Effects of Progressive Resistance Training on Cognition and IGF-1 Levels in Elder Women Who Live in Areas with High Air Pollution
Abstract
:1. Introduction
2. Methods
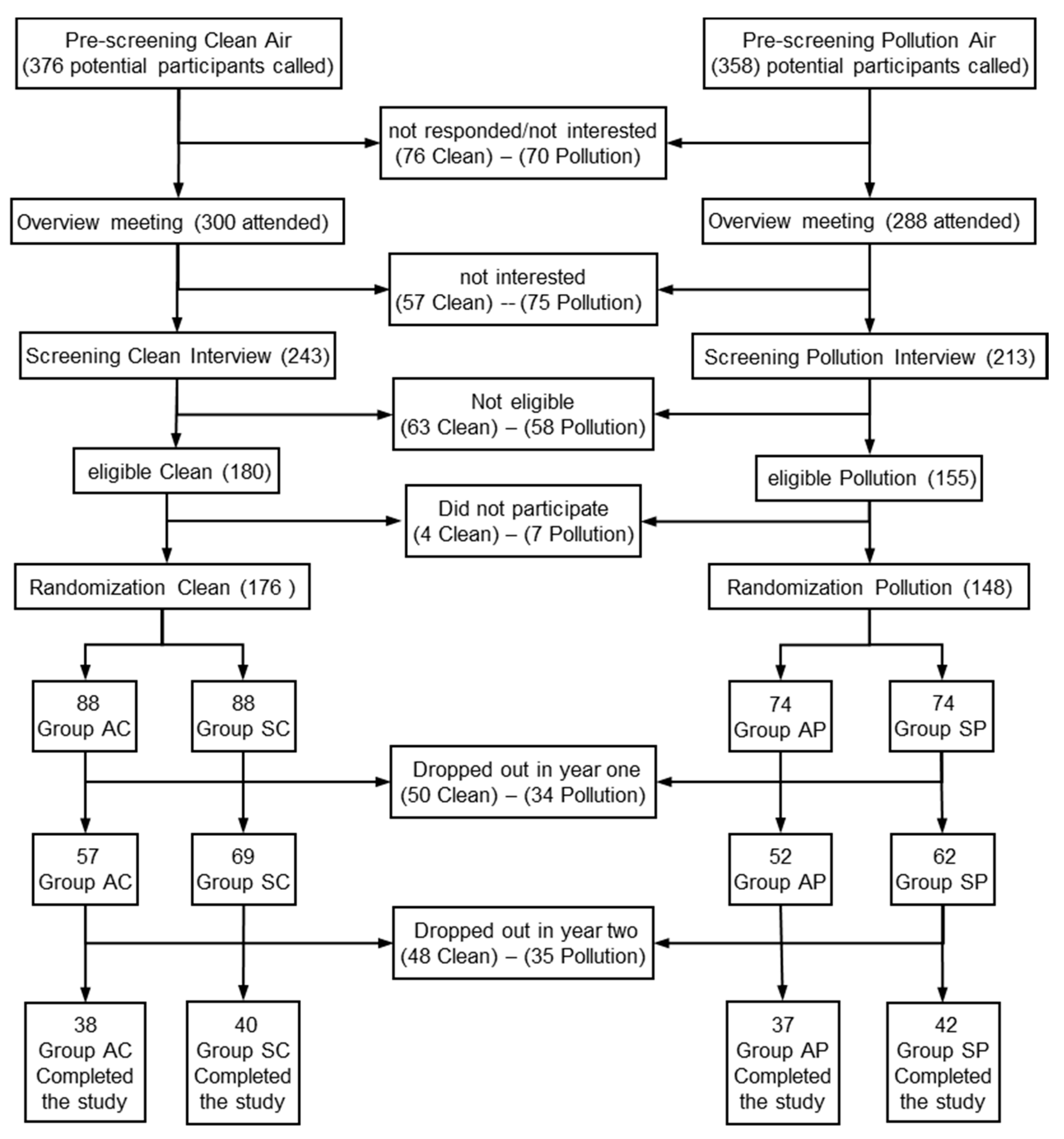
2.1. Sample
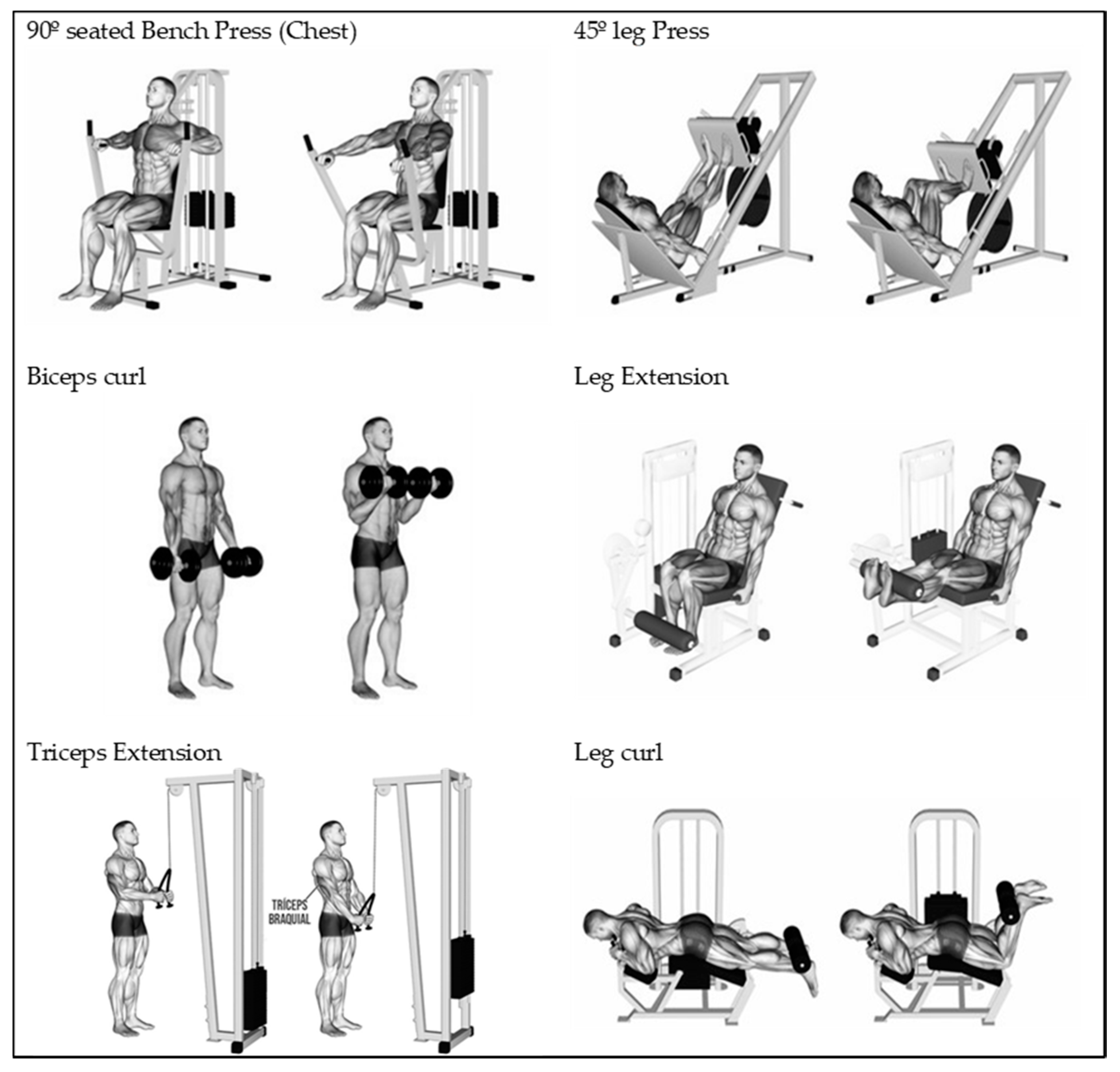
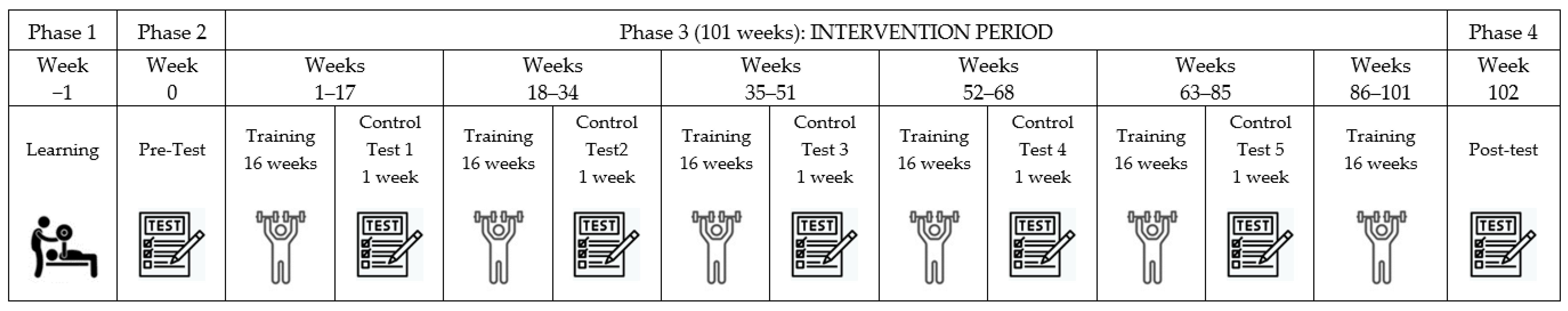
2.2. Procedures and Temporalisation.
2.3. Statistical Analysis
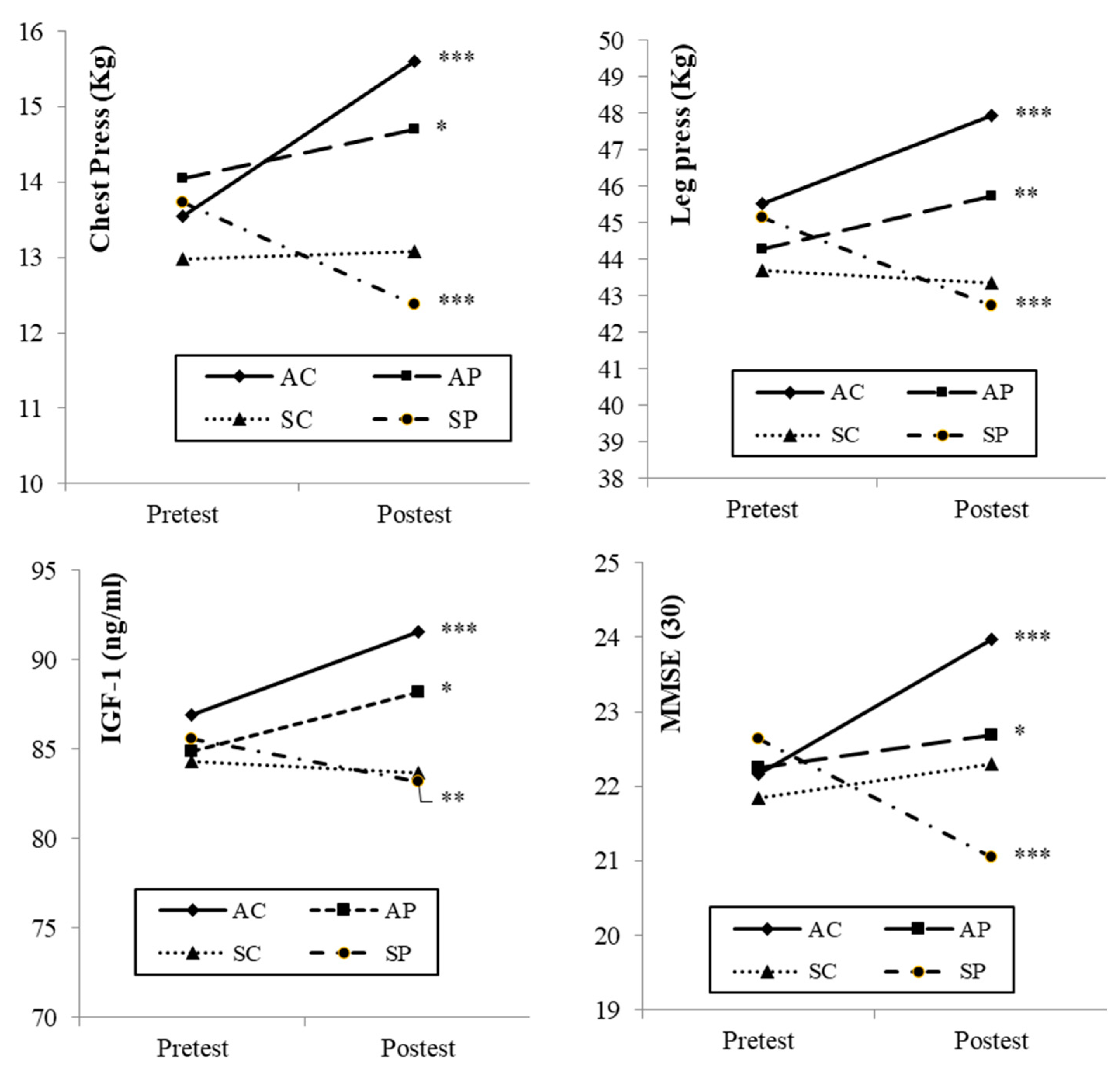
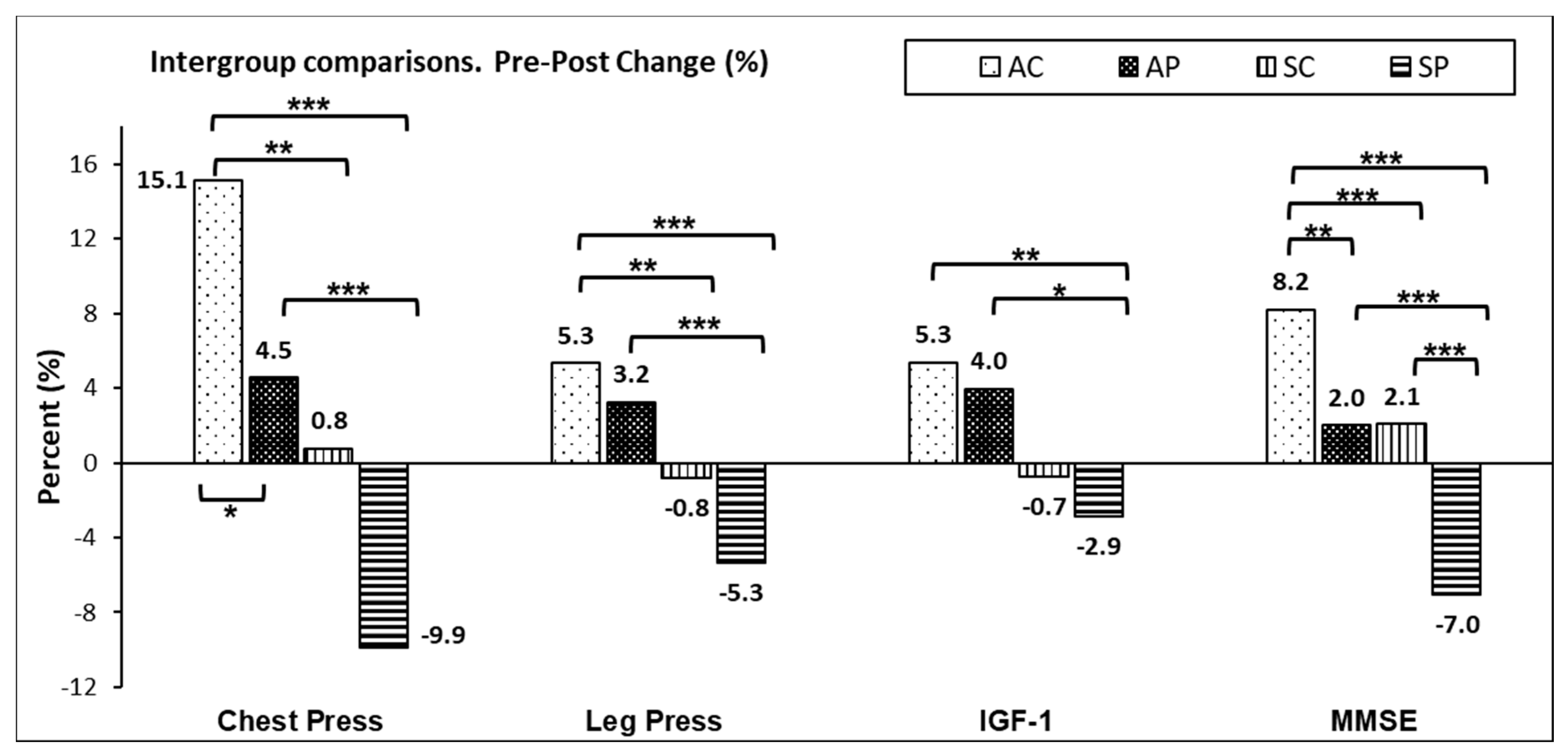
3. Results
4. Discussion
4.1. Muscular Strength
4.2. Insulin-Like Growth Factor-1
4.3. Cognitive State (Mini-Mental State Examination)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raynor, A.J.; Iredale, F.; Crowther, R.; White, J.; Dare, J. It’s Not Just Physical: Exercise Physiologist-Led Exercise Program Promotes Functional and Psychosocial Health Outcomes in Aged Care. J. Aging Phys. Act. 2020, 28, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Tolea, M.I.; Galvin, J.E. Sarcopenia and impairment in cognitive and physical performance. Clin. Interv. Aging 2015, 10, 663–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szlejf, C.; Suemoto, C.K.; Lotufo, P.A.; Benseñor, I.M. Association of Sarcopenia With Performance on Multiple Cognitive Domains: Results From the ELSA-Brasil Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Ishikawa, J.; Fujiwara, Y.; Tanaka, M.; Kanazawa, N.; Chiba, Y.; Iizuka, A.; Kaito, S.; Tanaka, J.; Sugie, M.; et al. Prevalence of frailty, cognitive impairment, and sarcopenia in outpatients with cardiometabolic disease in a frailty clinic. BMC Geriatr. 2018, 18, 264. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Hwang, A.-C.; Liu, L.-K.; Lee, W.-J.; Chen, L.-Y.; Peng, L.-N.; Lin, M.-H.; Chen, L.-K. Association of Dynapenia, Sarcopenia, and Cognitive Impairment Among Community-Dwelling Older Taiwanese. Rejuvenation Res. 2016, 19, 71–78. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, K.-I.; Paik, N.-J.; Kim, K.-W.; Jang, H.C.; Lim, J.-Y. Muscle strength: A better index of low physical performance than muscle mass in older adults. Geriatr. Gerontol. Int. 2016, 16, 577–585. [Google Scholar] [CrossRef]
