A.M. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl. Microbiol. Biotechnol. 2018, 102, 7669–7678. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef] [PubMed]
- Hantoro, I.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Widianarko, B.; Ragas, A.M.J. Microplastics in coastal areas and seafood: Implications for food safety. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 674–711. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Dick Vethaak, A.; Lavorante, B.R.B.O.; Lundebye, A.K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Dauvergne, P. The power of environmental norms: Marine plastic pollution and the politics of microbeads. Environ. Polit. 2018, 27, 579–597. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Freshwater Microplastics; Spriger: Berlin/Heidelberg, Germany, 2018; Volume 58, ISBN 978-3-319-61614-8. [Google Scholar]
- Wu, W.M.; Yang, J.; Criddle, C.S. Microplastics pollution and reduction strategies. Front. Environ. Sci. Eng. 2017, 11, 1–4. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection. Fate and Effects of Microplastics in the Marine Environment: Part 2 of a Global Assessment; International Maritime Organisation: London, UK, 2016; Available online: http://www.gesamp.org/site/assets/files/1275/sources-fate-and-effects-of-microplastics-in-the-marine-environment-part-2-of-a-global-assessment-en.pdf (accessed on 17 November 2020).
- Shim, W.J.; Thomposon, R.C. Microplastics in the ocean. Arch. Environ. Contam. Toxicol. 2015, 69, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Duwez, A.S.; Nysten, B. Mapping aging effects on polymer surfaces: Specific detection of additives by chemical force microscopy. Langmuir 2001, 17, 8287–8292. [Google Scholar] [CrossRef]
- Kooi, M.; Van Nes, E.H.; Scheffer, M.; Koelmans, A.A. Ups and downs in the ocean: Effects of biofouling on vertical transport of microplastics. Environ. Sci. Technol. 2017, 51, 7963–7971. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(nano)plastics: A threat to human health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, H.J.; Kim, S.K.; Kim, H.J. Global pattern of Microplastics (MPs) in commercial food-grade salts: Sea salt as an indicator of seawater MP pollution. Environ. Sci. Technol. 2018, 52, 12819–12828. [Google Scholar] [CrossRef]
- Chang, M. Reducing microplastics from facial exfoliating cleansers in wastewater through treatment versus consumer product decisions. Mar. Pollut. Bull. 2015, 101, 330–333. [Google Scholar] [CrossRef]
- Waller, C.L.; Griffiths, H.J.; Waluda, C.M.; Thorpe, S.E.; Loaiza, I.; Moreno, B.; Pacherres, C.O.; Hughes, K.A. Microplastics in the Antarctic marine system: An emerging area of research. Sci. Total Environ. 2017, 598, 220–227. [Google Scholar] [CrossRef]
- Chen, M.; Jin, M.; Tao, P.; Wang, Z.; Xie, W.; Yu, X.; Wang, K. Assessment of microplastics derived from mariculture in Xiangshan Bay, China. Environ. Pollut. 2018, 242, 1146–1156. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Estahbanati, S.; Fahrenfeld, N.L. Influence of wastewater treatment plant discharges on microplastic concentrations in surface water. Chemosphere 2016, 162, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Courtene-Jones, W.; Quinn, B.; Gary, S.F.; Mogg, A.O.M.; Narayanaswamy, B.E. Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environ. Pollut. 2017, 231, 271–280. [Google Scholar] [CrossRef]
- Peng, J.; Wang, J.; Cai, L. Current understanding of microplastics in the environment: Occurrence, fate, risks, and what we should do. Integr. Environ. Assess. Manag. 2017, 13, 476–482. [Google Scholar] [CrossRef]
- Klein, S.; Dimzon, I.K.; Eubeler, J.; Knepper, T.P. Analysis, occurrence, and degradation of microplastics in the aqueous environment. In Handbook of Environmental Chemistry; Wagner, M., Lambert, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 58, pp. 51–67. ISBN 978-3-319-61615-5. [Google Scholar]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Lv, W.; Zhou, W.; Lu, S.; Huang, W.; Yuan, Q.; Tian, M.; Lv, W.; He, D. Microplastic pollution in rice-fish co-culture system: A report of three farmland stations in Shanghai, China. Sci. Total Environ. 2019, 652, 1209–1218. [Google Scholar] [CrossRef]
