Tools to Support Self-Care Monitoring at Home: Perspectives of Patients with Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting
2.3. Sample and Recruitment
2.4. Data Collection
2.5. Deductive Content Analysis
2.6. Rigor
2.7. Ethical Considerations
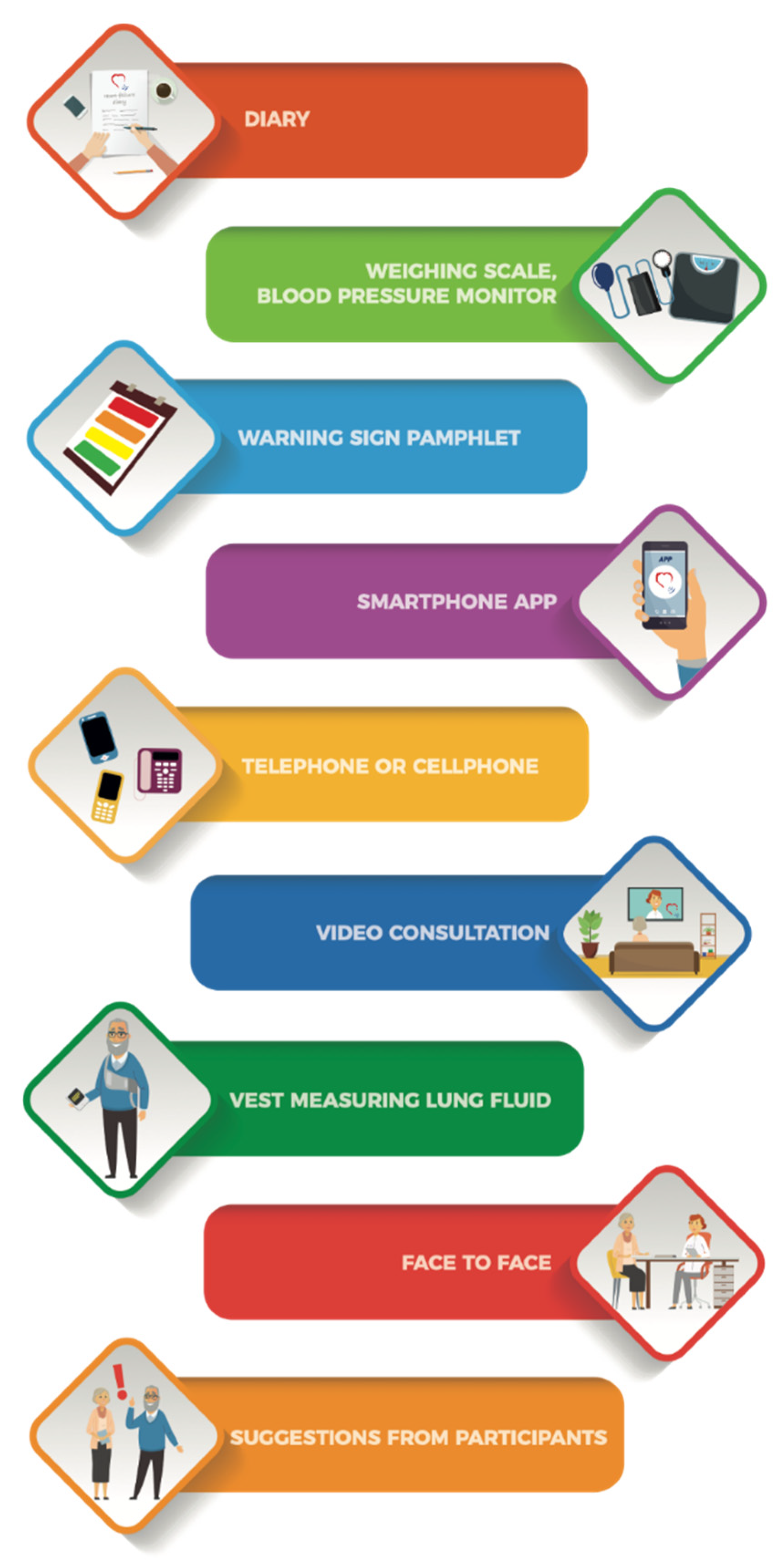
3. Results
3.1. Mhealth Tools to Identify HF Symptoms
Smartphone App
3.2. Paper-Based Tools to Identify HF Symptoms
3.2.1. Diary
3.2.2. Pamphlet with Schematic Warning Signs
3.3. Medical Devices to Identify HF Symptoms
3.3.1. Weighing Scale
3.3.2. Vest for Measuring Lung Fluid
3.4. Tools to Consult With HCP to Identify HF Symptoms
3.4.1. Video Consultation
3.4.2. Telephone
3.4.3. Face-to-Face
4. Additional Participant Suggestions for Supporting Self-Care at Home
5. Participants’ Prioritizing of the Eight Self-Care Tools
6. Discussion
7. Strengths and Limitations
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riegel, B.; Jaarsma, T.; Stromberg, A. A middle-range theory of self-care of chronic illness. ANS Adv. Nurs. Sci. 2012, 35, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Riegel, B.; Jaarsma, T.; Lee, C.S.; Stromberg, A. Integrating Symptoms Into the Middle-Range Theory of Self-Care of Chronic Illness. ANS Adv. Nurs. Sci. 2019, 42, 206. