Prevalence of Hypoproteinemia and Hypoalbuminemia in Pregnant Women from Three Different Socioeconomic Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Total Protein and Albumin Level Evaluation
2.3. Statistical Analysis
2.4. Ethical Consideration
3. Results
3.1. Socio-Demographic Characteristics
3.2. Anthropometric Characteristics and Obstetric Data
3.3. Prevalence of Deficit of Plasma Total Protein and Albumin
3.4. Relationship between Complications during Pregnancy and Plasma Total Protein and Albumin Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Veena, S.R.; Gale, C.R.; Krishnaveni, G.V.; Kehoe, S.H.; Srinivasan, K.; Fall, C.H. Association between maternal nutritional status in pregnancy and offspring cognitive function during childhood and adolescence; a systematic review. BMC Pregnancy Childbirth 2016, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Ball, R.O. Protein and amino acid requirements during pregnancy. Adv. Nutr. 2016, 7, 839S–844S. [Google Scholar] [CrossRef]
- Maslova, E.; Rytter, D.; Bech, B.H.; Henriksen, T.B.; Rasmussen, M.A.; Olsen, S.F.; Halldorsson, T.I. Maternal protein intake during pregnancy and offspring overweight 20 y later. Am. J. Clin. Nutr. 2014, 100, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Switkowski, K.M.; Jacques, P.F.; Must, A.; Kleinman, K.P.; Gillman, M.W.; Oken, E. Maternal protein intake during pregnancy and linear growth in the offspring. Am. J. Clin. Nutr. 2016, 104, 1128–1136. [Google Scholar] [CrossRef]
- Brion, M.J.; Ness, A.R.; Rogers, I.; Emmett, P.; Cribb, V.; Davey Smith, G.; Lawlor, D.A. Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 y: Exploring parental comparisons and prenatal effects. Am. J. Clin. Nutr. 2010, 91, 748–756. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity, 2011. Available online: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed on 15 December 2019).
- Flores-Quijano, M.E.; Vega-Sanchez, R.; Tolentino-Dolores, M.C.; Lopez-Alarcon, M.G.; Flores-Urrutia, M.C.; Lopez-Olvera, A.D. Obesity is associated with changes in iron nutrition status and its homeostatic regulation in pregnancy. Nutrients 2019, 11, 693. [Google Scholar] [CrossRef]