- Lenaker, P.L.; Baldwin, A.K.; Corsi, S.R.; Mason, S.A.; Reneau, P.C.; Scott, J.W. Vertical distribution of microplastics in the water column and sur fi cial sediment from the Milwaukee River basin to Lake Michigan. Environ. Sci. Technol. 2019, 53. [Google Scholar] [CrossRef]
- Miller, R.Z.; Watts, A.J.R.; Winslow, B.O.; Galloway, T.S.; Barrows, A.P.W. Mountains to the sea: River study of plastic and non-plastic micro fi ber pollution in the northeast USA. Mar. Pollut. Bull. 2017. [Google Scholar] [CrossRef]
- Mani, T.; Primpke, S.; Lorenz, C.; Gerdts, G.; Burkhardt-Holm, P. Microplastic pollution in benthic midstream sediments of the Rhine River. Environ. Sci. Technol. 2019, 53, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and spatial distribution of microplastics in river shore sediments of the rhine-main area in Germany. Environ. Sci. Technol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lahens, L.; Strady, E.; Kieu-Le, T.; Dris, R.; Boukerma, K.; Rinnert, E.; Gasperi, J.; Tassin, B. Macroplastic and microplastic contamination assessment of a tropical river ( Saigon River, Vietnam ) transversed by a developing megacity. Environ. Pollut. 2018, 236, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Kapp, K.J.; Yeatman, E. Microplastic hotspots in the Snake and Lower Columbia rivers: A journey from the greater Yellowstone ecosystem to the Pacific Ocean. Environ. Pollut. 2018. [Google Scholar] [CrossRef]
- De Lucia, G.A.; Vianello, A.; Camedda, A.; Vani, D.; Tomassetti, P.; Coppa, S.; Palazzo, L.; Amici, M.; Romanelli, G.; Zampetti, G.; et al. Sea water contamination in the vicinity of the Italian Minor Islands caused by microplastic pollution. Water 2018, 10, 1108. [Google Scholar] [CrossRef]
- Su, L.; Sharp, S.M.; Pettigrove, V.J.; Craig, N.J.; Nan, B. Superimposed microplastic pollution in a coastal metropolis. Water Res. 2019, 115140. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Wang, J.; Wang, Y.; Mu, J.; Wang, P.; Lin, X.; Ma, D. Microplastic pollution in the surface waters of the Bohai Sea, China. Environ. Pollut. 2017, 231, 541–548. [Google Scholar] [CrossRef]
- Su, L.; Cai, H.; Kolandhasamy, P.; Wu, C.; Rochman, C.M.; Shi, H. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ. Pollut. 2018, 234, 347–355. [Google Scholar] [CrossRef]
- Amélineau, F.; Bonnet, D.; Heitz, O.; Mortreux, V.; Harding, A.M.A.; Karnovsky, N.; Walkusz, W.; Fort, J.; Grémillet, D. Microplastic pollution in the Greenland Sea: Background levels and selective contamination of planktivorous diving seabirds. Environ. Pollut. 2016, 219, 1131–1139. [Google Scholar] [CrossRef]
- Tsang, Y.Y.; Mak, C.W.; Liebich, C.; Lam, S.W.; Sze, E.T.P.; Chan, K.M. Microplastic pollution in the marine waters and sediments of Hong Kong. Mar. Pollut. Bull. 2017, 115, 20–28. [Google Scholar] [CrossRef]
- Qu, X.; Su, L.; Li, H.; Liang, M.; Shi, H. Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Sci. Total Environ. 2018, 621, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Graca, B.; Szewc, K.; Zakrzewska, D.; Dołęga, A.; Szczerbowska-Boruchowska, M. Sources and fate of microplastics in marine and beach sediments of the Southern Baltic Sea—A preliminary study. Environ. Sci. Pollut. Res. 2017, 24, 7650–7661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ran, W.; Teng, J.; Liu, Y.; Liu, H.; Yin, X.; Cao, R.; Wang, Q. Microplastic pollution in sediments from the Bohai Sea and the Yellow Sea, China. Sci. Total Environ. 2018, 640–641, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Kor, K.; Ghazilou, A.; Ershadifar, H.; Kor, K.; Ghazilou, A.; Ershadifar, H. Microplastic pollution in the littoral sediments of the northern part of the Oman Sea. Mar. Pollut. Bull. 2020, 155, 111166. [Google Scholar] [CrossRef] [PubMed]
