The EuRRECa Project as a Model for Data Access and Governance Policies for Rare Disease Registries That Collect Clinical Outcomes
Abstract
1. Introduction
2. Principles of Data Governance
3. EuRRECa Project
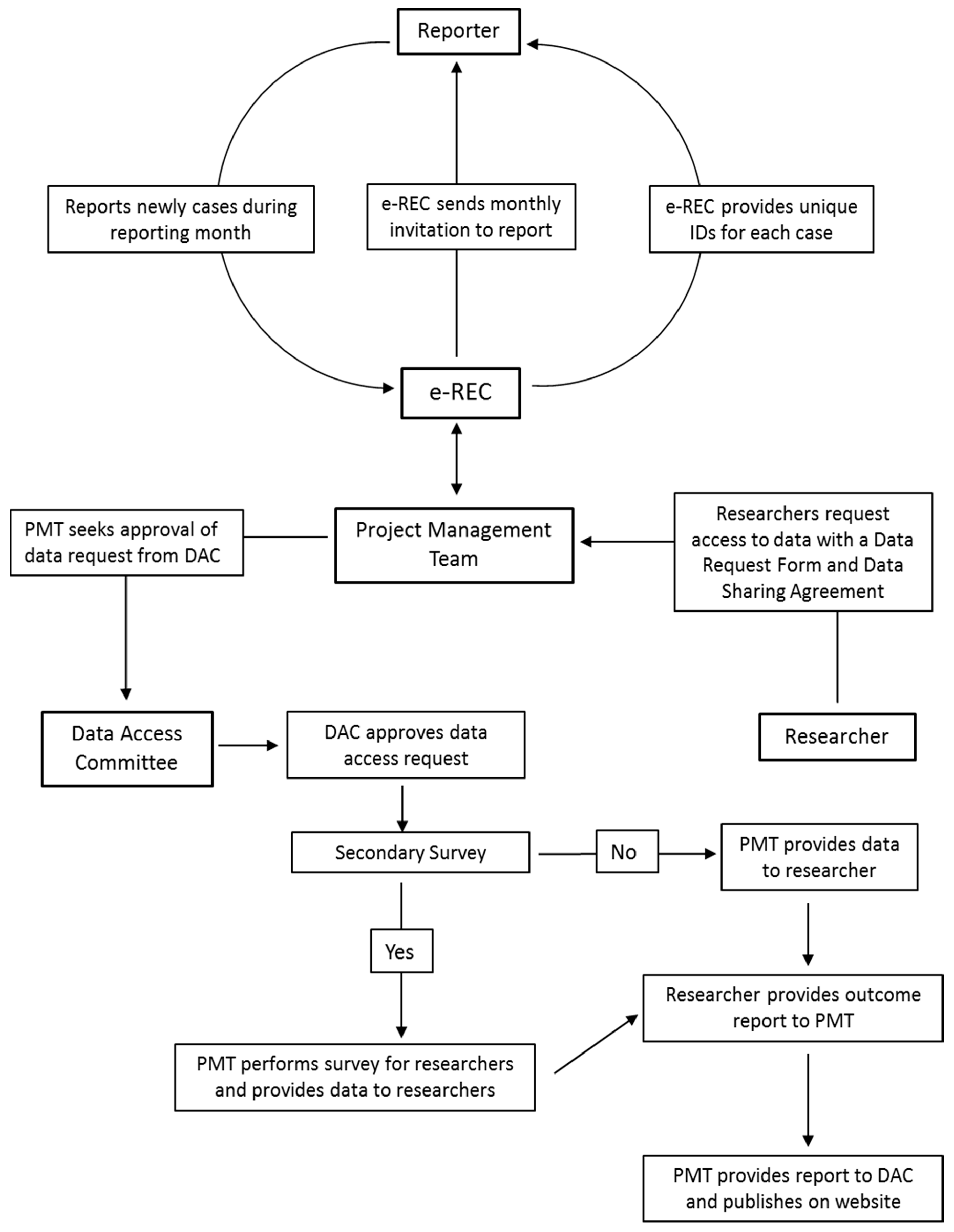
3.1. Platform for e-Reporting of Rare Conditions (e-REC)
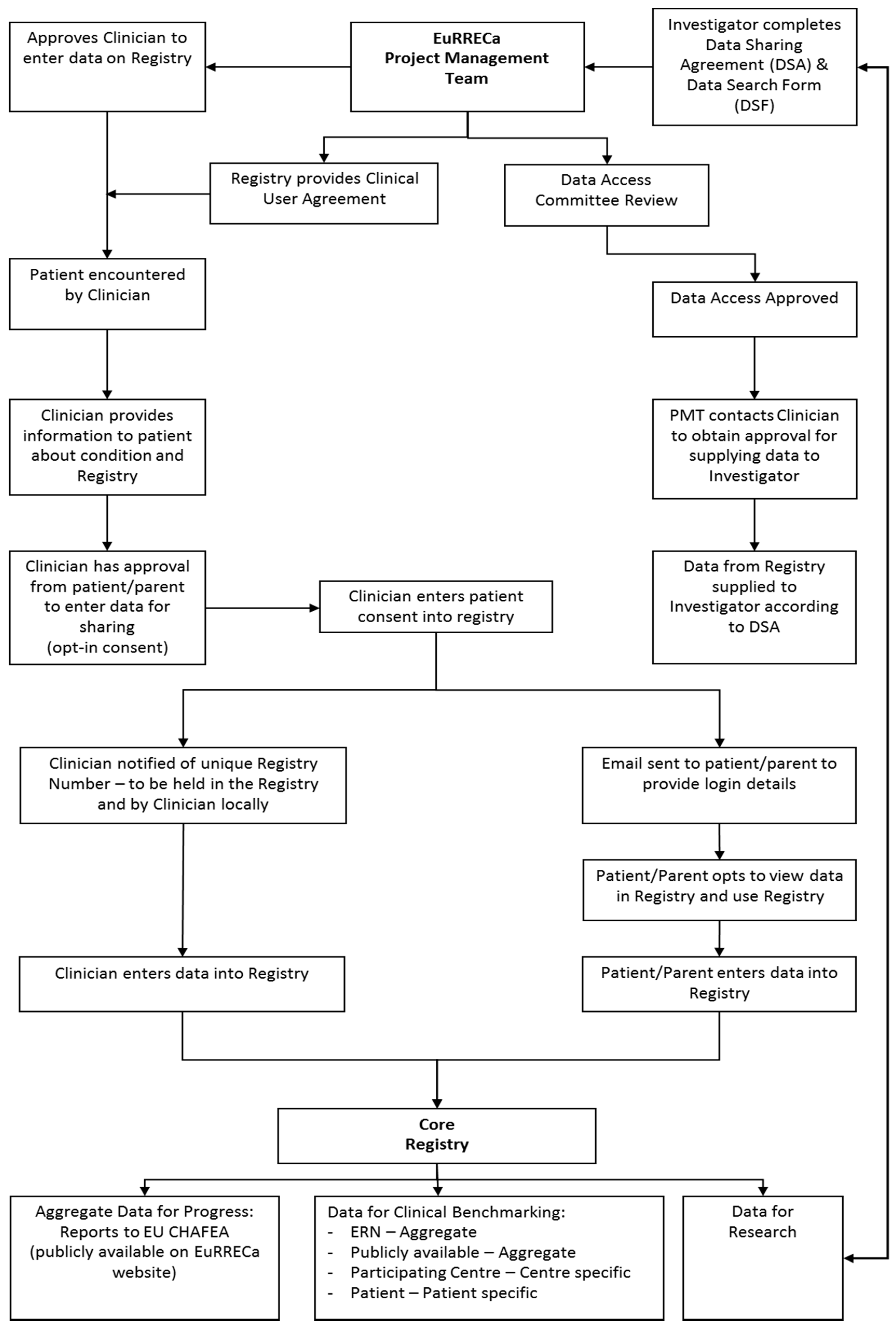
3.2. Core Registry
4. Data Access
4.1. EuRRECa Data Access Committee
4.2. Data Access Policy
4.3. Stakeholders Accessing Data
4.4. Data Ownership
4.5. Ethics
4.6. Procedures for Obtaining Data
4.7. Quality Assurance
4.8. Sustainability
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on Orphan Medicinal Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32000R0141 (accessed on 26 August 2020).
- EURCERD Core Recommendations on Rare Disease Patient Registration and Data Collection. Available online: http://www.eucerd.eu/wp-content/uploads/2013/06/EUCERD_Recommendations_RDRegistryDataCollection_adopted.pdf (accessed on 2 August 2020).
- Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the Application of Patients’ Rights in Cross-Border Healthcare. Off. J. Eur. Union 2011, L88, 45–65.
