Evaluation of the Feasibility of a Two-Week Course of Aquatic Therapy and Thalassotherapy in a Mild Post-Stroke Population
Abstract
1. Introduction
2. Materials and Methods
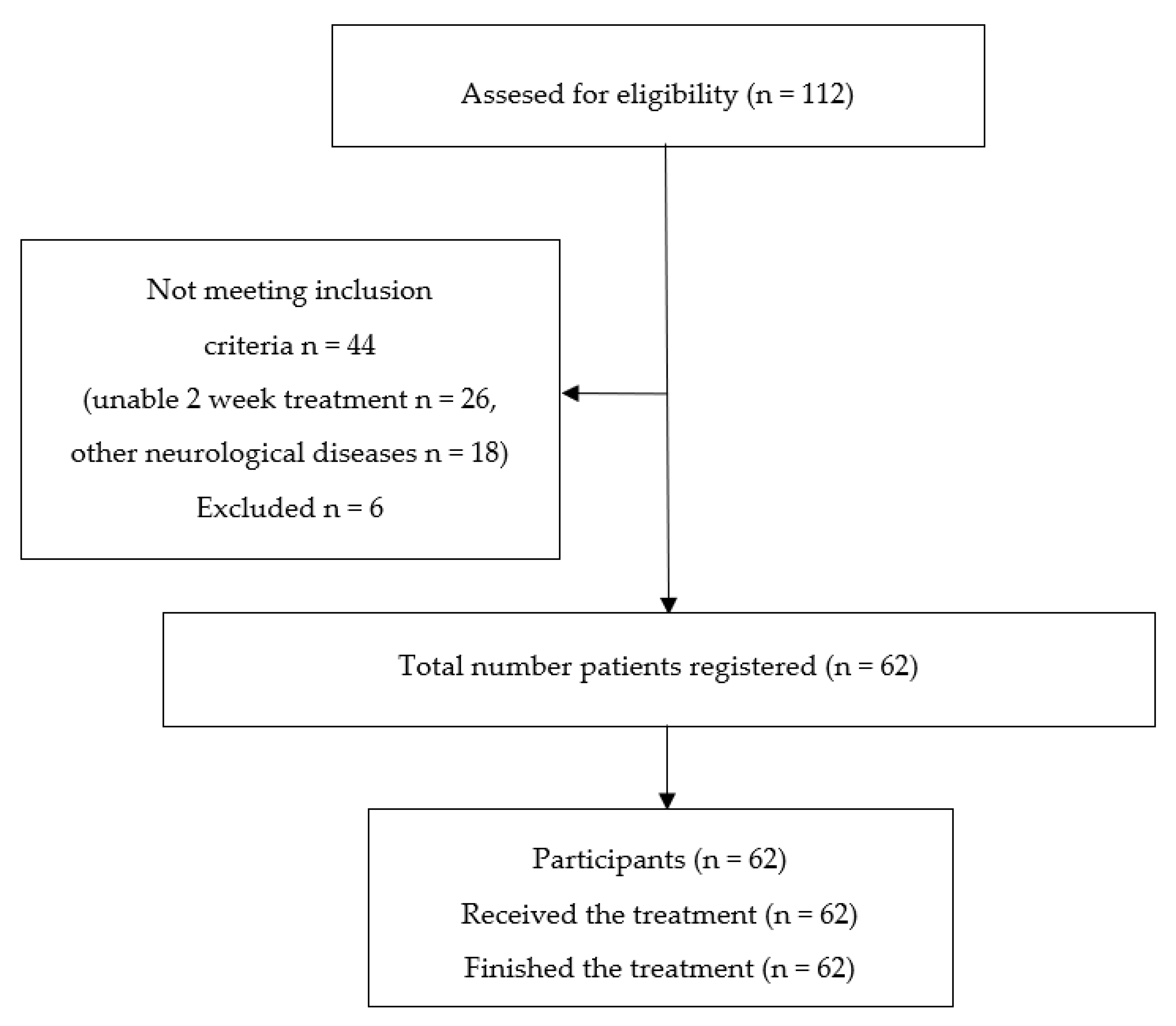
2.1. Participants
2.2. Methods
2.3. Measurements
2.4. Interventions
2.4.1. Thalassotherapy
- Seawater
- Sea climate
- Sea peloid
2.4.2. Aquatic Therapy (Halliwick)
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Balance
4.2. Mobility
4.3. Pain
4.4. Quality of Life
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bejot, Y.; Daubail, B.; Giroud, M. Epidemiology of stroke and transient ischemic attacks: Current knowledge and perspectives. Rev. Neurol. (Paris) 2016, 172, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Fundación Weber. El Atlas del Ictus España 2019. Available online: http://weber.org.es/wp-content/uploads/2019/10/Atlas-nacional-versio%CC%81n-web_low.pdf (accessed on 24 July 2020).