- Clunies-Ross, P.J.; Smith, G.P.S.; Gordon, K.C.; Gaw, S. Synthetic shorelines in New Zealand? Quantification and characterisation of microplastic pollution on Canterbury’s coastlines. N. Z. J. Mar. Freshw. Res. 2016, 50, 317–325. [Google Scholar] [CrossRef]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK–Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Mees, J.; Janssen, C.R. Assessment of marine debris on the Belgian Continental Shelf. Mar. Pollut. Bull. 2013, 73, 161–169. [Google Scholar] [CrossRef]
- Fok, L.; Lam, T.W.L.; Li, H.X.; Xu, X.R. A meta-analysis of methodologies adopted by microplastic studies in China. Sci. Total Environ. 2020, 718, 135371. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A. A procedure for measuring microplastics using pressurized fluid extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Palter, J.B.; Marinov, I.; Sarmiento, J.L.; Gruber, N. Large-Scale, persistent nutrient fronts of the world. Handb. Environ. Chem. 2006, 5, 1–12. [Google Scholar] [CrossRef]
- Prata, J.C.; Castro, J.L.; da Costa, J.P.; Duarte, A.C.; Cerqueira, M.; Rocha-Santos, T. An easy method for processing and identification of natural and synthetic microfibers and microplastics in indoor and outdoor air. MethodsX 2020, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Corti, A.; Vinciguerra, V.; Iannilli, V.; Pietrelli, L.; Manariti, A.; Bianchi, S.; Petri, A.; Cifelli, M.; Domenici, V.; Castelvetro, V. Thorough multianalytical characterization and quantification of micro-and nanoplastics from bracciano lake’s sediments. Sustainability 2020, 12, 878. [Google Scholar] [CrossRef]
- Fu, W.; Min, J.; Jiang, W.; Li, Y.; Zhang, W. Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci. Total Environ. 2020, 721, 137561. [Google Scholar] [CrossRef]
- Dehaut, A.; Cassone, A.L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef]
- Roch, S.; Brinker, A. Rapid and efficient method for the detection of microplastic in the gastrointestinal tract of fishes. Environ. Sci. Technol. 2017, 51, 4522–4530. [Google Scholar] [CrossRef]
- Barcelo, D. Microplastics analysis. MethodsX 2020, 7, 100884. [Google Scholar] [CrossRef]
- Altmann, K.; Goedecke, C.; Bannick, C.G.; Abusafia, A.; Steinmetz, H.S.C.; Braun, U.; Eichen, U. Identification and Quantification of Microplastic in Sewage Systems by TED-GC-MS. In Proceedings of the 16th International Conference Environmental Science and Technology, Rhodes, Greece, 4–7 September 2019; pp. 4–5. [Google Scholar]
- Frère, L.; Paul-Pont, I.; Moreau, J.; Soudant, P.; Lambert, C.; Huvet, A.; Rinnert, E. A semi-automated Raman micro-spectroscopy method for morphological and chemical characterizations of microplastic litter. Mar. Pollut. Bull. 2016, 113, 461–468. [Google Scholar] [CrossRef]
- Cocca, M.; Di Pace, E.; Errico, M.E.; Gentile, G.; Montarsolo, A.; Mossotti, R. Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea; Springer: Berlin/Heidelberg, Germany, 2018; Volume 22, ISBN 978-3-319-71278-9. [Google Scholar]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and quantification of microplastics in wastewater using focal plane Array-Based reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Quinn, B. The effects of microplastic on freshwater Hydra attenuata feeding, morphology & reproduction. Environ. Pollut. 2018, 234, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Deudero, S. Evidence of microplastic ingestion in the shark Galeus melastomus Rafinesque, 1810 in the continental shelf off the western Mediterranean Sea. Environ. Pollut. 2017, 223, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Steer, M.; Cole, M.; Thompson, R.C.; Lindeque, P.K. Microplastic ingestion in fish larvae in the western English Channel. Environ. Pollut. 2017, 226, 250–259. [Google Scholar] [CrossRef]