- Lindheimer, M.D.; Kanter, D. Interpreting abnormal proteinuria in pregnancy: The need for a more pathophysiological approach. Obs. Gynecol. 2010, 115, 365–375. [Google Scholar] [CrossRef]
- Woldeamanuel, G.G.; Geta, T.G.; Mohammed, T.P.; Shuba, M.B.; Bafa, T.A. Effect of nutritional status of pregnant women on birth weight of newborns at Butajira Referral Hospital, Butajira, Ethiopia. Sage Open Med. 2019, 7, 2050312119827096. [Google Scholar] [CrossRef]
- Faupel-Badger, J.M.; Hsieh, C.C.; Troisi, R.; Lagiou, P.; Potischman, N. Plasma volume expansion in pregnancy: Implications for biomarkers in population studies. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1720–1723. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Haas, S.; Ghossein-Doha, C.; van Kuijk, S.M.; van Drongelen, J.; Spaanderman, M.E. Physiological adaptation of maternal plasma volume during pregnancy: A systematic review and meta-analysis. Ultrasound Obs. Gynecol. 2017, 49, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and clinical significance. J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Apgar, V. A proposal for a new method of evaluation of the newborn infant. Curr. Res. Anesth. Analg. 1953, 32, 260–267. [Google Scholar] [CrossRef]
- Lean, S.C.; Derricott, H.; Jones, R.L.; Heazell, A.E.P. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186287. [Google Scholar] [CrossRef]
- Varea, C.; Bernis, C.; González González, A. Maternal characteristics and temporal trends in birth outcomes: Comparison between Spanish and migrant mothers. Int. J. Popul. Res. 2012, 2012, 412680. [Google Scholar] [CrossRef]
- Mills, M.; Rindfuss, R.R.; McDonald, P.; te Velde, E. ESHRE reproduction and society task force. Why do people postpone parenthood? Reasons and social policy incentives. Hum. Reprod. Update 2011, 17, 848–860. [Google Scholar] [CrossRef]
- International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: Meta-analysis of individual participant data from randomised trials. BMJ 2017, 358, j3991. [Google Scholar] [CrossRef]
- King, J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000, 71 (Suppl. S5), 1218S–1225S. [Google Scholar] [CrossRef]
- Morikawa, M.; Mayama, M.; Saito, Y.; Akabane-Nakagawa, K.; Umazume, T.; Chiba, K.; Kawaguchi, S.; Cho, K.; Watari, H. Hypoproteinemia as a parameter of poor perinatal/neonatal outcomes in women with preeclampsia diagnosed as hypertension plus proteinuria. Pregnancy Hypertens. 2020, 21, 111–117. [Google Scholar] [CrossRef]