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Tsuchihashi-Makaya, M.; Kang, J.; Aoki, Y.; Fukawa, M.; Matsuoka, S. Symptom Perception, Evaluation, Response to Symptom, and Delayed Care Seeking in Patients With Acute Heart Failure: An Observational Study. J. Cardiovasc. Nurs. 2019, 34, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Gjestsen, M.T.; Bronnick, K.; Testad, I. Characteristics and predictors for hospitalizations of home-dwelling older persons receiving community care: A cohort study from Norway. BMC Geriatr. 2018, 18, 203. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Jaarsma, T.; Hill, L.; Bayes-Genis, A.; La Rocca, H.B.; Castiello, T.; Celutkiene, J.; Marques-Sule, E.; Plymen, C.M.; Piper, S.E.; Riegel, B.; et al. Self-care of heart failure patients: Practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy on Human Resources for Health: Workforce 2030. Available online: http://www.who.int/hrh/resources/pub_globstrathrh-2030/en/ (accessed on 4 April 2020).
- World Health Organization. Global Diffusion of eHealth: Making Universal Health Coverage Achievable; Report of the Third Global Survey on eHealth; World Health Organization: Geneva, Switzerland, 2016; Available online: http://www.who.int/goe/publications/global_diffusion/en/ (accessed on 4 April 2020).