- Vendel, A.L.; Bessa, F.; Alves, V.E.N.; Amorim, A.L.A.; Patrício, J.; Palma, A.R.T. Widespread microplastic ingestion by fish assemblages in tropical estuaries subjected to anthropogenic pressures. Mar. Pollut. Bull. 2017, 117, 448–455. [Google Scholar] [CrossRef]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Foley, C.J.; Feiner, Z.S.; Malinich, T.D.; Höök, T.O. A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci. Total Environ. 2018, 631–632, 550–559. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef]
- Powell, M.D.; Berry, A.J. Ingestion and regurgitation of living and inert materials by the estuarine copepod Eurytemora affinis (Poppe) and the influence of salinity. Estuar. Coast. Shelf Sci. 1990, 31, 763–773. [Google Scholar] [CrossRef]
- Collignon, A.; Hecq, J.H.; Galgani, F.; Collard, F.; Goffart, A. Annual variation in neustonic micro- and meso-plastic particles and zooplankton in the Bay of Calvi (Mediterranean-Corsica). Mar. Pollut. Bull. 2014, 79, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Bråte, I.L.N.; Hurley, R.; Iversen, K.; Beyer, J.; Thomas, K.V.; Steindal, C.C.; Green, N.W.; Olsen, M.; Lusher, A. Mytilus spp. as sentinels for monitoring microplastic pollution in Norwegian coastal waters: A qualitative and quantitative study. Environ. Pollut. 2018, 243, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Alimba, C.G.; Faggio, C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Magni, S.; Della Torre, C.; Garrone, G.; D’Amato, A.; Parenti, C.C.; Binelli, A. First evidence of protein modulation by polystyrene microplastics in a freshwater biological model. Environ. Pollut. 2019, 250, 407–415. [Google Scholar] [CrossRef] [PubMed]
- da Costa Araújo, A.P.; Malafaia, G. Microplastic ingestion induces behavioral disorders in mice: A preliminary study on the trophic transfer effects via tadpoles and fish. J. Hazard. Mater. 2021, 401. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, S.; Liu, Q.; Wei, J.; Jin, Y.; Wang, X.; Zhang, L. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/β-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ. Pollut. 2020, 265, 115025. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Cong, Y.; Jin, F.; Tian, M.; Wang, J.; Shi, H.; Wang, Y.; Mu, J. Ingestion, egestion and post-exposure effects of polystyrene microspheres on marine medaka (Oryzias melastigma). Chemosphere 2019, 228, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Kržan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Wang, X.; Luo, X.; Liu, G.; Zheng, H. Effects of polystyrene microplastics on the fitness of earthworms in an agricultural soil. IOP Conf. Ser. Earth Environ. Sci. 2017, 61. [Google Scholar] [CrossRef]
- Handy, R.D.; Henry, T.B.; Scown, T.M.; Johnston, B.D.; Tyler, C.R. Manufactured nanoparticles: Their uptake and effects on fish - A mechanistic analysis. Ecotoxicology 2008, 17, 396–409. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Jabeen, K.; Li, B.; Chen, Q.; Su, L.; Wu, C.; Hollert, H.; Shi, H. Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere 2018, 213, 323–332. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yang, G.; Lu, L.; Zheng, Y.; Zhang, Q.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Low level of polystyrene microplastics decreases early developmental toxicity of phenanthrene on marine medaka (Oryzias melastigma). J. Hazard. Mater. 2020, 385, 121586. [Google Scholar] [CrossRef]
- Mak, C.W.; Yeung, K.C.F.; Chan, K.M. Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf. 2019, 182, 1–10. [Google Scholar] [CrossRef]