- Lu, J.; Zhai, H. Exacerbation of primary intestinal lymphangiectasia during late pregnancy and recovery after delivery: A case report and literature review. Medicine 2017, 96, e7928. [Google Scholar] [CrossRef] [PubMed]
- Hronek, M.; Kudlácková, Z. Deficient intake of nutrients and the resulting health complications in vegetarians in the course of pregnancy and lactation. Ceska Gynekol. 2005, 70, 161–164. [Google Scholar] [PubMed]
- Bernal-Delgado, E.; Garcia-Armesto, S.; Oliva, J.; Sanchez Martinez, F.I.; Repullo, J.R.; Pena-Longobardo, L.M.; Ridao-López, M.; Hernández-Quevedo, C. Spain: Health system review. Health Syst. Transit. 2018, 20, 1–179. [Google Scholar] [PubMed]
- OECD; European Observatory on Health Systems and Policies. State of Health in the EU; OECD Publishing: Paris, France; European Observatory on Health Systems and Policies: Brussels, Belgium, 2019; Available online: https://www.oecd.org/health/country-health-profiles-eu.htm (accessed on 30 November 2019).
| Total | Urban | Semi-Urban | Rural | p * | |
|---|---|---|---|---|---|
| N | 215 | 34 | 52 | 129 | |
| Maternal age (years) | 31.78 ± 5.14 | 34.09 ± 3.16 | 32.35 ± 4.71 | 30.94 ± 5.52 | 0.004 1 |
| Housewife | 57 (26.51) | 3 (8.82) | 5 (9.62) | 49 (37.98) | 0.000 2 |
| Education level (n, %) | 7 (5.42) | 0.034 | |||
| Illiterate | 7 (3.26) | 0 | 0 | 41 (31.78) | |
| Primary school | 59 (27.44) | 3 (8.82) | 15 (28.85) | 60 (46.51) | |
| Secondary school | 108 (50.23) | 23 (67.65) | 25 (48.07) | 21 (16.28) | |
| Bachelor or higher | 41 (19.07) | 8 (23.53) | 12 (23.08) | ||
| Socioeconomic level | 0.000 | ||||
| Low | 102 (47.44) | 0 | 17 (32.69) | 85 (65.89) | |
| Medium | 74 (34.42) | 12 (35.30) | 30 (57.69) | 32 (24.81) | |
| High | 39 (18.14) | 22 (64.70) | 5 (9.62) | 12 (9.30) | |
| Primigravidae (n, %) | 110 (51.16) | 18 (52.94) | 25 (48.08) | 67 (51.94) | 0.873 |
| Newborn weight (kg) | 3.32 ± 0.46 | 3.38 ± 0.45 | 3.31 ± 0.44 | 3.31 ± 0.47 | 0.763 |
| Apgar score (1–10; a.u.) 3 | 8.95 ± 0.36 | 9.00 ± 0.35 | 8.92 ± 0.48 | 8.95 ± 0.30 | 0.618 |
| BMI (kg/m2) | |||||
| T1 | 24.11 ± 4.04 | 22.89 ± 2.83 | 24.20 ± 4.64 | 24.39 ± 4.03 | 0.118 |