- Eriksen, C.S.; Garde, E.; Reislev, N.L.; Wimmelmann, C.L.; Bieler, T.; Ziegler, A.K.; Gylling, A.T.; Dideriksen, K.J.; Siebner, H.R.; Mortensen, E.L.; et al. Physical activity as intervention for age-related loss of muscle mass and function: Protocol for a randomised controlled trial (the LISA study). BMJ Open 2016, 6, e012951. [Google Scholar] [CrossRef]
- Van Dam, R.; van Ancum, J.M.; Verlaan, S.; Scheerman, K.; Meskers, C.G.M.; Maier, A.B. Lower Cognitive Function in Older Patients with Lower Muscle Strength and Muscle Mass. Dement. Geriatr. Cogn. Disord. 2018, 45, 243–250. [Google Scholar] [CrossRef]
- Steves, C.J.; Spector, T.D.; Jackson, S.H.D. 114 Physical Strength Predicts Both Change In Cognition And Brain Volumes Ten Years Later, Even Between Identical Twins. Age Ageing 2014, 43, i32. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Guo, Y.; Gong, J.; Deng, M.; Yang, N.; Yan, Y. Relationships between functional fitness and cognitive impairment in Chinese community-dwelling older adults: A cross-sectional study. BMJ Open 2018, 8, e020695. [Google Scholar] [CrossRef]
- Adcock, M.; Fankhauser, M.; Post, J.; Lutz, K.; Zizlsperger, L.; Luft, A.R.; Guimarães, V.; Schättin, A.; de Bruin, E.D. Effects of an In-home Multicomponent Exergame Training on Physical Functions, Cognition, and Brain Volume of Older Adults: A Randomized Controlled Trial. Front. Med. 2020, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Marston, K.J.; Peiffer, J.J.; Rainey-Smith, S.R.; Gordon, N.; Teo, S.Y.; Laws, S.M.; Sohrabi, H.R.; Martins, R.N.; Brown, B.M. Resistance training enhances delayed memory in healthy middle-aged and older adults: A randomised controlled trial. J. Sci. Med. Sport 2019, 22, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Zammit, A.R.; Robitaille, A.; Piccinin, A.M.; Muniz-Terrera, G.; Hofer, S.M. Associations Between Aging-Related Changes in Grip Strength and Cognitive Function in Older Adults: A Systematic Review. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 519–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.; Jung, S.; Bang, H.; Kim, H.; Kim, Y. The association between muscular strength and depression in Korean adults: A cross-sectional analysis of the sixth Koreast National Health and Nutrition Examination Survey (KNHANES VI) 2014. BMC Public Health 2018, 18, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herold, F.; Törpel, A.; Schega, L.; Müller, N.G. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements – a systematic review. Eur. Rev. Aging Phys. Act. 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, S.; Wallis, M.; Polit, D.; Steele, M.; Shum, D.; Morris, N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: A randomised controlled trial. Age Ageing 2014, 43, 623–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramnath, U.; Rauch, L.; Lambert, E.V.; Kolbe-Alexander, T.L. The relationship between functional status, physical fitness and cognitive performance in physically active older adults: A pilot study. PLoS ONE 2018, 13, e0194918. [Google Scholar] [CrossRef]
- Medeiros, L.B.; Ansai, J.H.; de Souza Buto, M.S.; de Vassimon Barroso, V.; Farche, A.C.S.; Rossi, P.G.; de Andrade, L.P.; de Medeiros Takahashi, A.C. Impact of a dual task intervention on physical performance of older adults who practice physical exercise. Impacto de uma intervenção de dupla tarefa no desempenho físico de idosos praticantes de exercício físico. Braz. J. Kineanthropometry Hum. Perform. 2018, 20, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Picillo, M.; Pivonello, R.; Santangelo, G.; Pivonello, C.; Savastano, R.; Auriemma, R.; Amboni, M.; Scannapieco, S.; Pierro, A.; Colao, A.; et al. Serum IGF-1 is associated with cognitive functions in early, drug-naïve Parkinson’s disease. PLoS ONE 2017, 12, e0186508. [Google Scholar] [CrossRef] [Green Version]
- Doi, T.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T.; Shimada, H. Association between insulin-like growth factor-1 and frailty among older adults. J. Nutr. Health Aging 2018, 22, 68–72. [Google Scholar] [CrossRef]