- Field, M.J. Institute of Medicine Committee on Evaluating Clinical Applications of Telemedicine. The National Academies Collection: Reports funded by National Institutes of Health. In Telemedicine: A Guide to Assessing Telecommunications in Health Care; Field, M.J., Ed.; National Academies Press: Washington, DC, USA, 1996. [Google Scholar] [CrossRef]
- Inglis, S.C.; Clark, R.A.; Dierckx, R.; Prieto-Merino, D.; Cleland, J.G. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Heart 2017, 103, 255–257. [Google Scholar] [CrossRef]
- Zanaboni, P.; Wootton, R. Adoption of routine telemedicine in Norwegian hospitals progress over 5 years. BMC Health Serv. Res. 2016, 16, 496. [Google Scholar] [CrossRef]
- Kropf, M.; Modre-Osprian, R.; Hayn, D.; Fruhwald, F.; Schreier, G. Telemonitoring in heart failure patients with clinical decision support to optimize medication doses based on guidelines. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2014, 2014, 3168–3171. [Google Scholar] [CrossRef]
- Koole, M.A.C.; Kauw, D.; Winter, M.M.; Dohmen, D.A.J.; Tulevski, I.I.; de Haan, R.; Somsen, G.A.; Schijven, M.P.; Robbers-Visser, D.; Mulder, B.J.M.; et al. First real-world experience with mobile health telemonitoring in adult patients with congenital heart disease. Neth. Heart J. 2019, 27, 30–37. [Google Scholar] [CrossRef]
- Aamodt, I.T.; Lycholip, E.; Celutkiene, J.; Stromberg, A.; Atar, D.; Falk, R.S.; von Lueder, T.; Helleso, R.; Jaarsma, T.; Lie, I. Health Care Professionals’ Perceptions of Home Telemonitoring in Heart Failure Care: Cross-Sectional Survey. J. Med. Internet Res. 2019, 21, e10362. [Google Scholar] [CrossRef] [PubMed]
- Gensini, G.F.; Alderighi, C.; Rasoini, R.; Mazzanti, M.; Casolo, G. Value of Telemonitoring and Telemedicine in Heart Failure Management. Card. Fail. Rev. 2017, 3, 116–121. [Google Scholar] [CrossRef]
- Greenhalgh, T.; A’Court, C.; Shaw, S. Understanding heart failure; explaining telehealth—A hermeneutic systematic review. BMC Cardiovasc. Disord. 2017, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Bidwell, J.T.; Paturzo, M.; Alvaro, R.; Cocchieri, A.; Jaarsma, T.; Stromberg, A.; Riegel, B.; Vellone, E. Patterns of self-care and clinical events in a cohort of adults with heart failure: 1 year follow-up. Heart Lung 2018, 47, 40–46. [Google Scholar] [CrossRef]
- Bashi, N.; Karunanithi, M.; Fatehi, F.; Ding, H.; Walters, D. Remote Monitoring of Patients With Heart Failure: An Overview of Systematic Reviews. J. Med. Internet Res. 2017, 19, e18. [Google Scholar] [CrossRef]
- Flodgren, G.; Rachas, A.; Farmer, A.J.; Inzitari, M.; Shepperd, S. Interactive telemedicine: Effects on professional practice and health care outcomes. Cochrane Database Syst. Rev. 2015, 9, CD002098. [Google Scholar] [CrossRef]
- Polit, D.F.; Beck, C.T. Nursing Research: Generating and Assessing Evidence for Nursing Practice, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2017. [Google Scholar]
- Palinkas, L.A.; Horwitz, S.M.; Green, C.A.; Wisdom, J.P.; Duan, N.; Hoagwood, K. Purposeful Sampling for Qualitative Data Collection and Analysis in Mixed Method Implementation Research. Adm. Policy Ment. Health 2015, 42, 533–544. [Google Scholar] [CrossRef]
- Harper, D. Talking About Pictures: A Case for Photo Elicitation. Vis. Stud. 2002, 17, 13–26. [Google Scholar] [CrossRef]
- Hurworth, R.; Clark, E.; Martin, J.; Thomsen, S. The Use of Photo-interviewing: Three Examples from Health Evaluation and Research. Eval. J. Australas. 2005, 4, 52–62. [Google Scholar] [CrossRef]
- Kvale, S.; Brinkmann, S. Det Kvalitative Forskningsintervju, 2nd ed.; Gyldendal Akademisk: Oslo, Norway, 2010; p. 344. [Google Scholar]
- The Norwegian Directorate of eHealth. Nasjonal e-Helsestrategi 2017–2022. Available online: https://ehelse.no/Documents/Nasjonal%20e-helsestrategi%20og%20handlingsplan/Nasjonal%20e-helsestrategi%202017-2022%20%28PDF%29.pdf (accessed on 27 September 2020).
