Changes in Markers of Oxidative Stress and α-Amylase in Saliva of Children Associated with a Tennis Competition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Subjects
- duration and RPE average of the first match: 23.6 ± 12.4 min and 11 ± 4;
- duration and RPE average of the second match: 23.1 ± 12.7 min and 13 ± 4;
- duration and RPE average of the third match: 20.4 ± 11.9 min and 13 ± 5;
- duration and RPE average of the fourth match: 16.6 ± 8.9 min and 12 ± 5.
2.2. Procedure and Variables
2.3. Statistical Analysis
dfchange = kold − knew
3. Results
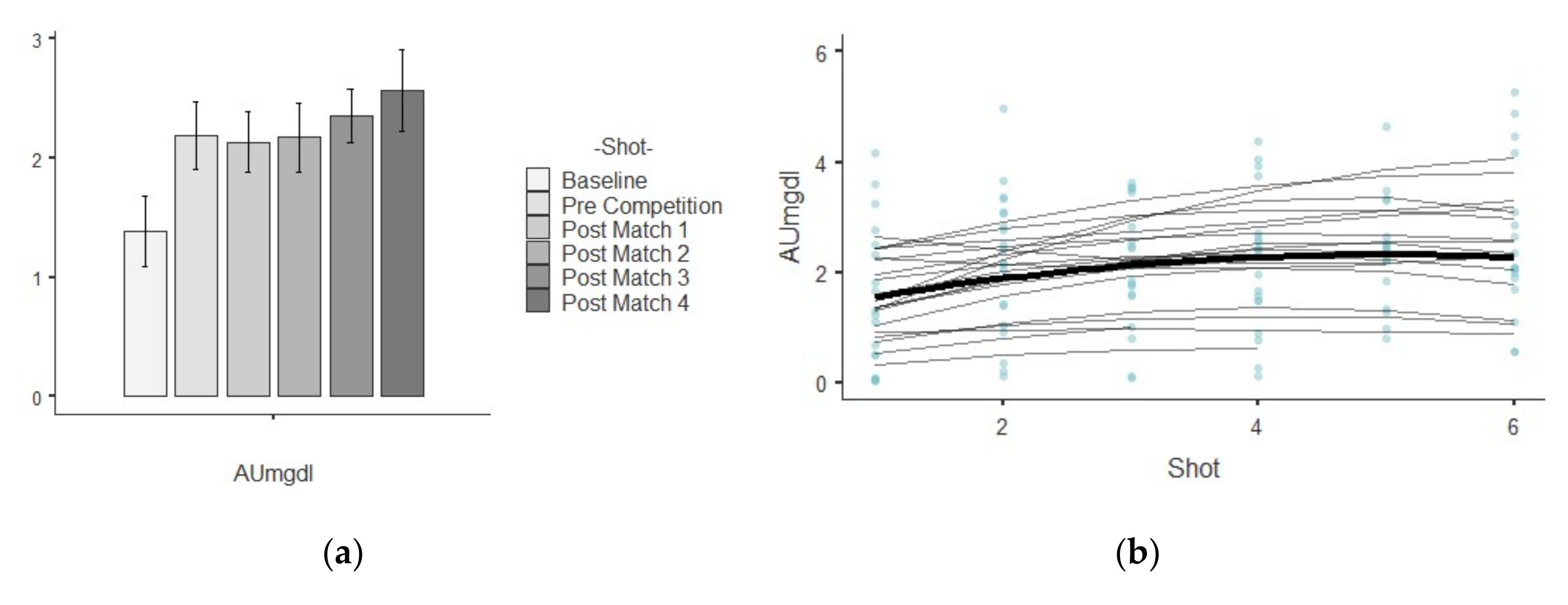
3.1. AU
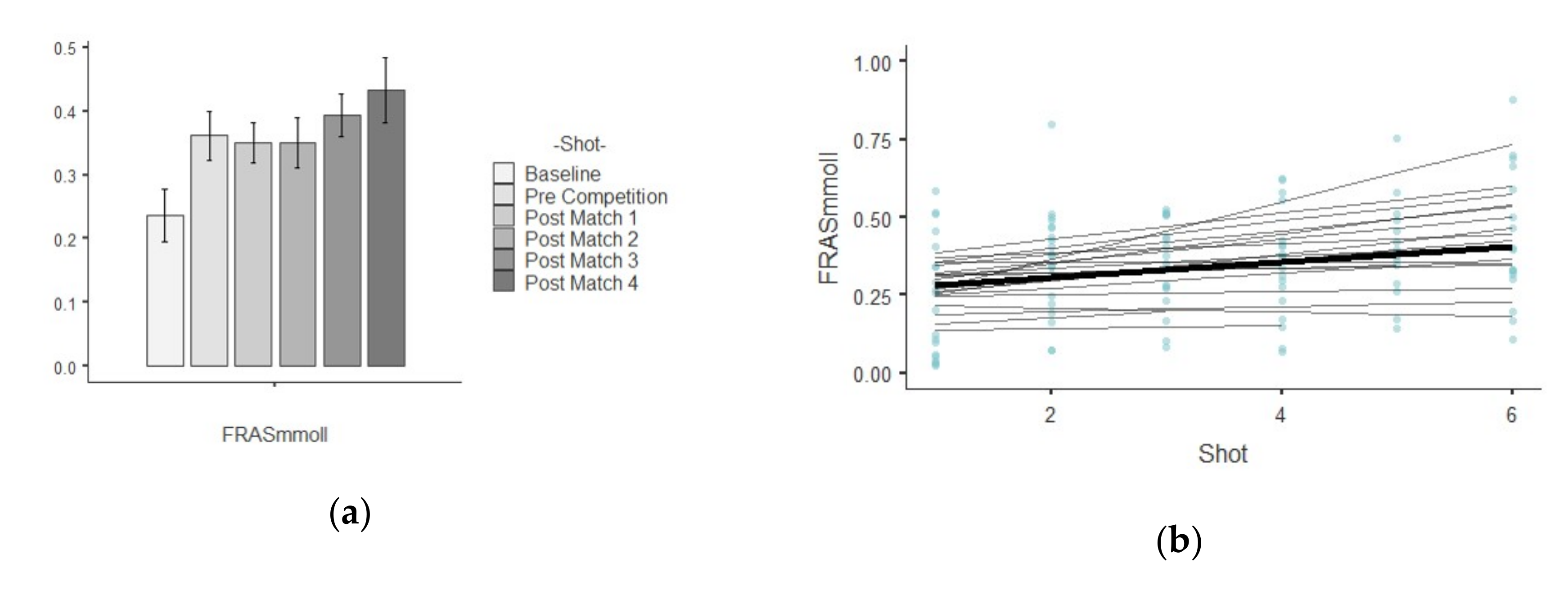
3.2. FRAS
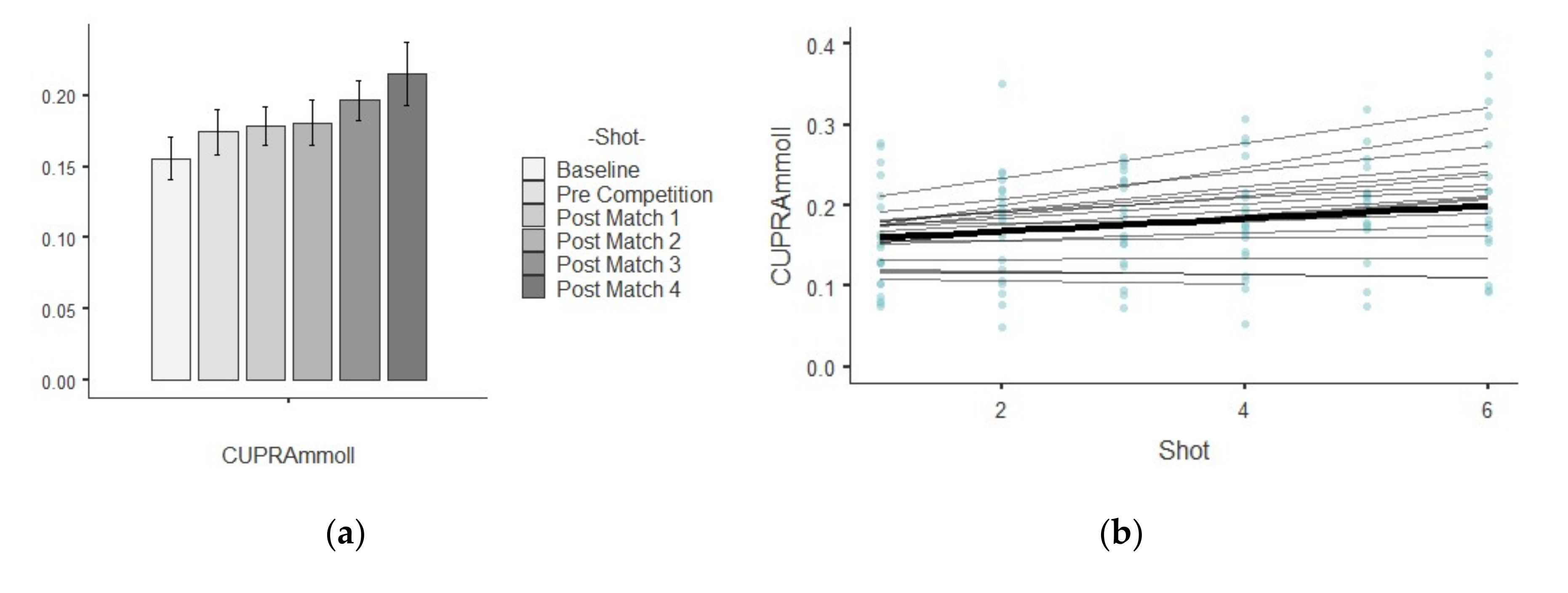
3.3. CUPRA
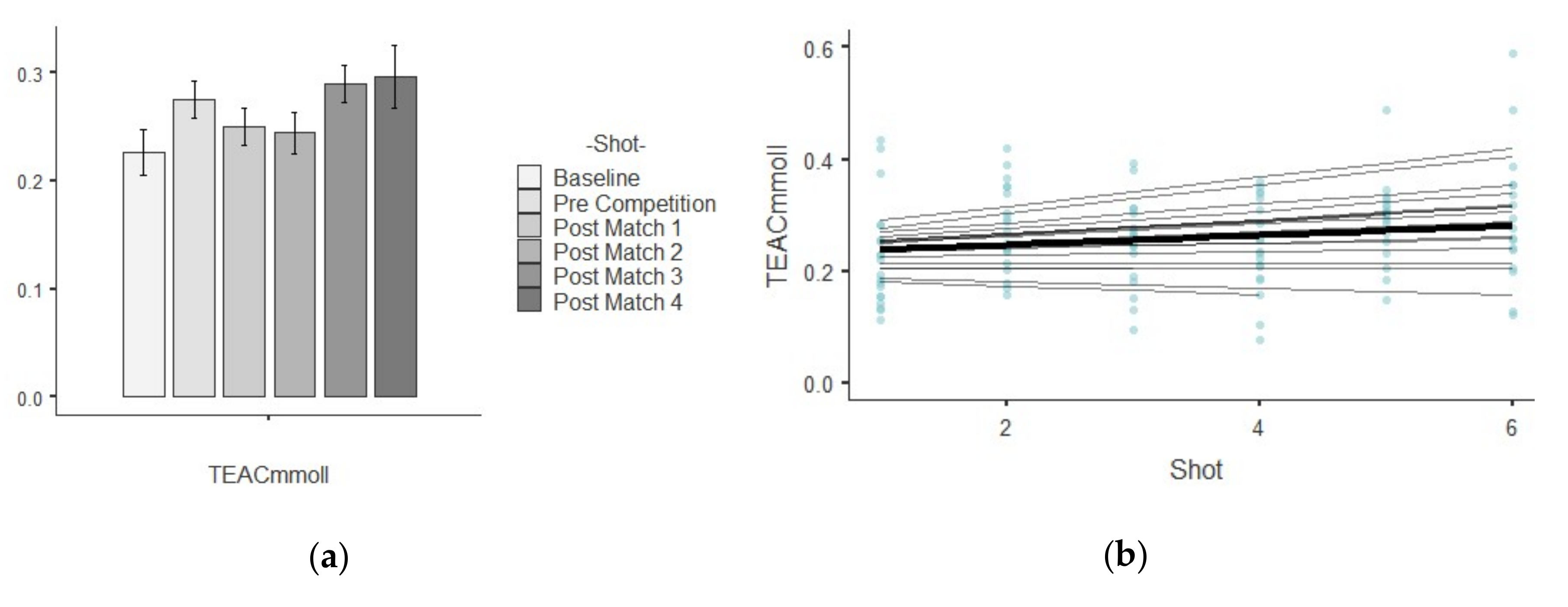
3.4. TEAC
3.5. sAA
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lopez-Samanes, A.; Pallares, J.G.; Perez-Lopez, A.; Mora-Rodriguez, R.; Ortega, J.F. Hormonal and neuromuscular responses during a singles match in male professional tennis players. PLoS ONE 2018, 13, e0195242. [Google Scholar] [CrossRef]
- Steinbacher, P.; Eckl, P. Impact of Oxidative Stress on Exercising Skeletal Muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef]
- Matias, C.N.; Bicho, M.; Laires, M.J.; Monteiro, C.P. Athletes have more susceptibility to oxidative stress: Truth or myth? A study in swimmers. Sci. Sports 2020, 35, 20–28. [Google Scholar] [CrossRef]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: Past, present and future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef] [Green Version]
- Ammar, A.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Chiboub, J.; Turki, M.; Abdelkarim, O.; El Abed, K.; Ben Ali, M.; Hoekelmann, A.; et al. Acute and delayed responses of C-reactive protein, malondialdehyde and antioxidant markers after resistance training session in elite weightlifters: Effect of time of day. Chronobiol. Int. 2015, 32, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Gamberi, T.; Magherini, F.; Fiaschi, T.; Modesti, P.A.; Gulisano, M.; Marella, M.; Bosi, P.; Spicuglia, P.; Radini, M.; Modesti, A. Postactivation potentiation improves athletic performance without affecting plasma oxidative level. J. Sports Med. Phys. Fit. 2019, 59, 975–981. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Koltai, E.; Taylor, A.W.; Goto, S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsic, A.; Vucic, V.; Glibetic, M.; Popovic, T.; Debeljak-Martacic, J.; Cubrilo, D.; Ahmetovic, Z.; Peric, D.; Borozan, S.; Djuric, D.; et al. Redox balance in elite female athletes: Differences based on sport types. J. Sports Med. Phys. Fit. 2016, 56, 1–8. [Google Scholar]
- Guilhem, G.; Hanon, C.; Gendreau, N.; Bonneau, D.; Guevel, A.; Chennaoui, M. Salivary Hormones Response to Preparation and Pre-competitive Training of World-class Level Athletes. Front. Physiol. 2015, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chennaoui, M.; Bougard, C.; Drogou, C.; Langrume, C.; Miller, C.; Gomez-Merino, D.; Vergnoux, F. Stress Biomarkers, Mood States, and Sleep during a Major Competition: “Success” and “Failure” Athlete’s Profile of High-Level Swimmers. Front. Physiol. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avloniti, A.; Chatzinikolaou, A.; Deli, C.K.; Vlachopoulos, D.; Gracia-Marco, L.; Leontsini, D.; Draganidis, D.; Jamurtas, A.Z.; Mastorakos, G.; Fatouros, I.G. Exercise-Induced Oxidative Stress Responses in the Pediatric Population. Antioxidants 2017, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasca, M.M.; Zhang, R.L.; Super, D.M.; Hazen, S.L.; Hall, H.R. Increased Oxidative Stress in Healthy Children Following an Exercise Program: A Pilot Study. J. Dev. Behav. Pediatr. 2010, 31, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benitez-Sillero, J.D.; Perez-Navero, J.L.; Tasset, I.; Castillo, M.G.-D.; Gil-Campos, M.; Tunez, I. Cardiorespiratory fitness and oxidative stress: Effect of acute maximal aerobic exercise in children and adolescents. J. Sports Med. Phys. Fit. 2011, 51, 204–210. [Google Scholar]