- Rogers, S.D.; Jarrott, S.E. Cognitive impairment and effects on upper body strength of adults with dementia. J. Aging Phys. Act. 2008, 16, 61–68. [Google Scholar] [CrossRef]
- Castilla-Cortázar, I.; Iturrieta, I.; García-Magariño, M.; Puche, J.E.; Martín-Estal, I.; Aguirre, G.A.; Femat-Roldan, G.; Cantu-Martinez, L.; Muñoz, Ú. Neurotrophic Factors and Their Receptors Are Altered by the Mere Partial IGF-1 Deficiency. Neuroscience 2019, 404, 445–458. [Google Scholar] [CrossRef]
- Puche, J.E.; Muñoz, Ú.; García-Magariño, M.; Sádaba, M.C.; Castilla-Cortázar, I. Partial IGF-1 deficiency induces brain oxidative damage and edema, which are ameliorated by replacement therapy. Biofactors 2016, 42, 60–79. [Google Scholar] [CrossRef]
- Molina-Sotomayor, E.; Orb, M.G.; de la Fuente, F.P.; Figueroa, G.C.; Sánchez-Oliver, A.J.; González-Jurado, J.A. Effects of Cardiorespiratory Exercise on Cognition in Older Women Exposed to Air Pollution. Int. J. Environ. Res. Public Health 2019, 16, 245. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.J.; Scribbans, T.D.; Bentley, R.F.; Kellawan, J.M.; Gurd, B.; Tschakovsky, M.E. Neurotrophic growth factor responses to lower body resistance training in older adults. Appl. Physiol. Nutr. Metab. 2016, 41, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chung, Y.; Chen, Y.; Ho, S.; Wu, H. Effects of different types of exercise on body composition, muscle strength, and IGF-1 in the elderly with sarcopenic obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Klersy, C.; Terracol, G.; Talluri, J.; Maugeri, R.; Guido, D.; Faliva, M.A.; Solerte, B.S.; Fioravanti, M.; Lukaski, H.; et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016, 103, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Guo, Y.; Ruan, Z.; Kowal, P.; Di, Q.; Zheng, Y.; Xiao, J.; Hoogendijk, E.O.; Dent, E.; Vaughn, M.G.; et al. Association of Indoor and Outdoor Air Pollution With Hand-Grip Strength Among Adults in Six Low- and Middle-Income Countries. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Nußbaum, R.; Lucht, S.; Jockwitz, C.; Moebus, S.; Engel, M.; Jöckel, K.-H.; Caspers, S.; Hoffmann, B. Associations of Air Pollution and Noise with Local Brain Structure in a Cohort of Older Adults. Environ. Health Perspect. 2020, 128, 067011–067012. [Google Scholar] [CrossRef]