- World Health Organization. Be he@Lthy be Mobile Annual Report 2016; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Aamodt, I.T.; Lycholip, E.; Celutkiene, J.; von Lueder, T.; Atar, D.; Falk, R.S.; Helleso, R.; Jaarsma, T.; Stromberg, A.; Lie, I. Self-Care Monitoring of Heart Failure Symptoms and Lung Impedance at Home Following Hospital Discharge: Longitudinal Study. J. Med. Internet Res. 2020, 22, e15445. [Google Scholar] [CrossRef]
- Heart Failure Association of the European Society of Cardiology. Warning Signs. Available online: https://www.heartfailurematters.org/en_GB/Understanding-heart-failure/What-is-heart-failure (accessed on 13 September 2020).
- Elo, S.; Kyngas, H. The qualitative content analysis process. J. Adv. Nurs. 2008, 62, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Polit, D.P.; Beck, C.T. Nursing Research Generating and Assessing Evidence for Nursing Practice, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Shochat, M.K.; Shotan, A.; Blondheim, D.S.; Kazatsker, M.; Dahan, I.; Asif, A.; Rozenman, Y.; Kleiner, I.; Weinstein, J.M.; Frimerman, A.; et al. Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients: A Randomized Controlled Trial (IMPEDANCE-HF Trial). J. Card. Fail. 2016, 22, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Coorey, G.M.; Neubeck, L.; Mulley, J.; Redfern, J. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: Systematic review with meta-synthesis of quantitative and qualitative data. Eur. J. Prev. Cardiol. 2018, 25, 505–521. [Google Scholar] [CrossRef]
- Hagglund, E.; Lynga, P.; Frie, F.; Ullman, B.; Persson, H.; Melin, M.; Hagerman, I. Patient-centred home-based management of heart failure. Findings from a randomised clinical trial evaluating a tablet computer for self-care, quality of life and effects on knowledge. Scand. Cardiovasc. J. 2015, 49, 193–199. [Google Scholar] [CrossRef]
- Allida, S.; Du, H.; Xu, X.; Prichard, R.; Chang, S.; Hickman, L.D.; Davidson, P.M.; Inglis, S.C. mHealth education interventions in heart failure. Cochrane Database Syst. Rev. 2020, 7, CD011845. [Google Scholar] [CrossRef]
- Trivedi, R.B.; Slightam, C.; Nevedal, A.; Guetterman, T.C.; Fan, V.S.; Nelson, K.M.; Rosland, A.M.; Heidenreich, P.A.; Timko, C.; Asch, S.M.; et al. Comparing the Barriers and Facilitators of Heart Failure Management as Perceived by Patients, Caregivers, and Clinical Providers. J. Cardiovasc. Nurs. 2019, 34, 399–409. [Google Scholar] [CrossRef]
- Takeda, A.; Martin, N.; Taylor, R.S.; Taylor, S.J. Disease management interventions for heart failure. Cochrane Database Syst. Rev. 2019, 1, CD002752. [Google Scholar] [CrossRef]
- Van Spall, H.G.; Rahman, T.; Mytton, O.; Ramasundarahettige, C.; Ibrahim, Q.; Kabali, C.; Coppens, M.; Brian Haynes, R.; Connolly, S. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: A systematic review and network meta-analysis. Eur. J. Heart Fail. 2017, 19, 1427–1443. [Google Scholar] [CrossRef]
- Savarese, G.; Lund, L.H.; Dahlstrom, U.; Stromberg, A. Nurse-Led Heart Failure Clinics Are Associated With Reduced Mortality but Not Heart Failure Hospitalization. J. Am. Heart Assoc. 2019, 8, e011737. [Google Scholar] [CrossRef]
- Wali, S.; Demers, C.; Shah, H.; Wali, H.; Lim, D.; Naik, N.; Ghany, A.; Vispute, A.; Wali, M.; Keshavjee, K. Evaluation of Heart Failure Apps to Promote Self-Care: Systematic App Search. JMIR Mhealth Uhealth 2019, 7, e13173. [Google Scholar] [CrossRef]