- Tvarijonaviciute, A.; Martinez-Lozano, N.; Rios, R.; Marcilla de Teruel, M.C.; Garaulet, M.; Ceron, J.J. Saliva as a non-invasive tool for assessment of metabolic and inflammatory biomarkers in children. Clin. Nutr. 2019, 39, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- Wing, C. A Brief Review of Salivary Biomarkers as Stress Indicators in Sport and Exercise. Strength Cond. J. 2019, 41, 80–88. [Google Scholar] [CrossRef]
- Deminice, R.; Sicchieri, T.; Payao, P.O.; Jordao, A.A. Blood and Salivary Oxidative Stress Biomarkers Following an Acute Session of Resistance Exercise in Humans. Int. J. Sports Med. 2010, 31, 599–603. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, J.M.; Franco, L.; Colomer-Poveda, D.; Martinez-Subiela, S.; Cugat, R.; Ceron, J.J.; Marquez, G.; Martinez-Aranda, L.M.; Jiménez-Reyes, P.; Tvarijonaviciute, A. Influence of Sampling Conditions, Salivary Flow, and Total Protein Content in Uric Acid Measurements in Saliva. Antioxidants 2019, 8, 389. [Google Scholar] [CrossRef] [Green Version]
- Rubio, C.P.; Hernandez-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Menezes, E.F.; Peixoto, L.G.; Teixeira, R.R.; Justino, A.B.; Puga, G.M.; Espindola, F.S. Potential Benefits of Nitrate Supplementation on Antioxidant Defense System and Blood Pressure Responses after Exercise Performance. Oxidative Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Vidal, K.; Robinson, N.; Ives, S.J. Exercise performance and physiological responses: The potential role of redox imbalance. Physiol. Rep. 2017, 5, e13225. [Google Scholar] [CrossRef] [PubMed]
- Pani, S.C.; Al Khabbaz, H.J.; Bin Enayeg, S.H.; Bin Zouman, A.H. The relationship between examination-related academic stress, salivary antioxidant capacity and exercise patterns of final-year Saudi dental students. Eur. J. Dent. Educ. 2017, 21, e83–e88. [Google Scholar] [CrossRef] [PubMed]