- Rainieri, S.; Conlledo, N.; Larsen, B.K.; Granby, K.; Barranco, A. Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ. Res. 2018, 162, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chernick, M.; Rittschof, D.; Hinton, D.E. Chronic dietary exposure to polystyrene microplastics in maturing Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2020, 220, 105396. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Jin, Y.; Xia, J.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. Pollut. 2018, 235, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Limonta, G.; Mancia, A.; Benkhalqui, A.; Bertolucci, C.; Abelli, L.; Fossi, M.C.; Panti, C. Microplastics induce transcriptional changes, immune response and behavioral alterations in adult zebrafish. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Hollman, P.; Mandoza-Hill, J.J. Microplastics in Fisheries and Aquaculture; Food and Agriculture Organziation of the United Nation: Rome, Italy, 2017; Volume 615. [Google Scholar]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Xu, J.; Zhu, L.; Peng, G.; Xu, P.; Li, D. Food-web transfer of microplastics between wild caught fish and crustaceans in East China Sea. Mar. Pollut. Bull. 2019, 146, 173–182. [Google Scholar] [CrossRef]
- Phillips, M.B.; Bonner, T.H. Occurrence and amount of microplastic ingested by fishes in watersheds of the Gulf of Mexico. Mar. Pollut. Bull. 2015, 100, 264–269. [Google Scholar] [CrossRef]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef]
- Karbalaei, S.; Golieskardi, A.; Hamzah, H.B.; Abdulwahid, S.; Hanachi, P.; Walker, T.R.; Karami, A. Abundance and characteristics of microplastics in commercial marine fish from Malaysia. Mar. Pollut. Bull. 2019, 148, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Ekvall, M.T.; Hansson, L.A.; Linse, S.; Malmendal, A.; Cedervall, T. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ. Sci. Technol. 2015, 49, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Peixoto, D.; Pinheiro, C.; Amorim, J.; Oliva-Teles, L.; Guilhermino, L.; Vieira, M.N. Microplastic pollution in commercial salt for human consumption: A review. Estuar. Coast. Shelf Sci. 2019, 219, 161–168. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Arias-Andres, M.; Klümper, U.; Rojas-Jimenez, K.; Grossart, H.P. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ. Pollut. 2018, 237, 253–261. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.; Wang, J.; Zhang, Y.; Zhang, P.; Li, X.; Zou, J.; Zhou, A. Interactions of microplastics and antibiotic resistance genes and their effects on the aquaculture environments. J. Hazard. Mater. 2021, 403, 123961. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, C.; Zhou, J.; Shen, M.; Wang, X.; Fu, Z.; Jin, Y. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef]
- Scopetani, C.; Cincinelli, A.; Martellini, T.; Lombardini, E.; Ciofini, A.; Fortunati, A.; Pasquali, V.; Ciattini, S.; Ugolini, A. Ingested microplastic as a two-way transporter for PBDEs in Talitrus saltator. Environ. Res. 2018, 167, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zhang, J.; Zhao, X.; Feng, J.; Teng, Y.; Chen, B.; Sun, X.; Zhu, L.; Sun, X.; Qu, K. Polystyrene microplastics increase uptake, elimination and cytotoxicity of decabromodiphenyl ether (BDE-209) in the marine scallop Chlamys farreri. Environ. Pollut. 2020, 258, 113657. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Ma, R.; Barrett, H.; Wang, B.; Han, J.; Wang, F.; Chen, P.; Wang, W.; Peng, G.; Yu, G. How microplastics affect chiral illicit drug methamphetamine in aquatic food chain? From green alga (Chlorella pyrenoidosa) to freshwater snail (Cipangopaludian cathayensis). Environ. Int. 2020, 136, 105480. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Lin, L.; Wang, X.; Yu, A.; Sun, X. Interfacial interactions between collected nylon microplastics and three divalent metal ions (Cu(II), Ni(II), Zn(II)) in aqueous solutions. J. Hazard. Mater. 2021, 403, 123548. [Google Scholar] [CrossRef]
- Wu, P.; Tang, Y.; Jin, H.; Song, Y.; Liu, Y.; Cai, Z. Consequential fate of bisphenol-attached PVC microplastics in water and simulated intestinal fluids. Environ. Sci. Ecotechnol. 2020, 2, 100027. [Google Scholar] [CrossRef]
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food exposure and impact on human health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef]
- Michałowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Wu, P.; Cai, Z.; Jin, H.; Tang, Y. Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci. Total Environ. 2019, 650, 671–678. [Google Scholar] [CrossRef]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Wong, C.K.C.; Zheng, J.S.; Bouwman, H.; Barra, R.; Wahlström, B.; Neretin, L.; Wong, M.H. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012, 42, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, D.; Li, L.; Jabeen, K.; Shi, H. Microplastics in commercial bivalves from China. Environ. Pollut. 2015, 207, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Keong Choo, C.; Larat, V.; Galloway, T.S.; Salamatinia, B. The presence of microplastics in commercial salts from different countries. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Bin Ho, Y.; Larat, V.; Salamatinia, B. Microplastics in eviscerated flesh and excised organs of dried fish. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Bessa, F.; Barría, P.; Neto, J.M.; Frias, J.P.G.L.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef]
- Abidli, S.; Lahbib, Y.; Trigui El Menif, N. Microplastics in commercial molluscs from the lagoon of Bizerte (Northern Tunisia). Mar. Pollut. Bull. 2019, 142, 243–252. [Google Scholar] [CrossRef]
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129. [Google Scholar] [CrossRef]
- Haward, M. Plastic pollution of the world’s seas and oceans as a contemporary challenge in ocean governance. Nat. Commun. 2018, 9, 9–11. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made–Supplementary information. Sci. Adv. 2017, 3, 19–24. [Google Scholar] [CrossRef]
- Dauvergne, P. Why is the global governance of plastic failing the oceans? Glob. Environ. Chang. 2018, 51, 22–31. [Google Scholar] [CrossRef]
- Rose, A. A solution to plastic pollution? Using international law to shape plastic regulation in the United States. Hast. Environ. Law J. 2020, 26, 127. [Google Scholar]
- McDevitt, J.P.; Criddle, C.S.; Morse, M.; Hale, R.C.; Bott, C.B.; Rochman, C.M. Addressing the issue of microplastics in the wake of the microbead-free waters act-a new standard can facilitate improved policy. Environ. Sci. Technol. 2017, 51, 6611–6617. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution–Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shi, H.; Peng, J.; Wang, Y.; Xiong, X.; Wu, C.; Lam, P.K.S. Microplastic pollution in China’s inland water systems: A review of findings, methods, characteristics, effects, and management. Sci. Total Environ. 2018, 630, 1641–1653. [Google Scholar] [CrossRef]
- Carlini, G.; Kleine, K. Advancing the international regulation of plastic pollution beyond the united nations environment assembly resolution on marine litter and microplastics. Rev. Eur. Comp. Int. Environ. Law 2018, 27, 234–244. [Google Scholar] [CrossRef]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- MESTECC. Malaysia’s Roadmap Towards Zero Disposable Plastic Use 2018–2030. Available online: https://www.pmo.gov.my/ms/2019/07/pelan-hala-tuju-malaysia-ke-arah-sifar-penggunaan-plastik-sekali-guna-2018-2030/ (accessed on 26 August 2020).
- DAWE. Australia Recycling and Waste Reduction Bill. Available online: http://www.environment.gov.au/protection/waste-resource-recovery/recycling-waste-reduction-bill-2020 (accessed on 3 September 2020).
- Vince, J.; Hardesty, B.D. Plastic pollution challenges in marine and coastal environments: From local to global governance. Restor. Ecol. 2017, 25, 123–128. [Google Scholar] [CrossRef]
| Location | Density | Size | Polymer Type | References |
|---|---|---|---|---|
| Milwaukee River basin, Wisconsin, USA | 1.1 g/cm3 | 0.355–4.749 mm | LDPP, PET | [32] |
| Hudson River, New York State, USA | 0.98 items/L | 1.000–4.749 mm | PET, PP | [33] |
| Rhine riverbed, Koblenz, Germany | 0.26–11.07 × 103/kg weight | 11−5033 μm | AC, PU, APV, PE, EPDM, PES | [34] |