- Wallstrom, S.; Ali, L.; Ekman, I.; Swedberg, K.; Fors, A. Effects of a person-centred telephone support on fatigue in people with chronic heart failure: Subgroup analysis of a randomised controlled trial. Eur. J. Cardiovasc. Nurs. 2020, 19, 393–400. [Google Scholar] [CrossRef]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Ponikowski, P.; Anker, S.D.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.F.; de Boer, R.A.; Drexel, H.; Ben Gal, T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 1169–1186. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, A.L.; Leppanen, J.; Kaijanranta, H.; Kulju, M.; Helio, T.; van Gils, M.; Lahteenmaki, J. Use of home telemonitoring to support multidisciplinary care of heart failure patients in Finland: Randomized controlled trial. J. Med. Internet Res. 2014, 16, e282. [Google Scholar] [CrossRef] [PubMed]
- Neubeck, L.; Hansen, T.; Jaarsma, T.; Klompstra, L.; Gallagher, R. Delivering healthcare remotely to cardiovascular patients during COVID-19: A rapid review of the evidence. Eur. J. Cardiovasc. Nurs. 2020. [Google Scholar] [CrossRef]
- Vandekerckhove, P.; Vandekerckhove, Y.; Tavernier, R.; De Jaegher, K.; de Mul, M. Leveraging User Experience to Improve Video Consultations in a Cardiology Practice During the COVID-19 Pandemic: Initial Insights. J. Med. Internet Res. 2020, 22, e19771. [Google Scholar] [CrossRef]
- Buffel du Vaure, C.; Ravaud, P.; Baron, G.; Barnes, C.; Gilberg, S.; Boutron, I. Potential workload in applying clinical practice guidelines for patients with chronic conditions and multimorbidity: A systematic analysis. BMJ Open 2016, 6, e010119. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Shaw, S.; Wherton, J.; Vijayaraghavan, S.; Morris, J.; Bhattacharya, S.; Hanson, P.; Campbell-Richards, D.; Ramoutar, S.; Collard, A.; et al. Real-World Implementation of Video Outpatient Consultations at Macro, Meso, and Micro Levels: Mixed-Method Study. J. Med. Internet Res. 2018, 20, e150. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. Report on the WHO Symposium on the Future of Digital Health Systems in the European Region, Copenhagen, Denmark, 6–8 February 2019; WHO European Region: Copenhagen, Denmark, 2019. [Google Scholar]
- The United Nations. International Telecommunication Union Administrative Council (ITU Councel). Available online: https://sustainabledevelopment.un.org/index.php?page=view&type=30022&nr=1932&menu=3170 (accessed on 4 April 2020).
- Norwegian Ministry of Health and Care Sercices. National Health and Hospital Plan 2020–2023; Norwegian Ministry of Health and Care Services: Oslo, Norway, 2020. [Google Scholar]
- Santos, G.C.; Liljeroos, M.; Dwyer, A.A.; Jaques, C.; Girard, J.; Stromberg, A.; Hullin, R.; Schafer-Keller, P. Symptom perception in heart failure: A scoping review on definition, factors and instruments. Eur. J. Cardiovasc. Nurs. 2020, 19, 100–117. [Google Scholar] [CrossRef]
- Nordfonn, O.K.; Morken, I.M.; Lunde Husebø, A.M. A qualitative study of living with the burden from heart failure treatment: Exploring the patient capacity for self-care. Nurs. Open 2020, 7, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma, T.; Cameron, J.; Riegel, B.; Stromberg, A. Factors Related to Self-Care in Heart Failure Patients According to the Middle-Range Theory of Self-Care of Chronic Illness: A Literature Update. Curr. Heart Fail. Rep. 2017, 14, 71–77. [Google Scholar] [CrossRef] [PubMed]
| Self-care monitoring | Category | Tool | Unit of Analysis |
| Paper-based tools to identify HF symptoms | Diary | Male, 60 years, high school, welfare. “I was hospitalized with swollen legs with wounds and everybody thought I had diabetes. I read in the diary about HF symptoms e.g., swollen legs, shortness of breath. I know the symptoms now and use the diary less” | |