- Kim, J.; Thayabaranathan, T.; Donnan, G.A.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.; Norrving, B.; Owolabi, M.; Pandian, J.; et al. Global Stroke Statistics 2019. Int. J. Stroke 2020, 15, 819–838. [Google Scholar] [CrossRef]
- Salamon, L.A.; Victory, M.; Bobay, K. Identification of patients at risk for falls in an inpatient rehabilitation program. Rehabil. Nurs. 2012, 37, 292–297. [Google Scholar] [CrossRef]
- Pollock, A.; Baer, G.; Campbell, P.; Choo, P.L.; Forster, A.; Morris, J.; Pomeroy, V.M.; Langhorne, P. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst. Rev. 2014, 2014, CD001920. [Google Scholar] [CrossRef] [PubMed]
- Lucchetta, M.C.; Monaco, G.; Valenzi, V.I.; Russo, M.V.; Campanella, J.; Nocchi, S.; Mennuni, G.; Fraioli, A. Le basi storico-scientifiche della talassoterapia: Stato dell’arte. Clin. Ter. 2007, 158, 533–541. [Google Scholar]
- Maraver, F.; Michán, A.; Morer, C.; Aguilera, L. Is thalassotherapy simply a type of climatotherapy? Int. J. Biometeorol. 2011, 55, 107–108. [Google Scholar] [CrossRef]
- Alonso, M. Principios básicos y fundamentos de la terapia acuática. In Terapia Acuática. Abordajes Desde la Fisioterapia y la Terapia Ocupacional; Gueita, J., Alonso, M., Fernandez, C., Eds.; Elservier: Barcelona, Spain, 2015; pp. 3–15. ISBN 978-84-9022-810-4. [Google Scholar]
- Becker, B.E. Aquatic therapy: Scientific foundations and clinical rehabilitation applications. Am. J. Phys. Med. Rehabil. 2009, 1, 859–872. [Google Scholar] [CrossRef]
- Becker, B.E. Aquatic Therapy in Contemporary Neurorehabilitation: An Update. PM R 2020. [Google Scholar] [CrossRef] [PubMed]
- Giuriati, S.; Servadio, A.; Temperoni, G.; Curcio, A.; Valente, D.; Galeoto, G. The effect of aquatic physical therapy in patients with stroke: A systematic review and meta-analysis. Top. Stroke Rehabil. 2020. [Google Scholar] [CrossRef] [PubMed]
- Morer, C.; Boestad, C.; Zuluaga, P.; Alvarez-Badillo, A.; Maraver, F. Efectos de un programa intensivo de talasoterapia y terapia acuática en pacientes con ictus. Estudio piloto. Rev. Neurol. 2017, 65, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Bonsignori, F. La Talassoterapia. Cure e Benessere Alle Terme Marine e al Mare; ETS: Pisa, Italy, 2011; ISBN 978-88-4672-912-5. [Google Scholar]
- Morer, C. Talasoterapia. Bol. Soc. Esp. Hidrol. Med. 2016, 31, 119–146. [Google Scholar] [CrossRef]
- Kollen, B.; van de Port, I.; Lindeman, E.; Twisk, J.; Kwakkel, G. Predicting improvement in gait after stroke: A longitudinal prospective study. Stroke 2005, 36, 2676–2680. [Google Scholar] [CrossRef]
- Lord, S.E.; Rochester, L. Measurement of community ambulation after stroke: Current status and future developments. Stroke 2005, 36, 1457–1461. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, P.; Molina-Rueda, F.; Cuesta-Gomez, A.; Carratala-Tejada, M.; Miangolarra-Page, J.C. Instrumental gait analysis in stroke patients. Rev. Neurol. 2016, 63, 433–439. [Google Scholar]
- Downie, W.W.; Leatham, P.A.; Rhind, V.M.; Wright, V.; Branco, J.; Anderson, J.A. Studies with pain rating scales. Ann. Rheum. Dis. 1978, 37, 378–381. [Google Scholar] [CrossRef]
- WHO-5 Webside. WHO-5. Available online: https://www.psykiatri-regionh.dk/who-5/Pages/default.aspx (accessed on 25 July 2020).