- Shin, J.; Han, S.-H.; Choi, J. Exposure to Ambient Air Pollution and Cognitive Impairment in Community-Dwelling Older Adults: The Korean Frailty and Aging Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 3767. [Google Scholar] [CrossRef] [Green Version]
- Shehab, M.A.; Pope, F.D. Effects of short-term exposure to particulate matter air pollution on cognitive performance. Sci. Rep. 2019, 9, 8237. [Google Scholar] [CrossRef] [PubMed]
- De Zwart, F.; Brunekreef, B.; Timmermans, E.; Deeg, D.; Gehring, U. Air Pollution and Performance-Based Physical Functioning in Dutch Older Adults. Environ. Health Perspect. 2018, 126, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Slezakova, K.; Peixoto, C.; Pereira, M.d.C.; Morais, S. (Ultra) Fine particle concentrations and exposure in different indoor and outdoor microenvironments during physical exercising. J. Toxicol. Environ. Health Part A 2019, 82, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Peters, J.; Booth, A.; Mudway, I.A.N. Is air pollution associated with increased risk of cognitive decline? A systematic review. Age Ageing 2015, 44, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Ren, T.; Yu, X.; Yang, W. Do cognitive and non-cognitive abilities mediate the relationship between air pollution exposure and mental health? PLoS ONE 2019, 14, e0223353. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, G.; Danti, S.; Carlesi, C.; Borin, G. Danger in the Air: Air Pollution and Cognitive Dysfunction. Am. J. Alzheimers Dis. Other Dement. 2018, 33, 333–341. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Zhang, X. The impact of exposure to air pollution on cognitive performance. Proc. Natl. Acad. Sci. USA 2018, 115, 9193–9197. [Google Scholar] [CrossRef] [Green Version]
- World Medical Association. WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects (accessed on 20 July 2020).