- Capranica, L.; Lupo, C.; Cortis, C.; Chiodo, S.; Cibelli, G.; Tessitore, A. Salivary cortisol and alpha-amylase reactivity to taekwondo competition in children. Eur. J. Appl. Physiol. 2012, 112, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef]
- Gimenez-Egido, J.M.; Ortega-Toro, E.; Palao, J.M.; Verdú-Conesa, I.; Torres-Luque, G. Effect of Modification Rules in Competition on Technical–Tactical Action in Young Tennis Players (Under-10). Front. Psychol. 2020, 10, 2789. [Google Scholar] [CrossRef] [Green Version]
- Slosar, L.; Simunic, B.; Pisot, R.; Marusic, U. Validation of a Tennis Rating Score to evaluate the technical level of children tennis players. J. Sports Sci. 2019, 37, 100–107. [Google Scholar] [CrossRef]
- Ishihara, T.; Kuroda, Y.; Mizuno, M. Competitive achievement may be predicted by executive functions in junior tennis players: An 18-month follow-up study. J. Sports Sci. 2019, 37, 755–761. [Google Scholar] [CrossRef]
- Ato, M.; Lopez, J.J.; Benavente, A. A classification system for research designs in psychology. An. Psicol. 2013, 29, 1038–1059. [Google Scholar]
- Thomas, J.R.; Nelson, J.K.; Silverman, S.J. Research Methods in Physical Activity; Human Kinetics Australia: Lower Mitcham, Australia, 2015. [Google Scholar]
- Otzen, T.; Manterola, C. Sampling Techniques on a Population Study. Int. J. Morphol. 2017, 35, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Borg, G.; Hassmen, P.; Lagerstrom, M. Perce Perceived exertion related to heart-rate and blood lactate during arm and leg exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 679–685. [Google Scholar] [CrossRef]
- Barranco, T.; Rubio, C.P.; Tvarijonayiciute, A.; Rubio, M.; Damia, E.; Lamy, E.; Cugat, R.; Cerón, J.J.; Tecles, F.; Escribano, D. Changes of salivary biomarkers under different storage conditions: Effects of temperature and length of storage. Biochem. Med. 2019, 29, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesser, H. Modeling individual differences in randomized experiments using growth models: Recommendations for design, statistical analysis and reporting of results of internet interventions. Internet Interv. 