| T2 | 25.84 ± 4.18 | 24.17 ± 2.98 | 26.01 ± 4.34 | 26.21 ± 4.31 | 0.026 4 |
| T3 | 27.72 ± 4.17 | 26.06 ± 3.08 | 28.19 ± 4.35 | 27.97 ± 4.27 | 0.069 |
| Weight gain (kg) | 9.34 ± 4.12 | 9.09 ± 4.47 | 10.52 ± 4.52 | 8.93 ± 3.79 | 0.012 5 |
| Pregnancy complications (n, %) | |||||
| Edema | |||||
| T1 | |||||
| T2 | 2 (0.93) | 0 | 1 (1.92) | 1 (0.78) | 0.635 |
| T3 | 43 (20.00) | 0 | 24 (46.15) | 19 (14.73) | 0 |
| Cramps (n, %) | 58 (26.98) | 1 (2.94) | 31 (59.62) | 26 (20.16) | 0 |
| T1 | |||||
| T2 | 19 (8.84) | 4 (11.76) | 3 (5.77) | 12 (9.30) | 0.109 |
| T3 | 72 (33.49) | 13 (38.24) | 14 (26.92) | 45 (34.88) | 0.26 |
| Weakness (n, %) | 86 (40.00) | 14 (41.18) | 22 (42.31) | 50 (38.76) | 0.589 |
| T1 | |||||
| T2 | 181 (84.19) | 30 (88.24) | 50 (71.15) | 101 (78.58) | 0.107 |
| T3 | 166 (77.21) | 25 (73.53) | 41 (78.85) | 100 (77.52) | 0.122 |
| 180 (83.72) | 33 (97.06) | 43 (86.69) | 104 (80.62) | 0.166 |
| Total Protein (g/dL) | Albumin (g/dL) | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | p * | T1 | T2 | T3 | p * | |
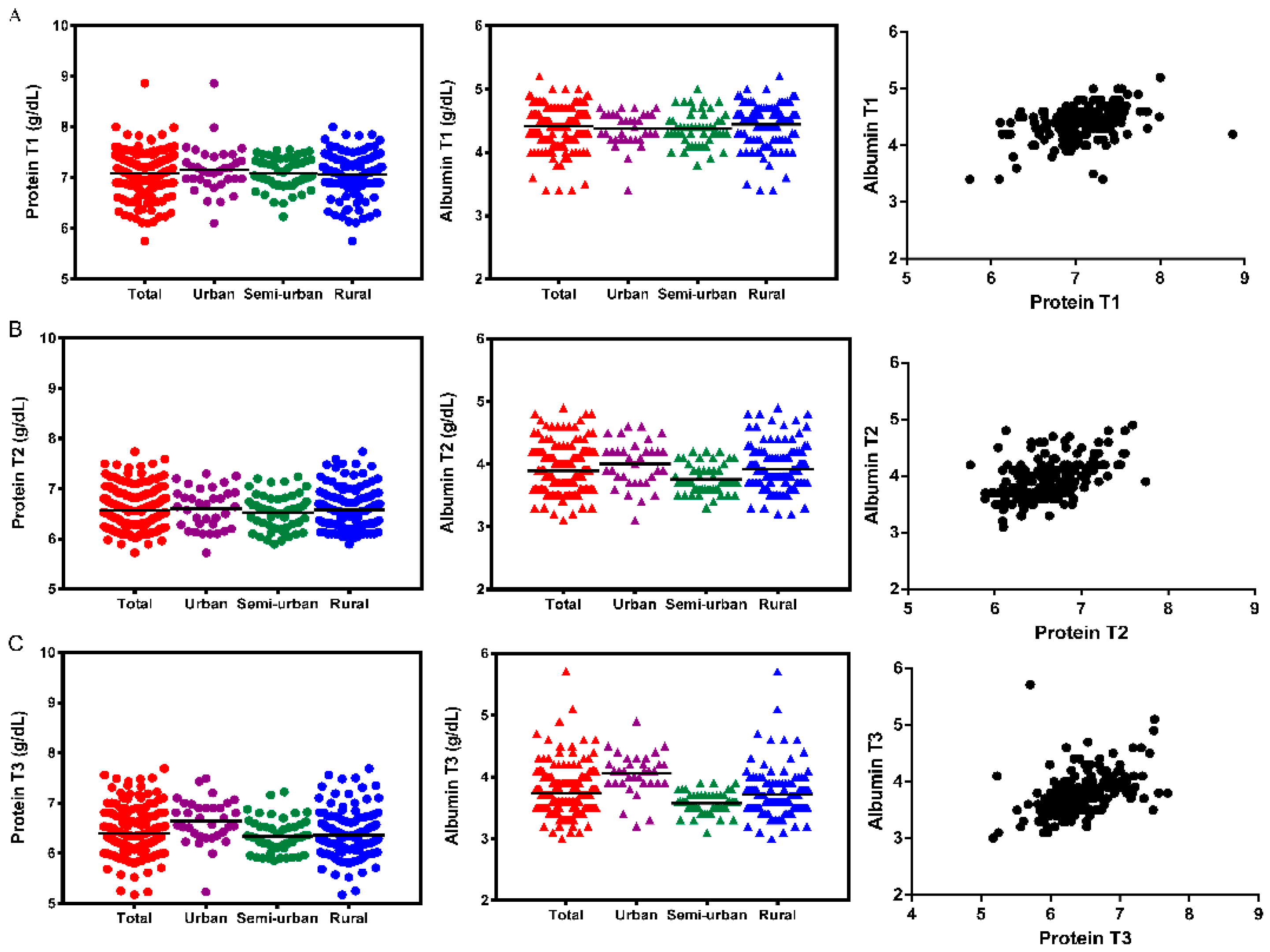
| Total | 7.082 ± 0.39 | 6.569 ± 0.39 | 6.398 ± 0.44 | 0.000 1 | 4.416 ± 0.29 | 3.895 ± 0.34 | 3.738 ± 0.36 | 0.000 1 |
| Urban | 7.161 ± 0.47 | 6.602 ± 0.40 | 6.644 ± 0.44 | 0.000 2 | 4.371 ± 0.27 | 4.003 ± 0.37 | 4.056 ± 0.34 | 0.000 5 |
| Semi-urb | 7.082 ± 0.31 | 6.527 ± 0.35 | 6.333 ± 0.33 | 0.000 3 | 4.378 ± 0.26 | 3.756 ± 0.23 | 3.575 ± 0.17 | 0.000 6 |
| Rural | 7.062 ± 0.40 | 6.578 ± 0.40 | 6.360 ± 0.46 | 0.000 4 | 4.444 ± 0.31 | 3.922 ± 0.35 | 3.719 ± 0.38 | 0.000 1 |
| p ** | 0.732 | 0.696 | 0.000 7 | 0.056 | 0.001 8 | 0.000 9 | ||
| Deficit of Protein (<6.4 g/dL) | Deficit of Albumin (<3.4 g/dL) | |||||
|---|---|---|---|---|---|---|
| T1 n (%) | T2 n (%) | T3 n (%) | T1 n (%) | T2 n (%) | T3 n (%) | |
| Total | 14 (6.51) | 81 (37.67) | 121 (56.28) | 0 | 7 (3.26) | 21 (9.77) |
| mean | 6.21 | 6.19 | 6.1 | 3.24 | 3.24 | |
| (95% CI) | (6.12–6.30) | (6.16–6.22) | (6.05–6.14) | (3.17–3.32) | (3.20–3.28) | |
| Urban | 1 (2.94) | 11 (32.35) | 9 (26.47) | 0 | 1 (2.94) | 2 (5.88) |
| mean | 6.14 | 6.13 | 3.25 | |||
| (95% CI) | (6.03–6.25) | (5.86–6.40) | (2.61–3.89) | |||
| Semi-urban | 1 (1.92) | 18 (34.62) | 34 (65.38) | 0 | 1 (1.92) | 5 (9.62) |