| River Rhine, River Main, Germany | 228–3763 particles/kg | 63–5000 μm | PE, PP, PS, PET, EPDM, PVC | [35] |
| Saigon River, Vietnam | 172,000–419,000 Items/m3 | 50–250 μm | PE, PP, PES, PET | [36] |
| Snake and lower Columbia rivers | 0.014–5.405 items/L | 100–333 µm | PP, PE, PET, PES | [37] |
| Italian coast, Italy | 0.641 to 0.119 items/m3 | ≤333 µm | PP, PE, PET, PES, EVA | [38] |
| Greater Melbourne Area and the Western Port area, Australia. | 0.06 to 2.5 items/L | 1.26 ± 0.93 mm | PES, PP, PE, PA | [39] |
| Bohai Sea China | 0.33 ± 0.36 m3 | 0.3–5 mm | PE, PP, PS, PET | [40] |
| Yangtze River Basin China | 0.5–3.1 items/L | 0.25–1 mm | PES, PP, PE | [41] |
| Greenland Sea | 0.81–4.52 particles m−3 | 0.5–4.5 mm | PES, PE | [42] |
| Hong Kong Marine waters | 413.38 particles m−3 | 0.2–4.9 mm | PP, PE, SAN | [43] |
| China coastal waters | 0.68 to 6.44 particles/L | 0.25–1 mm | PET, RY, PE, PVC, PP | [44] |
| Southern Baltic sea | 25 to 53 particles/kg dry weight | 0.1–5 mm | EPM, PVC, VCE, PAN, PVA, PES, EVA, PE | [45] |
| Bohai Sea China | 2.0–17.0/50 g dry weight | 66.25–4982.59 µm | RY, PE, PET, PP, PA | [46] |
| Northern Yellow Sea China | 4.0–14.0 particles/50 g dry weight | 66.25–4982.59 µm | RY, PE, PET, PP, PA | [46] |
| Southern Yellow Sea China | 2.0–7.0 items/50 g dry weight | 66.25–4982.5 µm | RY, PE, PET, PP, PA | [46] |
| Oman sea | 138.3–930.3 particles/kg | 100–1000 μm | PE, PP, PA, PET, PVA, PS, PVC | [47] |
| Canterbury’s coastlines | 0–45.4 particles/Kg of dry sediment. | 0.5–1 mm | PS, PE, PP | [48] |
| Xiangshan Bay, China | Water: 8.9 ± 4.7 items/m3Sediment: 1739 ± 2153 items/kg | 1.54 ± 1.53 mm 1.33 ± 1.69 mm | PE, PP, PS | [21] |
| River Thames, UK | 66 particles/100 g | 1 mm–4 mm | PP, PES, PAS | [49] |
| Type | Size (μm) | Concentration | Organisms | Tissue | Biomarker(s) | Response | References |
|---|---|---|---|---|---|---|---|
| PS | 1 and 10 | 50 mg/L | Zebra mussels (Dreissena polymorpha) | Gills | Proteome | Change in protein involved in oxidative stress, ribosomal function, energy metabolism, cellular trafficking, RNA binding and cytoskeleton | [80] |
| PE | 35.46 | 500 mg/mL | Mice | CNS | Stress locomotion | Reduced locomotion Anxiety | [81] |
| PS | 0.5 | 0.5, 5, 50 mg/L | Wistar rats | Heart | Troponin I Creatinine-kinas MB | Increase troponin I and creatinine-kinase MB Myocardial damage and apoptosis by induction of oxidative stress Collagen proliferation in heart by activation of Wnt/β-catenin pathway | [82] |
| PS | 5 and 20 | 0.1 mg/day | Mice | Liver | ATP, LDH, SOD, AChE | Decrease ATP, LDH and AChE Increased GSH-Px and SOD | [83] |
| PS | 5 | 100 and 1000 µg/L | Mice | Gut, liver, and feces | Gut damage, metabolic disorders, microbiota dysbiosis | [84] | |
| PE PS | <100 | 20 gm/L 0.5 mg/L 5 mg/L | Mytilus galloprovincialis | Gills, digestive glands, haemolymph | Immune cells functions NNRT, AChE, DNA MN, NA | Immunotoxicity, neurotoxicity, genotoxicity, changes in gene expression profile | [85] |
| PE | <400 | 0.02 gm/L 0.08 gm/mL 0.04 gm/mL 0.08 gm/mL | Hydra attenuata | Feeding habit | Reduced feeding | [67] | |
| PS | 10 | 1 × 105 particles/L | Medaka (Oryzias melastigma) | Mortality, growth and fecundity | Significant mortality, reduction in growth and egg production | [86] | |
| PS | 0.5 | 40,000 μg/L | Eriocheir sinensis | Liver | Inhibits growth Damage and oxidative stress induction in hepatopancreas | [87] | |
| PET | 62–1400 | Fresh water crustacean (Daphnia magna) | Gut | Mortality | Increased mortality Accumulation of PET in the gut | [88] | |
| PS | 58 | 0.25–2% | Earthworms (E. foetida) | Growth and mortality | Significant inhibition of growth and mortality | [89] |
| Products/Country | Concentration | Plastic Polymer | References |
|---|---|---|---|
| Bivalves/China | 2.1 to 10.5 items/g 4.3 to 57.2 items/individual | fibers, fragments, and pellets, | [128] |