2015, 2, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage Publications: London, UK, 2013. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage Publications: London, UK, 2018. [Google Scholar]
- Souza, A.V.; Giolo, J.S.; Teixeira, R.R.; Vilela, D.D.; Peixoto, L.G.; Justino, A.B.; Caixeta, D.C.; Puga, G.M.; Espindola, F.S. Salivary and Plasmatic Antioxidant Profile following Continuous, Resistance, and High-Intensity Interval Exercise: Preliminary Study. Oxidative Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chielle, E.O.; Casarin, J.N. Evaluation of salivary oxidative parameters in overweight and obese young adults. Arch. Endocrin. Metab. 2017, 61, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalavras, A.; Fatouros, I.G.; Deli, C.K.; Draganidis, D.; Theodorou, A.A.; Soulas, D.; Koutsioras, Y.; Koutedakis, Y.; Jamurtas, A.Z. Age-Related Responses in Circulating Markers of Redox Status in Healthy Adolescents and Adults during the Course of a Training Macrocycle. Oxidative Med. Cell. Longev. 2015, 17. [Google Scholar] [CrossRef]
- Benitez-Sillero, J.D.; Perez-Navero, J.; Tasset, I.; Castillo, M.G.-D.; Gil-Campos, M.; Tunez, I. Influence of intense exercise on saliva glutathione in prepubescent and pubescent boys. Eur. J. Appl. Physiol. 2009, 106, 181–186. [Google Scholar] [CrossRef]
- Perrea, A.; Vlachos, I.S.; Korou, L.M.; Doulamis, I.P.; Exarhopoulou, K.; Kypraios, G.; Kalofoutis, A.; Perrea, D.N. Comparison of the Short-Term Oxidative Stress Response in National League Basketball and Soccer Adolescent Athletes. Angiology 2014, 65, 624–629. [Google Scholar] [CrossRef]
- Yilmaz, N.; Erel, O.; Hazer, M.; Bagci, C.; Namiduru, E.; Gul, E. Biochemical assessments of retinol, alpha-tocopherol, pyridoxal-5-phosphate oxidative stress index and total antioxidant status in adolescent professional basketball players and sedentary controls. Int. J. Adolesc. Med. Health 2007, 19, 177–186. [Google Scholar] [CrossRef]