| mean | 6.15 | 6.14 | 3.26 | |||
| (95% CI) | (6.08–6.22) | (6.08–6.20) | (3.15–3.37) | |||
| Rural | 12 (9.30) | 52 (40.31) | 78 (60.46) | 0 | 5 (3.88) | 14 (10.85) |
| mean | 6.22 | 6.21 | 6.07 | 3.26 | 3.23 | |
| (95% CI) | (6.11–6.33) | (6.18–6.25) | (6.02–6.13) | (3.19–3.33) | (3.17–3.29) | |
| p * | 0.125 | 0.607 | 0.001 | 0.794 | 0.677 | |
| Total Protein (g/dL) | Albumin (g/dL) | |||||
|---|---|---|---|---|---|---|
| Edema + | Edema − | p | Edema + | Edema − | p | |
| T1 | 7.38 (5.28–9.47) | 7.08 (7.03–7.13) | 0.178 | 4.50 (3.23–5.77) | 4.42 (4.38–4.46) | 0.679 |
| T2 | 6.44 (6.35–6.53) | 6.60 (6.54–6.66) | 0.036 * | 3.78 (3.71–3.85) | 3.92 (3.87–3.98) | 0.007 * |
| T3 | 6.29 (6.18–6.39) | 6.44 (6.37–6.51) | 0.008 * | 3.61 (3.55–3.68) | 3.79 (3.72–3.85) | 0.001 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Cantarino, S.; Agulló-Ortuño, M.T.; de Dios-Aguado, M.; Ugarte-Gurrutxaga, M.I.; Bouzas-Mosquera, C. Prevalence of Hypoproteinemia and Hypoalbuminemia in Pregnant Women from Three Different Socioeconomic Populations. Int. J. Environ. Res. Public Health 2020, 17, 6275. https://doi.org/10.3390/ijerph17176275
Gómez-Cantarino S, Agulló-Ortuño MT, de Dios-Aguado M, Ugarte-Gurrutxaga MI, Bouzas-Mosquera C. Prevalence of Hypoproteinemia and Hypoalbuminemia in Pregnant Women from Three Different Socioeconomic Populations. International Journal of Environmental Research and Public Health. 2020; 17(17):6275. https://doi.org/10.3390/ijerph17176275
Chicago/Turabian StyleGómez-Cantarino, Sagrario, M. Teresa Agulló-Ortuño, Mercedes de Dios-Aguado, M. Idoia Ugarte-Gurrutxaga, and Carmen Bouzas-Mosquera. 2020. "Prevalence of Hypoproteinemia and Hypoalbuminemia in Pregnant Women from Three Different Socioeconomic Populations" International Journal of Environmental Research and Public Health 17, no. 17: 6275. https://doi.org/10.3390/ijerph17176275
APA StyleGómez-Cantarino, S., Agulló-Ortuño, M. T., de Dios-Aguado, M., Ugarte-Gurrutxaga, M. I., & Bouzas-Mosquera, C. (2020). Prevalence of Hypoproteinemia and Hypoalbuminemia in Pregnant Women from Three Different Socioeconomic Populations. International Journal of Environmental Research and Public Health, 17(17), 6275. https://doi.org/10.3390/ijerph17176275







