Clinical Characteristics and Survival Analysis in a Small Sample of Older COVID-19 Patients with Defined 60-Day Outcome
Abstract
1. Introduction
2. Method
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Henri, H.; Kluge, P. Statement—Older People Are at Highest Risk from COVID-19, but All Must Act to Prevent Community Spread. Available online: http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/statements/statement-older-people-are-at-highest-risk-from-covid-19,-but-all-must-act-to-prevent-community-spread (accessed on 30 July 2020).
- Bialek, S.; Boundy, E.; Bowen, V.; Chow, N.; Cohn, A.; Dowling, N.; Ellington, S.; Gierke, R.; Hall, A.; MacNeil, J.; et al. Severe Outcomes among Patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb. Mortal. Wkly Rep. 2020, 69, 343–346. [Google Scholar] [CrossRef]
- Du, R.-H.; Liang, L.-R.; Yang, C.-Q.; Wang, W.; Cao, T.-Z.; Li, M.; Guo, G.-Y.; Du, J.; Zheng, C.-L.; Zhu, Q.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A.; Axfors, C.; Contopoulos-Ioannidis, D.G. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ. Res. 2020, 188, 109890. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Y.; Lin, R.; Han, K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G.; Goronzy, J.J.; Grubeck-Loebenstein, B.; Lambert, P.-H.; Mrkvan, T.; Stoddard, J.J.; Doherty, T.M. Fighting against a protean enemy: Immunosenescence, vaccines, and healthy aging. NPJ Aging Mech. Dis. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar] [CrossRef]
- McMichael, T.M.; Currie, D.W.; Clark, S.; Pogosjans, S.; Kay, M.; Schwartz, N.G.; Lewis, J.; Baer, A.; Kawakami, V.; Lukoff, M.D.; et al. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N. Eng. J. Med. 2020, 382, 2005–2011. [Google Scholar] [CrossRef]
- Guan, W.; Liang, W.; Zhao, Y.; Liang, H.; Chen, Z.-S.; Li, Y.; Liu, X.; Chen, R.; Tang, C.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur. Respir. J. 2020, 2000547. [Google Scholar] [CrossRef]
- Sun, H.; Ning, R.; Tao, Y.; Yu, C.; Deng, X.; Zhao, C.; Meng, S.; Tang, F.; Xu, D. Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: A retrospective study. J. Am. Geriatr. Soc. 2020. [Google Scholar] [CrossRef]
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.G.T.; Fu, H.; et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Gémes, K.; Talbäck, M.; Modig, K.; Ahlbom, A.; Berglund, A.; Feychting, M.; Matthews, A.A. Burden and prevalence of prognostic factors for severe COVID-19 in Sweden. Eur. J. Epidemiol. 2020, 35, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available online: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report (accessed on 27 July 2020).