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Quiroga, L.P.; Albala, B.C.; Klaasen, P.G. Validación de un test de tamizaje para el diagnóstico de demencia asociada a edad, en Chile. Rev. Médica De Chile 2004, 132, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Ortiz, C.A.; Luque, J.S.; Eriksson, C.K.; Soto, L. Self-reported tooth loss and cognitive function: Data from the Hispanic established populations for epidemiologic studies of the elderly (Hispanic EPESE). Colomb. Médica 2013, 44, 139–145. [Google Scholar] [CrossRef]
- Lourenço, R.A.; Veras, R.P. Mini-Mental State Examination: Psychometric characteristics in elderly outpatients. Rev. De Saúde Pública 2006, 40, 712–719. [Google Scholar] [CrossRef]
- Cortés, N.A.R.; Villarreal, R.E.; Galicia, R.L.; Martínez, G.L.; Vargas, D.E.R. Evaluación geriátrica integral del adulto mayor. Rev. Médica De Chile 2011, 139, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.A.M.; Orellana, P.A.R.; Marzuca-Nassr, G.N. Criterios de valoración geriátrica integral en adultos mayores con dependencia moderada y severa en Centros de Atención Primaria en Chile. Rev. Médica De Chile 2015, 143, 612–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knutzen, K.M.; Brilla, L.R.; Caine, D.J. Validity of 1RM prediction equations for older adults. J. Strength Cond. Res. 1999, 13, 242–246. [Google Scholar]
- García-Ramos, A.; Barboza-González, P.; Ulloa-Díaz, D.; Rodriguez-Perea, A.; Martinez-Garcia, D.; Guede-Rojas, F.; Hinojosa-Riveros, H.; Chirosa-Ríos, L.J.; Cuevas-Aburto, J.; Janicijevic, D.; et al. Reliability and validity of different methods of estimating the one-repetition maximum during the free-weight prone bench pull exercise. J. Sports Sci. 2019, 37, 2205–2212. [Google Scholar] [CrossRef]
- Tan, S.; Wang, J.; Liu, S. Establishment of the Prediction Equations of 1RM Skeletal Muscle Strength in 60- to 75-Year-Old Chinese Men and Women. J. Aging Phys. Act. 2015, 23, 640–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morishita, S.; Tsubaki, A.; Nakamura, M.; Nashimoto, S.; Fu, J.B.; Onishi, H. Rating of perceived exertion on resistance training in elderly subjects. Expert Rev. Cardiovasc. Ther. 2019, 17, 135–142. [Google Scholar] [CrossRef]
- Préndez, M.; Calderón, V. Análisis de Contaminantes en la Cuenca del Río Aconcagua en Chile. Evaluación de Riesgo Humano y Ambiental. Anal. Pollut. Watershed Aconcagua River Chile. Eval. Hum. Environ. Risks. (Engl.) 2013, 24, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Fiogbé, E.; Carnavale, B.F.; de Medeiros Takahashi, A.C. Exercise training in older adults, what effects on muscle force control? A systematic review of randomized clinical trials. Arch. Gerontol. Geriatr. 2019, 83, 138–150. [Google Scholar] [CrossRef]
- Lacroix, A.; Hortobágyi, T.; Beurskens, R.; Granacher, U. Effects of Supervised vs. Unsupervised Training Programs on Balance and Muscle Strength in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 2341–2361. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Latham, N.K. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, F.; Yang, Y.; Wang, S.T.; Dong, Y.N.; Xu, M.X.; Wang, Z.W.; Zhao, J.X. Exercise and air pollutants exposure: A systematic review and meta-analysis. Life Sci. 2019, 218, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Bucci, L.; Yani, S.L.; Fabbri, C.; Bijlsma, A.Y.; Maier, A.B.; Meskers, C.G.; Narici, M.V.; Jones, D.A.; McPhee, J.S.; Seppet, E.; et al. Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology 2013, 14, 261–272. [Google Scholar] [CrossRef]
- Van Nieuwpoort, I.C.; Vlot, M.C.; Schaap, L.A.; Lips, P.; Drent, M.L. The relationship between serum IGF-1, handgrip strength, physical performance and falls in elderly men and women. Eur. J. Endocrinol. 2018, 179, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Ahtiainen, J.P.; Hulmi, J.J.; Lehti, M.; Kraemer, W.J.; Nyman, K.; Selänne, H.; Alen, M.; Komulainen, J.; Kovanen, V.; Mero, A.A.; et al. Effects of resistance training on expression of IGF-I splice variants in younger and older men. Eur. J. Sport Sci. 2016, 16, 1055–1063. [Google Scholar] [CrossRef]
- Cunha, P.M.; Nunes, J.P.; Tomeleri, C.M.; Nascimento, M.A.; Schoenfeld, B.J.; Antunes, M.; Gobbo, L.A.; Teixeira, D.; Cyrino, E.S. Resistance training performed with single and multiple sets induces similar improvements in muscular strength, muscle mass, muscle quality, and IGF-1 in older women: A randomized controlled trial. J. Strength Cond. Res. 2018, 34, 1008–1016. [Google Scholar] [CrossRef]