- Badia, X.; Roset, M.; Montserrat, S.; Herdman, M.; Segura, A. The Spanish version of EuroQol: A description and its applications. European Quality of Life scale. Med. Clin. (Barc.) 1999, 112 (Suppl. 1), 79–85. [Google Scholar] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 978-16-2576-240-5. [Google Scholar]
- Clima España–Climate-Data.org: San Pedro del Pinatar Clima (España). Available online: https://es.climate-data.org/europe/espana/region-de-murcia/san-pedro-del-pinatar-31364/ (accessed on 25 July 2020).
- Gomes, C.S.F.; Carretero, M.I.; Pozo, M.; Maraver, F.; Cantista, P.; Armijo, F.; Legido, J.L.; Teixeira, F.; Rautureau, M.; Delgado, R. Peloids and pelotherapy: Historical evolution, classification and glossary. Appl. Clay Sci. 2013, 75–76, 28–38. [Google Scholar] [CrossRef]
- Carretero, M.I. Clays in pelotherapy. A review. Part II: Organic compounds, microbiology and medical applications. Appl. Clay Sci. 2020, 189, 105531. [Google Scholar] [CrossRef]
- Armijo, F.; Maraver, F.; Carretero, M.; Pozo, M.; Ramos, M.; Fernandez-Torán, M.; Corvillo, I. The water effect on instrumental hardness and adhesiveness of clay mixtures for pelotherapy. Appl. Clay Sci. 2015, 114, 395–401. [Google Scholar] [CrossRef]
- Armijo, F.; Maraver, F.; Pozo, M.; Carretero, M.; Armijo, O.; Fernández-Torán, M.; Fernández-González, M.; Corvillo, I. Thermal behaviour of clays and clay-water mixtures for pelotherapy. Appl. Clay Sci. 2016, 126, 50–56. [Google Scholar] [CrossRef]
- Lambeck, J.F.; Gamper, U.N. The Halliwick Concept. In Comprehensive Aquatic Therapy, 3rd Ed.; Becker, B.E., Cole, A.J., Eds.; Washington State University Publishing: Washington, DC, USA, 2010; pp. 77–107. ISBN 978-06-1536-567-1. [Google Scholar]
- Mehrholz, J.; Pohl, M.; Elsner, B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst. Rev. 2014, 2014, CD002840. [Google Scholar] [CrossRef]
- Jette, D.U.; Latham, N.K.; Smout, R.J.; Gassaway, J.; Slavin, M.D.; Horn, S.D. Physical therapy interventions for patients with stroke in inpatient rehabilitation facilities. Phys. Ther. 2005, 85, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Azurdia, D.; Jeng, B.; Jung, T. Influence of water depth on energy expenditure during aquatic walking in people post stroke. Physiother. Res. Int. 2018, 23, e1717. [Google Scholar] [CrossRef]
- Gueita-Rodriguez, J.; Hoyas-Avila, S.; Palacios-Cena, D.; Molina-Rueda, F. Efectos de la inmersion vertical en el agua sobre el sistema nervioso: Revision sistematica. Rev. Neurol. 2019, 68, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Pugh, C.J.; Sprung, V.S.; Ono, K.; Spence, A.L.; Thijssen, D.H.; Carter, H.H.; Green, D.J. The effect of water immersion during exercise on cerebral blood flow. Med. Sci. Sports Exerc. 2015, 47, 299–306. [Google Scholar] [CrossRef]