- Decreased, G. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef]
- Bradburn, M.J.; Clark, T.G.; Love, S.B.; Altman, D.G. Survival Analysis Part II: Multivariate data analysis—An introduction to concepts and methods. Br. J. Cancer 2003, 89, 431–436. [Google Scholar] [CrossRef]
- Beloosesky, Y.; Weiss, A.; Hershkovitz, A.; Grinblat, J. Atypical illness presentation in the elderly. IMAJ 2000, 2, 540–543. [Google Scholar]
- Azarpazhooh, M.R.; Amiri, A.; Morovatdar, N.; Steinwender, S.; Rezaei Ardani, A.; Yassi, N.; Biller, J.; Stranges, S.; Tokazebani Belasi, M.; Neya, S.K.; et al. Correlations between COVID-19 and burden of dementia: An ecological study and review of literature. J. Neurol. Sci. 2020, 416, 117013. [Google Scholar] [CrossRef]
- Cipriani, G.; Danti, S.; Nuti, A.; Carlesi, C.; Lucetti, C.; Di Fiorino, M. A complication of coronavirus disease 2019: Delirium. Acta Neurol. Belgica 2020, 1–6. [Google Scholar] [CrossRef]
- Chen, T.; Dai, Z.; Mo, P.; Li, X.; Ma, Z.; Song, S.; Chen, X.; Luo, M.; Liang, K.; Gao, S.; et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): A single-centered, retrospective study. J. Gerontol. Ser. A 2020. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, S.; He, Y.; Zuo, Q.; Liu, D.; Xiao, M.; Fan, J.; Li, X. COVID-19 Is Distinct from SARS-CoV-2-Negative Community-Acquired Pneumonia. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 1–2. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Mohiddin, S.A.; Dimarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Flack, J.M.; Ito, S.; Muntner, P.; Webb, R.C. Hypertension and COVID-19. Am. J. Hypertens. 2020, 33, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Pio-Abreu, A.; Lopes, R.D.; Bortolotto, L.A. Is Hypertension a Real Risk Factor for Poor Prognosis in the COVID-19 Pandemic? Curr. Hypertens. Rep. 2020, 22. [Google Scholar] [CrossRef] [PubMed]
- Neumann-Podczaska, A.; Al-Saad, S.R.; Karbowski, L.M.; Chojnicki, M.; Tobis, S.; Wieczorowska-Tobis, K. COVID 19—Clinical Picture in the Elderly Population: A Qualitative Systematic Review. Aging Dis. 2020, 11, 988. [Google Scholar] [CrossRef]
- Kumar, A.; Arora, A.; Sharma, P.; Anikhindi, S.A.; Bansal, N.; Singla, V.; Khare, S.; Srivastava, A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabet. Metab. Syndr. 2020, 14, 535–545. [Google Scholar] [CrossRef]
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin. Pract. 2020, 10. [Google Scholar] [CrossRef]