- Tecles, F.; Fuentes-Rubio, M.; Tvarijonaviciute, A.; Martinez-Subiela, S.; Fatjo, J.; Ceron, J.J. Assessment of Stress Associated with an Oral Public Speech in Veterinary Students by Salivary Biomarkers. J. Vet. Med. Educ. 2014, 41, 37–43. [Google Scholar] [CrossRef]
- Santos-Silva, A.; Rebelo, M.I.; Castro, E.M.B.; Belo, L.; Guerra, A.; Rego, C.; Quintanilha, A. Leukocyte activation, erythrocyte damage, lipid profile and oxidative stress imposed by high competition physical exercise in adolescents. Clin. Chim. Acta 2001, 306, 119–126. [Google Scholar] [CrossRef]
- Dehghan, F.; Khodaei, F.; Afshar, L.; Shojaei, F.K.; Poorhakimi, E.; Soori, R.; Fatolahi, H.; Azarbayjani, M.A. Effect of competition on stress salivary biomarkers in elite and amateur female adolescent inline skaters. Sci. Sports 2019, 34, E37–E44. [Google Scholar] [CrossRef]
- Tsuber, V.; Kadamov, Y.; Tarasenko, L. Activation of Antioxidant Defenses in Whole Saliva by Psychosocial Stress Is More Manifested in Young Women than in Young Men. PLoS ONE 2014, 9, e115048. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, K.; Wurst, R.; von Dawans, B.; Strahler, J.; Kasten, N.; Fuchs, R. Habitual and acute exercise effects on salivary biomarkers in response to psychosocial stress. Psychoneuroendocrinology 2019, 106, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Kamodyova, N.; Tothova, L.; Celec, P. Salivary markers of oxidative stress and antioxidant status: Influence of external factors. Dis. Markers 2013, 34, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Escribano, D.; Martinez-Subiela, S.; Martinez-Miro, S.; Rubio, M.; Tvarijonaviciute, A.; Tecles, F.; Cerón, J.J. Influence of the way of reporting alpha-Amylase values in saliva in different naturalistic situations: A pilot study. PLoS ONE 2017, 12, e0180100. [Google Scholar] [CrossRef] [Green Version]