- Coelho, L.; Hauck, K.; McKenzie, K.; Copeland, J.L.; Kan, I.P.; Gibb, R.L.; Gonzalez, C.L.R. The association between sedentary behavior and cognitive ability in older adults. Aging Clin. Exp. Res. 2020. [Google Scholar] [CrossRef]
- Vancampfort, D.; Stubbs, B.; Lara, E.; Vandenbulcke, M.; Swinnen, N.; Koyanagi, A. Correlates of sedentary behavior in middle-aged and old age people with mild cognitive impairment: A multinational study. Int. Psychogeriatr. 2019, 31, 579–589. [Google Scholar] [CrossRef]
- Ku, P.W.; Liu, Y.T.; Lo, M.K.; Chen, L.J.; Stubbs, B. Higher levels of objectively measured sedentary behavior is associated with worse cognitive ability: Two-year follow-up study in community-dwelling older adults. Exp. Gerontol. 2017, 99, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.; Ramel, A.; Jonsson, P.V.; Thorsdottir, I.; Geirsdottir, O.G. The effect of cognitive function on mobility improvement among community-living older adults: A 12-week resistance exercise intervention study. Aging Neuropsychol. Cogn. 2020, 27, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, X.; Xiang, W.; Han, J.; Li, K. The effect of resistance training on cognitive function in the older adults: A systematic review of randomized clinical trials. Aging Clin. Exp. Res. 2018, 30, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; Gonçalves, I.O.; Sampaio, R.A.C.; Sampaio, P.Y.S.; Cadore, E.L.; Calvani, R.; Picca, A.; Izquierdo, M.; Marzetti, E.; Uchida, M.C. Effects of combined resistance and power training on cognitive function in older women: A randomized controlled trial. Int. J. Environ. Res. Public Health 2020, 17, 3435. [Google Scholar] [CrossRef]
- Kulick, E.R.; Wellenius, G.A.; Boehme, A.K.; Joyce, N.R.; Schupf, N.; Kaufman, J.D.; Mayeux, R.; Sacco, R.L.; Manly, J.J.; Elkind, M.S.V. Long-term exposure to air pollution and trajectories of cognitive decline among older adults. Neurology 2020, 94, E1782–E1792. [Google Scholar] [CrossRef]
- Lo, Y.T.C.; Lu, Y.C.; Chang, Y.H.; Kao, S.; Huang, H.B. Air pollution exposure and cognitive function in taiwanese older adults: A repeated measurement study. Int. J. Environ. Res. Public Health 2019, 16, 2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekblom, M.M.; Ekblom, Ö.B.; Börjesson, M.; Bergström, G.; Jern, C.; Wallin, A. Device-measured sedentary behavior, physical activity and aerobic fitness are independent correlates of cognitive performance in healthy middle-aged adults—Results from the SCAPIS pilot study. Int. J. Environ. Res. Public Health 2019, 16, 5136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olanrewaju, O.; Stockwell, S.; Stubbs, B.; Smith, L. Sedentary behaviours, cognitive function, and possible mechanisms in older adults: A systematic review. Aging Clin. Exp. Res. 2020, 32, 969–984. [Google Scholar] [CrossRef]
- Maasakkers, C.M.; Claassen, J.A.H.R.; Gardiner, P.A.; Olde Rikkert, M.G.M.; Lipnicki, D.M.; Scarmeas, N.; Dardiotis, E.; Yannakoulia, M.; Anstey, K.J.; Cherbuin, N.; et al. The Association of Sedentary Behaviour and Cognitive Function in People Without Dementia: A Coordinated Analysis Across Five Cohort Studies from COSMIC. Sports Med. 2020, 50, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
| Area | PM10 µg/m3 | PM2.5 µg/m3 | NO2 µg/m3 | O3 µg/m3 | SO2 µg/m3 |
|---|---|---|---|---|---|
| Pudahuel | 67.5 | 29.2 | 38.7 | 26.8 | NI |
| Viña del Mar | 34.3 | 12.8 | NI | NI | NI |
| Chile | 50 ** | 20 ** | 100 ** | 120 * | 60 ** |
| WHO | 20 ** | 10 ** | 40 ** | 100 * | 20/24 h |
| Weeks | 0 | 1–17 | 18–34 | 35–51 | 52–68 | 63–85 | 86–101 | 102 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | 1RM 30–40% | 1RM 35–40% | 1 RM 40–45% | 1RM 40–45% | 1RM 45–50% | 1RM 50–55% | |||||||
| Benchpress | Pre-test ¥ | 1–2 set; 6–8 rep 30–60 s * | Control test 1 ¥ | 2–3 set; 6–8 rep 30–60 s * | Control test 2 ¥ | 2–4 set; 5–7 rep 1–2 min * | Control test 3 ¥ | 3–4 set; 4–6 rep 2–3 min * | Control test 4 ¥ | 2–3 set; 3–5 rep 3–4 min * | Control test 5 ¥ | 2–3 set; 2–4 rep 3–4 min * | Post-test ¥ |
| Bicepscurl | 1–2 set; 6–8 rep 30–60 s * | 2–3 set; 6–8 rep 30–60 s * | 2–4 set; 5–7 rep 1–2 min * | 3–4 set; 4–6 rep 2–3 min * | 2–3 set; 3–5 rep 3–4 min * | 2–3 set; 2–4 rep 3–4 min * | |||||||