- Hewitt, J.; Carter, B.; Vilches-Moraga, A.; Quinn, T.J.; Braude, P.; Verduri, A.; Pearce, L.; Stechman, M.; Short, R.; Price, A.; et al. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. Lancet Public Health 2020. [Google Scholar] [CrossRef]
- Leung, P.-M.; Ejupi, A.; van Schooten, K.S.; Aziz, O.; Feldman, F.; Mackey, D.C.; Ashe, M.C.; Robinovitch, S.N. Association between Sedentary Behaviour and Physical, Cognitive, and Psychosocial Status among Older Adults in Assisted Living. BioMed Res. Int. 2017, 2017, 9160504. [Google Scholar] [CrossRef]
- WHO. Physical Inactivity a Leading Cause of Disease and Disability, Warns WHO. 2020. Available online: https://www.who.int/mediacentre/news/releases/release23/en/#:~:text=Sedentary%20lifestyles%20increase%20all%20causes (accessed on 1 July 2020).
- Costa, F.F.; Rosário, W.R.; Ribeiro Farias, A.C.; de Souza, R.G.; Duarte Gondim, R.S.; Barroso, W.A. Metabolic syndrome and COVID-19: An update on the associated comorbidities and proposed therapies. Diabet. Metab. Syndr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Bigley, A.B.; Spielmann, G.; LaVoy, E.C.P.; Simpson, R.J. Can exercise-related improvements in immunity influence cancer prevention and prognosis in the elderly? Maturitas 2013, 76, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, B.; Bergland, A.; Rydwik, E. The Importance of Physical Activity Exercise among Older People. BioMed Res. Int. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kanach, F.A.; Pastva, A.M.; Hall, K.S.; Pavon, J.M.; Morey, M.C. Effects of Structured Exercise Interventions for Older Adults Hospitalized with Acute Medical Illness: A Systematic Review. J. Aging Phys. Act. 2018, 26, 284–303. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Tanimoto, Y.; Imbe, A.; Inaba, Y.; Sakai, S.; Shishikura, K.; Tanimoto, K.; Hanafusa, T. Association between Functional Capacity Decline and Nutritional Status Based on the Nutrition Screening Initiative Checklist: A 2-Year Cohort Study of Japanese Community-Dwelling Elderly. PLoS ONE 2016, 11, e0166037. [Google Scholar] [CrossRef]
| Total (n = 50) | Survivors (n = 30) | Non-Survivors (n = 20) | p-Value | |
|---|---|---|---|---|
| Age | 74.8 ± 9.4 (72.5; 61–99) | 72.4 ± 8.5 (70; 61–89) | 78.3 ± 9.7 (78; 66–99) | <0.05 |
| Gender | ||||
| Male | 35 (70.0%) | 23 (76.7%) | 12 (60.0%) | 0.23 |
| Female | 15 (30.0%) | 7 (23.3%) | 8 (40.0%) | |
| Symptoms | ||||
| Fever * | 29 (58.0%) | 20 (66.7%) | 9 (45.0%) | 0.15 |
| Cough | 14 (28.0%) | 9 (30.0%) | 5 (25.0%) | 0.76 |
| Dyspnea | 17 (34.0%) | 10 (33.3%) | 7 (35.0%) | 1.0 |
| Muscular pain | 3 (6.0%) | 1 (3.3%) | 2 (10.0%) | 0.56 |