| Commercial fish/Malaysia | 56 particles/11 fish | PP, PE, PET | [107] |
| Commercial salt/China | 550−681 particles/kg in sea salts, 43−364 particles/kg in lake salts, and 7−204 particles/kg in rock/well salts | PET, PES, PE, PB, CP, PP | [3] |
| Commercial salt from Australia, France, Iran, Japan, Malaysia, New Zealand, Portugal and South Africa | 1 to 10 microplastics/kg | PET, PE, PP, PET | [129] |
| Commercial mussels/UK | 1.4 items/g | PP | [106] |
| Dried commercial fish/Malaysia | 0–3 particles/fish | PP, PE, PET, PS, PA | [130] |
| Commercial fish/Mondego estuary | 1.67 ± 0.27 (SD) | PP, PAN, PE, polyamide 6-nylon | [131] |
| Commercial molluscs from the lagoon of Bizerte (Northern Tunisia) | 703.95 ± 109.80 to 1482.82 ± 19.20 items/kg | PE, PP | [132] |
| Returnable water Single plastic bottled water Beverages All in grocery stores Germany. | 118 ± 88 particles/L 14 ± 14 particles/L 11 ± 8 particles/L | PET, PP | [133] |
| Country/Agency/Company | Policy/Strategy | Functions | References |
|---|---|---|---|
| Australia | Recycling and Waste Reduction Bill (2020) | Banning of plastic export. Provides flow chart of waste management and recycling. | [144] |
| China | Law on the Prevention and Control of Environmental Pollution by Solid Wastes (LPCEPSW) | Regulates waste dumping sites. Prohibition of plastic dumping in rivers, lakes, and reservoirs. Promotes circular energy | [140] |
| Malaysia | Road map for zero single plastic use | Taxation on single plastic use bags and plastic manufacturers. Communication, education and public awareness. Research and development on alternatives such as biobags. | [143] |
| USA | Microbeads Free Water Acts (2005) | Prohibition of sales of personal care products containing microbeads | [9] |
| United Nations Environment Assembly (UNEA) | To identify obstacles, grey areas and to adopt new strategies | Setting a committee to create response strategies. Strategies should target government policies and voluntary options to combat plastic pollution. | [141] |
| Plastic soup foundation and North Sea foundation | Information on microplastic containing products | Allows consumers to make an informed choice | [19] |
| Toyota, Walmart, Proctor and Gamble | Efficient plastic waste disposal | Disposing plastic waste to land fill | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, S.; Abdull Razis, A.F.; Shaari, K.; Amal, M.N.A.; Saad, M.Z.; Mat Isa, N.; Nazarudin, M.F.; Zulkifli, S.Z.; Sutra, J.; Ibrahim, M.A. Microplastics Pollution as an Invisible Potential Threat to Food Safety and Security, Policy Challenges and the Way Forward. Int. J. Environ. Res. Public Health 2020, 17, 9591. https://doi.org/10.3390/ijerph17249591
Usman S, Abdull Razis AF, Shaari K, Amal MNA, Saad MZ, Mat Isa N, Nazarudin MF, Zulkifli SZ, Sutra J, Ibrahim MA. Microplastics Pollution as an Invisible Potential Threat to Food Safety and Security, Policy Challenges and the Way Forward. International Journal of Environmental Research and Public Health. 2020; 17(24):9591. https://doi.org/10.3390/ijerph17249591
Chicago/Turabian StyleUsman, Sunusi, Ahmad Faizal Abdull Razis, Khozirah Shaari, Mohammad Noor Azmai Amal, Mohd Zamri Saad, Nurulfiza Mat Isa, Muhammad Farhan Nazarudin, Syaizwan Zahmir Zulkifli, Jumria Sutra, and Musa Adamu Ibrahim. 2020. "Microplastics Pollution as an Invisible Potential Threat to Food Safety and Security, Policy Challenges and the Way Forward" International Journal of Environmental Research and Public Health 17, no. 24: 9591. https://doi.org/10.3390/ijerph17249591
APA StyleUsman, S., Abdull Razis, A. F., Shaari, K., Amal, M. N. A., Saad, M. Z., Mat Isa, N., Nazarudin, M. F., Zulkifli, S. Z., Sutra, J., & Ibrahim, M. A. (2020). Microplastics Pollution as an Invisible Potential Threat to Food Safety and Security, Policy Challenges and the Way Forward. International Journal of Environmental Research and Public Health, 17(24), 9591. https://doi.org/10.3390/ijerph17249591