- Lomonaco, T.; Ghimenti, S.; Biagini, D.; Bramanti, E.; Onor, M.; Bellagambi, F.G.; Fuoco, R.; Di Francesco, F. The effect of sampling procedures on the urate in oral fluid and lactate concentration in oral fluid. Microchem. J. 2018, 136, 255–262. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Degano, I.; Ghimenti, S.; Lomonaco, T.; Dini, V.; Romanelli, M.; Mastorci, F.; Gemignani, A.; Salvo, P.; Fuoco, R.; et al. Determination of salivary alpha-amylase and cortisol in psoriatic subjects undergoing the Trier Social Stress Test. Microchem. J. 2018, 136, 177–184. [Google Scholar] [CrossRef]
- Morimoto, M.; Hashimoto, T.; Tsuda, Y.; Kitaoka, T.; Kyotani, S. Evaluation of oxidative stress and antioxidant capacity in healthy children. J. Chin. Med. Assoc. 2019, 82, 651–654. [Google Scholar] [CrossRef]
| Saliva Parameter | Shot | Mean ± SD | Variance | CV | Skewness | Kurtosis |
|---|---|---|---|---|---|---|
| AU (μmol/L) | Baseline | 1.38 ± 1.32 | 1.74 | 0.96 | 0.71 | 0.51 |
| Pre Competition | 2.19 ± 1.28 | 1.63 | 0.58 | 0.06 | 0.51 | |
| Post-match 1 | 2.14 ± 1.13 | 1.28 | 0.53 | −0.31 | 0.52 | |
| Post-match 2 | 2.17 ± 1.24 | 1.55 | 0.57 | 0.17 | 0.52 | |
| Post-match 3 | 2.36 ± 0.95 | 0.90 | 0.40 | 0.46 | 0.54 | |
| Post-match 4 | 2.57 ± 1.41 | 1.99 | 0.55 | 0.55 | 0.55 | |
| FRAS (mM/L) | Baseline | 0.23 ± 0.18 | 0.03 | 0.81 | 0.44 | −1.17 |
| Pre Competition | 0.36 ± 0.17 | 0.03 | 0.48 | 0.37 | 1.17 | |
| Post-match 1 | 0.35 ± 0.13 | 0.02 | 0.40 | −0.45 | −0.70 | |
| Post-match 2 | 0.35 ± 0.16 | 0.03 | 0.48 | 0.02 | −0.62 | |
| Post-match 3 | 0.39 ± 0.14 | 0.02 | 0.36 | 0.62 | 1.78 | |
| Post-match 4 | 0.43 ± 0.21 | 0.04 | 0.49 | 0.49 | −0.38 | |
| CUPRA (mM/L) | Baseline | 0.15 ± 0.06 | 0.00 | 0.40 | 0.63 | −0.74 |
| Pre Competition | 0.17 ± 0.07 | 0.01 | 0.41 | 0.36 | 0.60 | |
| Post-match 1 | 0.17 ± 0.05 | 0.00 | 0.35 | −0.23 | −1.08 | |
| Post-match 2 | 0.18 ± 0.07 | 0.00 | 0.38 | 0.20 | −0.32 | |
| Post-match 3 | 0.19 ± 0.06 | 0.00 | 0.32 | −0.16 | 0.55 | |
| Post-match 4 | 0.21 ± 0.09 | 0.01 | 0.43 | 0.48 | −0.63 | |