| Exacerbation of chronic diseases | 10 (20%) | 3 (10%) | 7 (35%) | 0.06 |
| Cognitive impairment | 11 (22%) | 4 (13.3%) | 7 (35%) | 0.09 |
| Comorbidities | ||||
| Cardiovascular diseases | 40 (80.0%) | 23 (76.6%) | 17 (85.0%) | 0.72 |
| Hypertension | 30 (60.0%) | 18 (60.0%) | 12 (60.0%) | 1.0 |
| Heart diseases | 26 (52.0%) | 11 (36.7%) | 15 (75.0%) | <0.05 |
| Diabetes | 19 (38.0%) | 8 (26.7%) | 11 (55.0%) | 0.07 |
| Chronic Obstructive Pulmonary Disease | 7 (14.0%) | 5 (16.7%) | 2 (10.0%) | 0.69 |
| Renal Dysfunction | 8 (16.0%) | 3 (10.0%) | 5 (25.0%) | 0.24 |
| Liver Dysfunction | 3 (6.0%) | 1 (3.3%) | 2 (10%) | 0.56 |
| Malignancy | 6 (12.0%) | 4 (13.3%) | 2 (10.0%) | 1.0 |
| Dementia | 2 (4.0%) | 0 (0.0%) | 2 (10.0%) | 0.16 |
| Stroke | 11(22.0%) | 6 (20.0%) | 5 (25.0%) | 0.74 |
| Lab Tests | Normal Range | Total (n = 50) | Survivors (n = 30) | Non-Survivors (n = 20) | p-Value |
|---|---|---|---|---|---|
| White Blood Cells (WBC) (×103/µL) | 4.0–11.0 | 8.4 ± 4.5 (7.3; 2.3–29) n = 50 | 7.1 ± 2.7 (7; 2.7–12.5) n = 30 | 10.2 ± 6.0 (8.8; 2.3–29) n = 20 | <0.05 |
| Red Blood Cells (RBC) (×106/µL) | 4.50–6.10 | 4.2 ± 0.7 (4.2; 1.9–5.6) n = 50 | 4.4 ± 0.6 (4.4; 3.2–5.6) n = 30 | 3.9 ± 0.7 (4; 1.9–4.8) n = 20 | <0.01 |
| Hemoglobin (g/dL) | 14.0–18.0 | 12.3 ± 2.1 (12.4; 6.6–17.3) n = 50 | 13.1 ± 1.9 (13.1; 9.1–17.3) n = 30 | 11.1 ± 1.8 (11.2; 6.6–14.1) n = 20 | <0.001 |
| Hematocrit (%) | 38.0–55.0 | 35.9 ± 5.9 (35.1; 18.5–54.3) n = 49 | 38.0 ± 5.4 (36.8; 28.5–54.3) n = 29 | 32.9 ± 5.4 (33.4; 18.5–40.2) n = 20 | <0.01 |
| Platelets (×103/µL) | 30–440 | 240.8 ± 108.3 (223.5; 55–502) n = 50 | 238.4 ± 101.8 (223.5; 98–502) n = 30 | 244.4 ± 120.1 (225; 55–477) n = 20 | 0.91 |
| Lymphocytes (×103/µL) | 1.0–4.0 | 1.3 ± 0.6 (1.2; 0–2.9) n = 33 | 1.3 ± 0.7 (1.2; 0–2.9) n = 23 | 1.3 ± 0.4 (1.2; 0.8–2.2) n = 10 | 0.74 |
| Neutrophils (×103/µL) | 1.5–7.7 | 5.1 ± 3.2 (4.6; 0.1–14.3) n = 33 | 4.4 ± 2.1 (4; 1.4–9.5) n = 23 | 6.7 ± 4.6 (6.8; 0.1–14.3) n = 10 | 0.12 |
| Urea (mmol/L) | 3.6–7.1 | 9.0 ± 5.1 (8; 3.1–26.2) n = 33 | 7.6 ± 3.8 (6; 3.1–15.4) n = 18 | 10.7 ± 6.1 (9.7; 3.2–26.2) n = 15 | 0.16 |
| Lactate Dehydrogenase (U/L) | 125–220 | 368.8 ± 145.6 (352.5; 141–660) n = 36 | 309.4 ± 110.1 (284.5; 141–560) n = 22 | 462.1 ± 14.9 (430.5; 193–660) n = 14 | <0.01 |
| CRP (mg/L) | <5.0 | 93.2 ± 86.1 (66; 1–384) n = 50 | 75.3 ± 75.3 (45.8; 1–290.5) n = 30 | 120.0 ± 96.0 (78.5; 33.2–384) n = 20 | <0.05 |
| PCT (ng/mL) | <0.10 | 1.0 ± 4.8 (0.1; 0–29.8) n = 38 | 0.1 ± 0.1 (0.1; 0–0.4) n = 19 | 1.8 ± 6.8 (0.3; 0–29.8) n = 19 | <0.01 |