- Nualnim, N.; Barnes, J.N.; Tarumi, T.; Renzi, C.P.; Tanaka, H. Comparison of central artery elasticity in swimmers, runners, and the sedentary. Am. J. Cardiol. 2011, 107, 783–787. [Google Scholar] [CrossRef]
- Sato, D.; Onishi, H.; Yamashiro, K.; Iwabe, T.; Shimoyama, Y.; Maruyama, A. Water immersion to the femur level affects cerebral cortical activity in humans: Functional near-infrared spectroscopy study. Brain Topogr. 2012, 25, 220–227. [Google Scholar] [CrossRef]
- Sato, D.; Yamashiro, K.; Onishi, H.; Shimoyama, Y.; Yoshida, T.; Maruyama, A. The effect of water immersion on short-latency somatosensory evoked potentials in human. BMC Neurosci. 2012, 13, 13. [Google Scholar] [CrossRef]
- Mehrholz, J.; Kugler, J.; Pohl, M. Water-based exercises for improving activities of daily living after stroke. Cochrane Database Syst. Rev. 2011, 2011, CD008186. [Google Scholar] [CrossRef]
- Stier-Jarmer, M.; Kus, S.; Frisch, D.; Sabariego, C.; Schuh, A. Health resort medicine in non-musculoskeletal disorders: Is there evidence of its effectiveness? Int. J. Biometeorol. 2015, 59, 1523–1544. [Google Scholar] [CrossRef]
- Marinho-Buzelli, A.R.; Bonnyman, A.M.; Verrier, M.C. The effects of aquatic therapy on mobility of individuals with neurological diseases: A systematic review. Clin. Rehabil. 2015, 29, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Tripp, F.; Krakow, K. Effects of an aquatic therapy approach (Halliwick-Therapy) on functional mobility in subacute stroke patients: A randomized controlled trial. Clin. Rehabil. 2014, 28, 432–439. [Google Scholar] [CrossRef]
- Zamparo, P.; Pagliaro, P. The energy cost of level walking before and after hydro-kinesi therapy in patients with spastic paresis. Scand. J. Med. Sci. Sports 1998, 8, 222–228. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, R.; Dong, M.; Lin, L. Effects of seawater physical exercise on cerebral and cardiac hemodynamics in patients with cerebral infarction. Chin. J. Clin. Rehabil. 2004, 8, 3006–3007. [Google Scholar]
- Iliescu, A.M.; McIntyre, A.; Wiener, J.; Iruthayarajah, J.; Lee, A.; Caughlin, S.; Teasell, R. Evaluating the effectiveness of aquatic therapy on mobility, balance, and level of functional independence in stroke rehabilitation: A systematic review and meta-analysis. Clin. Rehabil. 2020, 34, 56–68. [Google Scholar] [CrossRef]
- Nascimento, L.R.; Flores, L.C.; de Menezes, K.; Teixeira-Salmela, L.F. Water-based exercises for improving walking speed, balance, and strength after stroke: A systematic review with meta-analyses of randomized trials. Physiotherapy 2020, 107, 100–110. [Google Scholar] [CrossRef]