- Orphanet Report Series-Rare Disease Registries in Europe—May 2019. Available online: https://www.orpha.net/orphacom/cahiers/docs/GB/Registries.pdf (accessed on 23 August 2020).
- Kodra, Y.; Weinback, J.; Posada-de-la-Paz, M.; Coi, A.; Lemonnier, S.L.; van Enckvort, D.; Roos, M.; Jacobson, A.; Cornet, R.; Ahmed, S.F.; et al. Recommendations for improving the quality of rare disease registries. Int. J. Environ. Res. Public Health 2018, 15, 1644. [Google Scholar] [CrossRef] [PubMed]
- International Rare Disease Research Consortium. Available online: http://wwwirdirc.org (accessed on 26 August 2020).
- Orphanet: The Portal for Rare Diseases and Orphan Drugs. Available online: https://www.orpha.net/consor/cgi-bin/index.ph (accessed on 26 August 2020).
- RD Connect. Available online: http://catalogue.rd-connect.eu/ (accessed on 26 August 2020).
- Ali, S.R.; Bryce, J.; Cools, M.; Korbonits, M.; Beun, J.G.; Taruscio, D.; Danne, T.; Dattani, M.; Dekkers, O.M.; Linglart, A.; et al. The current landscape of European registries for rare endocrine conditions. Eur. J. Endocrinol. 2019, 180, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95/46/EC (General Data Protection Regulation) (Text with EEA Relevance). Available online: http://eur-lex.europa.eu/eli/reg/2016/679/oj (accessed on 25 August 2020).
- Coi, A.; Santoro, M.; Villaverde-Hueso, A.; Di Paola, M.L.; Gainotti, S.; Taruscio, D.; De La Paz, M.P.; Bianchi, F. The quality of rare disease registries: Evaluation and characterization. Public Health Genom. 2016, 19, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Cheah, P.Y.; Piasecki, J. Data Access Committees. BMC Med. Ethics 2020, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- European Registries for Rare Endocrine Conditions (EuRRECa), COVID-19 Surveillance. Available online: https://eurreca.net/covid-19-captured-in-e-rec/ (accessed on 31 August 2020).
- Brasil, S.; Pascoal, C.; Francisco, R.; Dos Reis Ferreira, V.; Videira, P.A.; Valadão, A.G. Artificial Intelligence (AI) in Rare Diseases: Is the Future Brighter? Genes 2019, 10, 978. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.R.; Bryce, J.; Tan, L.E.; Hiort, O.; Pereira, A.M.; van den Akker, E.L.T.; Appelman-Dijkstra, N.M.; Bertherat, J.; Cools, M.; Dekkers, O.M.; et al. The EuRRECa Project as a Model for Data Access and Governance Policies for Rare Disease Registries That Collect Clinical Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 8743. https://doi.org/10.3390/ijerph17238743
Ali SR, Bryce J, Tan LE, Hiort O, Pereira AM, van den Akker ELT, Appelman-Dijkstra NM, Bertherat J, Cools M, Dekkers OM, et al. The EuRRECa Project as a Model for Data Access and Governance Policies for Rare Disease Registries That Collect Clinical Outcomes. International Journal of Environmental Research and Public Health. 2020; 17(23):8743. https://doi.org/10.3390/ijerph17238743
Chicago/Turabian StyleAli, Salma R., Jillian Bryce, Li En Tan, Olaf Hiort, Alberto M. Pereira, Erica L. T. van den Akker, Natasha M. Appelman-Dijkstra, Jerome Bertherat, Martine Cools, Olaf M. Dekkers, and et al. 2020. "The EuRRECa Project as a Model for Data Access and Governance Policies for Rare Disease Registries That Collect Clinical Outcomes" International Journal of Environmental Research and Public Health 17, no. 23: 8743. https://doi.org/10.3390/ijerph17238743
APA StyleAli, S. R., Bryce, J., Tan, L. E., Hiort, O., Pereira, A. M., van den Akker, E. L. T., Appelman-Dijkstra, N. M., Bertherat, J., Cools, M., Dekkers, O. M., Kodra, Y., Persani, L., Smyth, A., Smythe, C., Taruscio, D., & Ahmed, S. F. (2020). The EuRRECa Project as a Model for Data Access and Governance Policies for Rare Disease Registries That Collect Clinical Outcomes. International Journal of Environmental Research and Public Health, 17(23), 8743. https://doi.org/10.3390/ijerph17238743