| IL-6 (pg/mL) | 1.5–7.0 | 128.2 ± 273.1 (54.7; 1.5–1592) n = 36 | 63.6 ± 113.1 (29.7; 1.5–500) n = 20 | 208.9 ± 381.2 (86.9; 6–1592) n = 16 | <0.01 |
| Assessed Variables | In-Hospital Survival | 60-Day Survival | ||
|---|---|---|---|---|
| Univariate Model | Multivariate Model | Univariate Model | Multivariate Model | |
| Age [≥75 yrs vs. 60–74 yrs] | 1.73 (0.69–4.31); p = 0.24 | - | 2.11 (0.86–5.18); p = 0.10 | - |
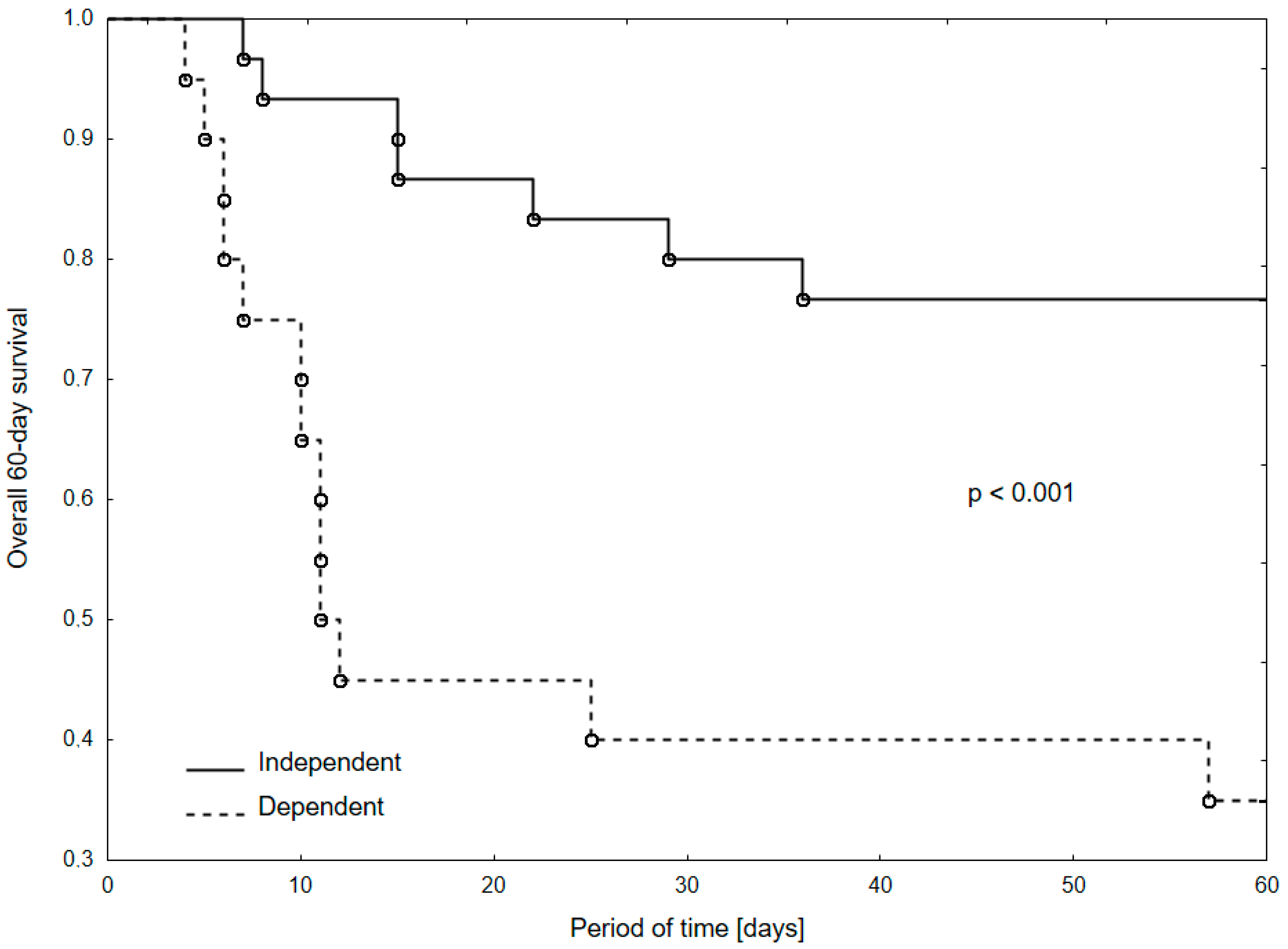
| Functional Capacity [Dependent vs. Independent] | 2.97 (1.16–7.59); p = 0.02 | 2.36 (0.90–6.23); p = 0.08 | 4.18 (1.66–10.54); p = 0.002 | 3.34 (1.29–8.63); p = 0.01 |
| Diabetes [Yes vs. No] | 1.63 (0.66–4.03); p = 0.29 | - | 2.35 (0.97–5.67); p = 0.06 | - |
| Heart Disease [Yes vs. No] | 3,05 (1.10–8.47); p = 0.03 | 2.42 (0.84–6.94); p =0.10 | 3.49 (1.27–9.63); p = 0.02 | 2.61 (0.92–7.39); p = 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann-Podczaska, A.; Chojnicki, M.; Karbowski, L.M.; Al-Saad, S.R.; Hashmi, A.A.; Chudek, J.; Tobis, S.; Kropinska, S.; Mozer-Lisewska, I.; Suwalska, A.; et al. Clinical Characteristics and Survival Analysis in a Small Sample of Older COVID-19 Patients with Defined 60-Day Outcome. Int. J. Environ. Res. Public Health 2020, 17, 8362. https://doi.org/10.3390/ijerph17228362
Neumann-Podczaska A, Chojnicki M, Karbowski LM, Al-Saad SR, Hashmi AA, Chudek J, Tobis S, Kropinska S, Mozer-Lisewska I, Suwalska A, et al. Clinical Characteristics and Survival Analysis in a Small Sample of Older COVID-19 Patients with Defined 60-Day Outcome. International Journal of Environmental Research and Public Health. 2020; 17(22):8362. https://doi.org/10.3390/ijerph17228362
Chicago/Turabian StyleNeumann-Podczaska, Agnieszka, Michal Chojnicki, Lukasz M. Karbowski, Salwan R. Al-Saad, Abbas A. Hashmi, Jerzy Chudek, Slawomir Tobis, Sylwia Kropinska, Iwona Mozer-Lisewska, Aleksandra Suwalska, and et al. 2020. "Clinical Characteristics and Survival Analysis in a Small Sample of Older COVID-19 Patients with Defined 60-Day Outcome" International Journal of Environmental Research and Public Health 17, no. 22: 8362. https://doi.org/10.3390/ijerph17228362
APA StyleNeumann-Podczaska, A., Chojnicki, M., Karbowski, L. M., Al-Saad, S. R., Hashmi, A. A., Chudek, J., Tobis, S., Kropinska, S., Mozer-Lisewska, I., Suwalska, A., Tykarski, A., & Wieczorowska-Tobis, K. (2020). Clinical Characteristics and Survival Analysis in a Small Sample of Older COVID-19 Patients with Defined 60-Day Outcome. International Journal of Environmental Research and Public Health, 17(22), 8362. https://doi.org/10.3390/ijerph17228362