| Tricepsextension | 1–2 set; 6–8 rep 30–60 s * | 2–3 set; 6–8 rep 30–60 s * | 2–4 set; 4–6 rep 1–2 min * | 3–4 set; 4–6 rep 2–3 min * | 2–3 set; 3–5 rep 3–4 min * | 2–3 set; 2–4 rep 3–4 min * | |||||||
| Legpress | 1–2 set; 5–7 rep 30–60 s * | 2–3 set; 5–7 rep 30–60 s * | 2–4 set; 4–6 rep 1–2 min * | 3–4 set; 3–5 rep 2–3 min * | 2–3 set; 2–4 rep 3–4 min * | 2–3 set; 2–3 rep 3–4 min * | |||||||
| Legextension | 1–2 set; 5–7 rep 30–60 s * | 2–3 set; 5–7 rep 30–60 s * | 2–4 set; 4–6 rep 1–2 min * | 3–4 set; 3–5 rep 2–3 min * | 2–3 set; 2–4 rep 3–4 min * | 2–3 set; 2–3 rep 3–4 min * | |||||||
| Legcurl | 1–2 set; 4–6 rep 30–60 s * | 2–3 set; 4–5 rep 30–60 s * | 2–4 set; 3–4 rep 1–2 min * | 3–4 set; 2–4 rep 2–3 min * | 2–3 set; 2–3 rep 3–4 min * | 2–3 set; 1–2 rep 3–4 min * | |||||||
| Variables | Active Clean (n = 38) | Active Pollution (n = 37) | Sedentary Clean (n = 40) | Sedentary Pollution (n = 42) | Intergroup Comparisons § | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M ± SE | CI (95%) | M ± SE | CI (95%) | M ± SE | CI (95%) | M ± SE | CI (95%) | F | p Value | PartialEta2 | |
| Chest Press (Kg) | 13.5 ± 0.35 | (12.8–14.2) | 14.1 ± 0.36 | (13.3–14.8) | 12.9 ± 0.34 | (12.3–13.7) | 13.7 ± 0.34 | (13.1–14.4) | 1.68 | 0.174 | 0.032 |
| Leg Press (Kg) | 45.5 ± 0.68 | (44.2–46.9) | 44.3 ± 0.70 | (42.9–45.7) | 43.7 ± 0.66 | (42.4–45.1) | 45.1 ± 0.64 | (43.9–46.4) | 1.53 | 0.210 | 0.029 |
| IGF-1 (ng/mL) | 86.9 ± 3.89 | (79.2–94.6) | 84.7 ± 4.00 | (76.9–92.8) | 84.3 ± 3.80 | (76.8–91.8) | 85.6 ± 3.70 | (78.3–92.9) | 0.084 | 0.968 | 0.002 |
| MMSE | 22.2 ± 0.3 | (21.6–22.7) | 22.2 ± 0.31 | (21.6–22.9) | 21.8 ± 0.29 | (21.1–22.7) | 22.6 ±0.29 | (22.1–23.2) | 1.87 | 0.136 | 0.036 |
| Variables | Active Clean (n = 38) | Active Pollution (n = 37) | Sedentary Clean (n = 40) | Sedentary Pollution (n = 42) | Intergroup Comparisons § | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M ± SE | CI (95%) | M ± SE | CI (95%) | M ± SE | CI (95%) | M ± SE | CI (95%) | F | p Value | PartialEta2 | |
| Chest Press (Kg) | 15.6 ± 0.43 a,b | (14.8–16.4) | 14.7 ± 0.44 c,d | (13.8–15.6) | 13.1 ± 0.41 a,c | (12.2–13.9) | 12.4 ± 0.41 b,d | (11.6–13.2) | 12.42 | 0.000 | 0.197 |
| Leg Press (Kg) | 47.9 ± 0.73 a,b | (46.5–49.4) | 45.7 ± 0.75 c | (44.2–47.2) | 43.3 ± 0.71 a | (41.9–44.7) | 42.7 ± 0.69 b,c | (41.4–44.1) | 11.11 | 0.000 | 0.180 |
| IGF-1 (ng/mL) | 91.6 ± 3.7 | (69.8–113.3) | 88.2 ± 3.8 | (65.9–110.6) | 83.7 ± 3.6 | (76.5–90.9) | 83.2 ± 3.5 | (62.5–103.8) | 1.173 | 0.322 | 0.023 |
| MMSE | 23.9 ± 0.29 a,b,c | (23.4–24.5) | 22.7 ± 0.3 a,d | (22.1–23.3) | 22.3 ± 0.28 b,e | (21.7–22.8) | 21.1 ± 0.27 c,d,e | (20.5–21.6) | 18.39 | 0.000 | 0.266 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Sotomayor, E.; Castillo-Quezada, H.; Martínez-Salazar, C.; González-Orb, M.; Espinoza-Salinas, A.; Gonzalez-Jurado, J.A. Effects of Progressive Resistance Training on Cognition and IGF-1 Levels in Elder Women Who Live in Areas with High Air Pollution. Int. J. Environ. Res. Public Health 2020, 17, 6203. https://doi.org/10.3390/ijerph17176203
Molina-Sotomayor E, Castillo-Quezada H, Martínez-Salazar C, González-Orb M, Espinoza-Salinas A, Gonzalez-Jurado JA. Effects of Progressive Resistance Training on Cognition and IGF-1 Levels in Elder Women Who Live in Areas with High Air Pollution. International Journal of Environmental Research and Public Health. 2020; 17(17):6203. https://doi.org/10.3390/ijerph17176203
Chicago/Turabian StyleMolina-Sotomayor, Edgardo, Humberto Castillo-Quezada, Cristian Martínez-Salazar, Marcelo González-Orb, Alexis Espinoza-Salinas, and Jose Antonio Gonzalez-Jurado. 2020. "Effects of Progressive Resistance Training on Cognition and IGF-1 Levels in Elder Women Who Live in Areas with High Air Pollution" International Journal of Environmental Research and Public Health 17, no. 17: 6203. https://doi.org/10.3390/ijerph17176203
APA StyleMolina-Sotomayor, E., Castillo-Quezada, H., Martínez-Salazar, C., González-Orb, M., Espinoza-Salinas, A., & Gonzalez-Jurado, J. A. (2020). Effects of Progressive Resistance Training on Cognition and IGF-1 Levels in Elder Women Who Live in Areas with High Air Pollution. International Journal of Environmental Research and Public Health, 17(17), 6203. https://doi.org/10.3390/ijerph17176203