- Chu, K.S.; Eng, J.J.; Dawson, A.S.; Harris, J.E.; Ozkaplan, A.; Gylfadottir, S. Water-based exercise for cardiovascular fitness in people with chronic stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2004, 85, 870–874. [Google Scholar] [CrossRef]
- Furnari, A.; Calabrò, R.S.; Gervasi, G.; La Fauci-Belponer, F.; Marzo, A.; Berbiglia, F.; Paladina, G.; De Cola, M.C.; Bramanti, P. Is hydrokinesitherapy effective on gait and balance in patients with stroke? A clinical and baropodometric investigation. Brain Inj. 2014, 28, 1109–1114. [Google Scholar] [CrossRef]
- Han, E.Y.; Im, S.H. Effects of a 6-Week Aquatic Treadmill Exercise Program on Cardiorespiratory Fitness and Walking Endurance in Subacute Stroke Patients: A PILOT TRIAL. J. Cardiopulm. Rehabil. Prev. 2018, 38, 314–319. [Google Scholar] [CrossRef] [PubMed]
- la Cruz, S.P.-d. Comparison of Aquatic Therapy vs. Dry Land Therapy to Improve Mobility of Chronic Stroke Patients. Int. J. Environ. Res. Public Health 2020, 17, 4728. [Google Scholar] [CrossRef] [PubMed]
- la Cruz, S.P.-d. Influence of an Aquatic Therapy Program on Perceived Pain, Stress, and Quality of Life in Chronic Stroke Patients: A Randomized Trial. Int. J. Environ. Res. Public Health 2020, 17, 4796. [Google Scholar] [CrossRef] [PubMed]
- Temperoni, G.; Curcio, A.; Iosa, M.; Mangiarotti, M.A.; Morelli, D.; De Angelis, S.; Vergano, S.; Tramontano, M. A Water-Based Sequential Preparatory Approach vs. Conventional Aquatic Training in Stroke Patients: A Randomized Controlled Trial With a 1-Month Follow-Up. Front. Neurol. 2020, 11, 466. [Google Scholar] [CrossRef]
- Carod-Artal, J.; Egido, J.A.; González, J.L.; de Seijas, E.V. Quality of life among stroke survivors evaluated 1 year after stroke: Experience of a stroke unit. Stroke 2000, 31, 2995–3000. [Google Scholar] [CrossRef]
- Gutenbrunner, C.; Bender, T.; Cantista, P.; Karagülle, Z. A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology and climatology. Int. J. Biometeorol. 2010, 54, 495–507. [Google Scholar] [CrossRef]
| Seawater | Jan | April | July | Oct | Mean |
|---|---|---|---|---|---|
| Conductivity to 25 °C (μS·cm−1) | 67,300 | 65,600 | 66,700 | 67,280 | 66,720 |
| pH | 7.4 | 7.5 | 7.5 | 7.6 | 7.5 |
| Li+ (mg/L) | 0.09 | 0.08 | 0.09 | 0.09 | 0.09 |
| Na+ (mg/L) | 11,343 | 11,570 | 11,643 | 11,426 | 11,496 |
| K+ (mg/L) | 472 | 459 | 470 | 444 | 462 |
| Mg2+ (mg/L) | 1405 | 1417 | 1431 | 1331 | 1396 |
| Ca2+ (mg/L) | 562 | 537 | 511 | 548 | 540 |
| F− (mg/L) | 1.5 | 1.5 | 1.4 | 1.5 | 1.5 |
| Cl− (mg/L) | 19,541 | 19,103 | 19,586 | 20,482 | 19,678 |
| Br− (mg/L) | 6.7 | 6.6 | 6.0 | 6.1 | 6.4 |
| HCO3− (mg/L) | 134 | 140 | 134 | 140 | 137 |
| NO3− (mg/L) | 10 | 10 | 10 | 10 | 10 |
| SO42− (mg/L) | 2833 | 2713 | 2837 | 2741 | 2782 |