| TEAC (mM/L)) | Baseline | 0.22 ± 0.09 | 0.01 | 0.41 | 1.05 | 0.40 |
| Pre Competition | 0.27 ± 0.07 | 0.01 | 0.27 | 0.26 | −0.85 | |
| Post-match 1 | 0.25 ± 0.07 | 0.01 | 0.31 | −0.16 | 0.12 | |
| Post-match 2 | 0.24 ± 0.08 | 0.01 | 0.34 | −0.36 | −0.51 | |
| Post-match 3 | 0.28 ± 0.07 | 0.01 | 0.25 | 0.61 | 2.72 | |
| Post-match 4 | 0.29 ± 0.11 | 0.01 | 0.40 | 0.87 | 1.22 | |
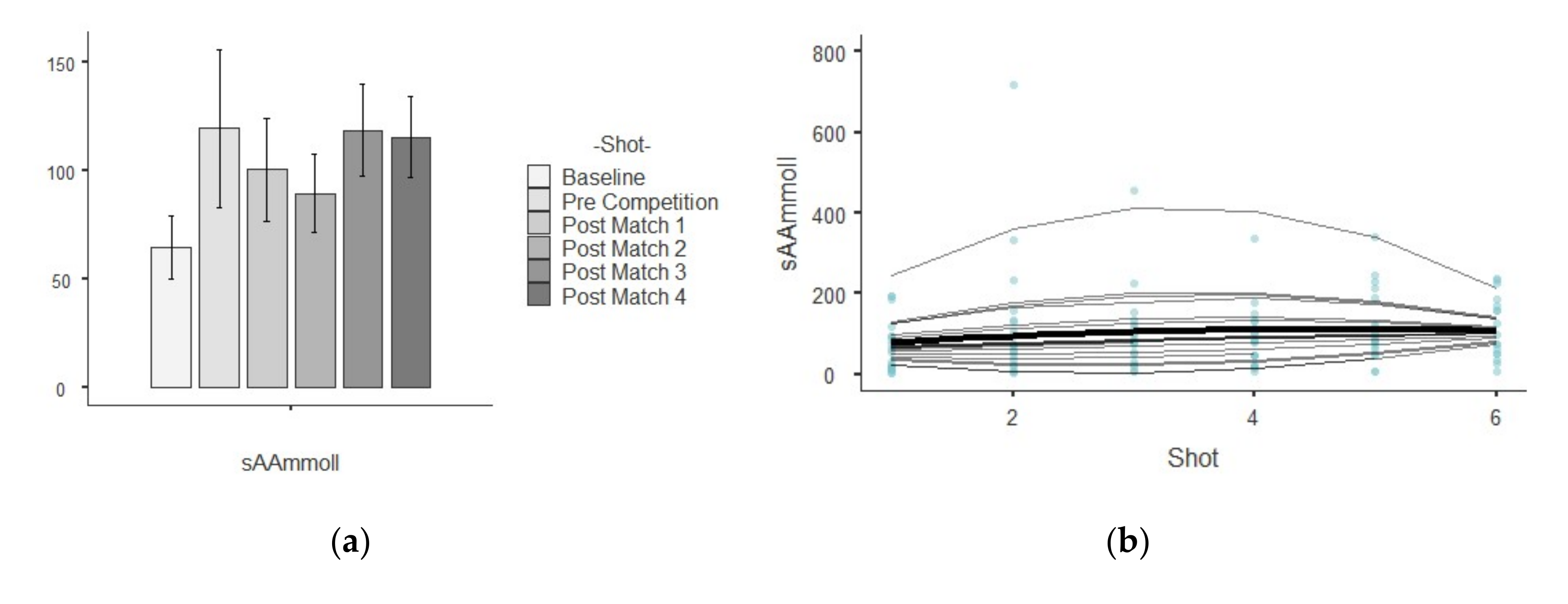
| sAA (U/L) | Baseline | 64.70 ± 64.50 | 4159.00 | 1.00 | 1.06 | 0.01 |
| Pre Competition | 120.00 ± 163.00 | 26,636.00 | 1.36 | 2.94 | 9.98 | |
| Post-match 1 | 101.00 ± 102.00 | 10,503.00 | 1.01 | 2.55 | 8.18 | |
| Post-match 2 | 89.50 ± 78.30 | 6133.00 | 0.87 | 1.82 | 4.78 | |
| Post-match 3 | 119.00 ± 89.90 | 8074.00 | 0.76 | 1.04 | 0.65 | |
| Post-match 4 | 116.00 ± 76.70 | 5880.00 | 0.66 | 0.31 | −1.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez-Egido, J.M.; Hernández-García, R.; Escribano, D.; Martínez-Subiela, S.; Torres-Luque, G.; Ortega-Toro, E.; Cerón, J.J. Changes in Markers of Oxidative Stress and α-Amylase in Saliva of Children Associated with a Tennis Competition. Int. J. Environ. Res. Public Health 2020, 17, 6269. https://doi.org/10.3390/ijerph17176269
Giménez-Egido JM, Hernández-García R, Escribano D, Martínez-Subiela S, Torres-Luque G, Ortega-Toro E, Cerón JJ. Changes in Markers of Oxidative Stress and α-Amylase in Saliva of Children Associated with a Tennis Competition. International Journal of Environmental Research and Public Health. 2020; 17(17):6269. https://doi.org/10.3390/ijerph17176269
Chicago/Turabian StyleGiménez-Egido, José María, Raquel Hernández-García, Damián Escribano, Silvia Martínez-Subiela, Gema Torres-Luque, Enrique Ortega-Toro, and José Joaquín Cerón. 2020. "Changes in Markers of Oxidative Stress and α-Amylase in Saliva of Children Associated with a Tennis Competition" International Journal of Environmental Research and Public Health 17, no. 17: 6269. https://doi.org/10.3390/ijerph17176269