| Warning signs | Male, 59 years, college, work. “I thought cough sirup would relive my breathing problems, but that did not happen. I ended up in hospital. I might have come to the hospital earlier by using the warning signs. I like the warnings signs, but it’s not available.” | ||
| Medical devices to identify HF symptoms | Weighing scale | Female, 77 years, university, retired. “I manage diuretics on-demand by myself, and I check my weight to see the effect of using and not using diuretics.” | |
| Vest measuring lung fluid | Male, 60 years, high school, welfare. “We should use technology, but there should be a healthcare professional receiving the information from the [measuring] vest. I am against being my own doctor and seeking answers to my questions online.” | ||
| mHealth to identify HF symptoms | Smartphone App | Female, 62 years, college, welfare. “I do not like too much app technology, but I know how to use it and will use it if needed.” | |
| Tools to consult with HCPs to identify HF symptoms | Face to face | Male, 59 years, college, welfare. “Nothing can replace to meeting in person. All the other things can only be supplemental. There are a lot of signs that are not possible to transfer by phone such as facial expressions and eye contact.” | |
| Telephone | Male, 72 years, university, retired. “I am a techno freak; I have experience with HCPs support when I had an alarm. I phoned and we solved it by phone. ” | ||
| Video consultation | Male, 78 years, college, retired. “If I experience shortness of breath, pain or chest pain, I can do it [video consultation]) from my home, using my iPad or computer to talk with the nurse and not having to travel to the hospital.” |
| Characteristics | n | |
|---|---|---|
| Demographic | Gender | |
| Female | 3 | |
| Male | 16 | |
| Highest education level | ||
| High school | 8 | |
| College/University | 11 | |
| Current work status | ||
| Retired or welfare | 15 | |
| Work fulltime | 4 | |
| Clinical | NYHA class | |
| NYHA 1 | 1 | |
| NYHA 2 | 11 | |
| NYHA 3 | 5 | |
| NYHA 4 | 2 | |
| HF Medications | ||
| ACEI/ARB | 19 | |
| Betablockers | 18 | |
| Diuretics | 17 | |
| Mineral corticoids | 12 | |
| HF Device therapy a | 8 | |
| Comorbidities | 18 | |
| ICT Experience | Experience of using | |
| Internet at home | 18 | |
| 18 | ||
| E-mail on smartphone | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aamodt, I.T.; Strömberg, A.; Hellesø, R.; Jaarsma, T.; Lie, I. Tools to Support Self-Care Monitoring at Home: Perspectives of Patients with Heart Failure. Int. J. Environ. Res. Public Health 2020, 17, 8916. https://doi.org/10.3390/ijerph17238916
Aamodt IT, Strömberg A, Hellesø R, Jaarsma T, Lie I. Tools to Support Self-Care Monitoring at Home: Perspectives of Patients with Heart Failure. International Journal of Environmental Research and Public Health. 2020; 17(23):8916. https://doi.org/10.3390/ijerph17238916
Chicago/Turabian StyleAamodt, Ina Thon, Anna Strömberg, Ragnhild Hellesø, Tiny Jaarsma, and Irene Lie. 2020. "Tools to Support Self-Care Monitoring at Home: Perspectives of Patients with Heart Failure" International Journal of Environmental Research and Public Health 17, no. 23: 8916. https://doi.org/10.3390/ijerph17238916
APA StyleAamodt, I. T., Strömberg, A., Hellesø, R., Jaarsma, T., & Lie, I. (2020). Tools to Support Self-Care Monitoring at Home: Perspectives of Patients with Heart Failure. International Journal of Environmental Research and Public Health, 17(23), 8916. https://doi.org/10.3390/ijerph17238916