| Sea climate | Jan | Feb | Mar | April | May | June | July | Aug | Sept | Oct | Nov | Dec | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | |||||||||||||
| Average temp. (°C) | 11.1 | 12 | 14.2 | 16.2 | 19.3 | 23.1 | 25.8 | 26.2 | 23.7 | 19.5 | 15.4 | 12.4 | 18.2 |
| Min. temperature (°C) | 6 | 6.7 | 8.8 | 11 | 13.9 | 17.5 | 19.9 | 20.4 | 18.1 | 14.2 | 10.2 | 7.6 | 12.9 |
| Max temperature (°C) | 16.2 | 17.3 | 19.7 | 21.5 | 24.8 | 28.8 | 31.7 | 32 | 29.4 | 24.8 | 20.6 | 17.3 | 23.7 |
| Pluviometry | |||||||||||||
| Precipitation (mm) | 24 | 19 | 23 | 27 | 25 | 13 | 4 | 7 | 29 | 55 | 38 | 35 | 24.9 |
| Sea peloid | |||||||||||||
| Centesimal composition | Instrumental texture | Thermal properties | |||||||||||
| Water (%) | 59.9 | Hardness (g) | 45 | Specific heat (Cp) | 2.9 | ||||||||
| Solids (%) | 40.1 | Adhesiveness (g s) | 548 | Relaxation time tr (s) | 498 | ||||||||
| Ash (%) | 35.8 | Cohesiveness | 0.96 | Heat amount Q (J) | 16,644 | ||||||||
| Ash/Solids | 0.88 | Adhesiveness/Hardness | 12.10 | Heat flow Φ (J/s) | 33.4 | ||||||||
| Characteristics | Participants |
|---|---|
| Sex | Females 24 (38.7) |
| Males 38 (61.3) | |
| Age (years) | Min. 49 |
| Max. 87 | |
| Mean 69 | |
| Stroke type | Ischemic 47 (75.8) |
| Hemorrhagic 15 (24.2) | |
| Stage | <6 months: 1 (1.6) |
| 6 months–12 months: 11 (17.7) | |
| >1 year: 50 (80.7) | |
| Barthel Scale | Min. 15 |
| Max. 100 | |
| Mean 70.3 | |
| Morse Fall Scale | Min. 20 |
| Max. 90 | |
| Mean 67.7 | |
| Downton Scale | Min. 3 |
| Max. 7 | |
| Mean 4.24 | |
| Rankin Scale | Min. 1 |
| Max. 3 | |
| Mean 1.92 |
| Min. | Max. | Mean | Sd | Median | p-Value | |
|---|---|---|---|---|---|---|
| BBS | ||||||
| Pre | 0.0 | 56.0 | 25.8 | 19.2 | 28.5 | |
| Post | 0.0 | 56.0 | 31.3 | 19.6 | 36.0 | |
| Mean diff. | −2.0 | 23.0 | 5.5 | 5.2 | 5.0 | p < 0.001 * |
| TUG | ||||||
| Pre | 6.0 | 240.0 | 49.2 | 45.8 | 43.0 | |
| Post | 6.0 | 144.0 | 39.7 | 31.3 | 36.0 | |
| Mean diff. | −5.0 | 96.0 | 9.4 | 18.9 | 3.0 | p < 0.001 * |
| T 10-MW | ||||||
| Pre | 6.9 | 240.0 | 43.0 | 47.8 | 26.5 | |
| Post | 5.9 | 165.0 | 38.1 | 36.4 | 21,5 | |
| Mean diff. | −71.0 | 75.0 | 4.8 | 25.2 | 1.0 | p = 0.115 |
| 6-MWT | ||||||
| Pre | 0.0 | 630.0 | 153.4 | 153.9 | 113.0 | |
| Post | 0.0 | 683.0 | 180.0 | 170.6 | 135.0 | |
| Mean diff. | −80.0 | 139.0 | 27.3 | 39.0 | 15.0 | p < 0.001 * |
| VAS | ||||||
| Pre | 0.0 | 10.0 | 3.0 | 3.4 | 0.0 | |
| Post | 0.0 | 9.0 | 1.7 | 2.4 | 0.0 | |
| Mean diff. | −3.0 | 8.0 | 1.2 | 2.1 | 0.0 | p < 0.001 * |
| WHO−5 | ||||||
| Pre | 20.0 | 100.0 | 62.8 | 21.9 | 70.0 | |
| Post | 28.0 | 100.0 | 78.4 | 16.6 | 80.0 | |
| Mean diff. | −4.0 | 48.0 | 15.5 | 15.0 | 12.0 | p < 0.001 * |
| Min. | Max. | Mean | Sd | Median | p-Value | ||
|---|---|---|---|---|---|---|---|
| EQ-VAS | |||||||
| Pre | 0.0 | 91.0 | 57.1 | 19.2 | 60.0 | ||
| Post | 20.0 | 95.0 | 66.1 | 15.4 | 65.0 | ||
| Mean diff. | –25.0 | 46.0 | 8.9 | 12.2 | 9.0 | p < 0.001 * | |
| Post | 0.0 | 1.0 | 2.0 | 3.0 | Total | p-Value | |
| EQ-5D Mobility | |||||||
| Pre | 0.0 | 0 | 0 | 0 | 0 | 0 | |
| 1.0 | 0 | 14 | 0 | 0 | 14 | ||
| 2.0 | 0 | 6 | 38 | 0 | 44 | ||
| 3.0 | 0 | 0 | 1 | 3 | 4 | ||
| Total | 0 | 20 | 39 | 3 | 62 | p = 0.030 ** | |
| EQ-5D Self-care | |||||||
| Pre | 0.0 | 1 | 0 | 0 | 0 | 1 | |
| 1.0 | 0 | 30 | 1 | 0 | 31 | ||
| 2.0 | 0 | 2 | 18 | 3 | 23 | ||
| 3.0 | 0 | 0 | 3 | 4 | 7 | ||
| Total | 1 | 32 | 22 | 7 | 62 | p = 0.846 | |
| EQ-5D Usual activities | |||||||
| Pre | 0.0 | 1 | 0 | 0 | 0 | 1 | |
| 1.0 | 0 | 15 | 4 | 1 | 20 | ||
| 2.0 | 0 | 8 | 19 | 1 | 28 | ||
| 3.0 | 0 | 3 | 3 | 7 | 13 | ||
| Total | 1 | 26 | 26 | 9 | 62 | p = 0.343 | |
| EQ-5D Pain/discomfort | |||||||
| Pre | 0.0 | 1 | 1 | 0 | 0 | 2 | |
| 1.0 | 0 | 21 | 2 | 0 | 23 | ||
| 2.0 | 0 | 2 | 26 | 0 | 28 | ||
| 3.0 | 0 | 0 | 4 | 5 | 9 | ||
| Total | 1 | 24 | 32 | 5 | 62 | p = 0.172 | |
| EQ-5D Anxiety/depression | |||||||
| Pre | 0.0 | 1 | 0 | 0 | 0 | 1 | |
| 1.0 | 1 | 30 | 3 | 0 | 34 | ||
| 2.0 | 0 | 6 | 17 | 0 | 23 | ||
| 3.0 | 0 | 0 | 1 | 3 | 4 | ||
| Total | 2 | 36 | 21 | 3 | 62 | p = 0.392 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morer, C.; Michan-Doña, A.; Alvarez-Badillo, A.; Zuluaga, P.; Maraver, F. Evaluation of the Feasibility of a Two-Week Course of Aquatic Therapy and Thalassotherapy in a Mild Post-Stroke Population. Int. J. Environ. Res. Public Health 2020, 17, 8163. https://doi.org/10.3390/ijerph17218163
Morer C, Michan-Doña A, Alvarez-Badillo A, Zuluaga P, Maraver F. Evaluation of the Feasibility of a Two-Week Course of Aquatic Therapy and Thalassotherapy in a Mild Post-Stroke Population. International Journal of Environmental Research and Public Health. 2020; 17(21):8163. https://doi.org/10.3390/ijerph17218163
Chicago/Turabian StyleMorer, Carla, Alfredo Michan-Doña, Antonio Alvarez-Badillo, Pilar Zuluaga, and Francisco Maraver. 2020. "Evaluation of the Feasibility of a Two-Week Course of Aquatic Therapy and Thalassotherapy in a Mild Post-Stroke Population" International Journal of Environmental Research and Public Health 17, no. 21: 8163. https://doi.org/10.3390/ijerph17218163
APA StyleMorer, C., Michan-Doña, A., Alvarez-Badillo, A., Zuluaga, P., & Maraver, F. (2020). Evaluation of the Feasibility of a Two-Week Course of Aquatic Therapy and Thalassotherapy in a Mild Post-Stroke Population. International Journal of Environmental Research and Public Health, 17(21), 8163. https://doi.org/10.3390/ijerph17218163






