Health Risk Assessment of Dietary Heavy Metals Intake from Fruits and Vegetables Grown in Selected Old Mining Areas—A Case Study: The Banat Area of Southern Carpathians
Abstract
:1. Introduction
2. Materials and Methods
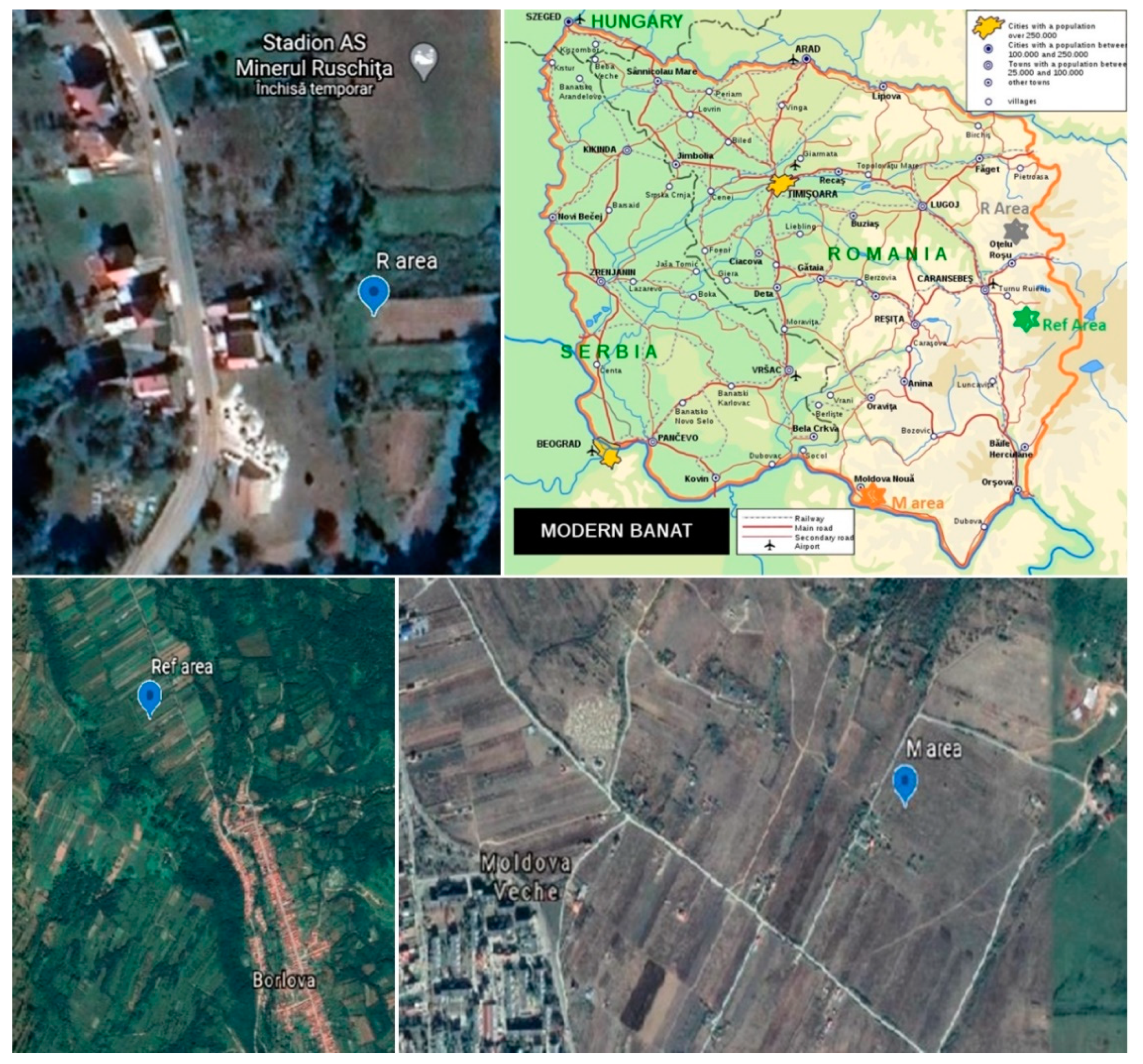
2.1. Sample Localization and Collection
2.2. Sample Preparation and Analytical Methods
2.3. Quality Assurance and Control
2.4. Methodology for Health Risk Assessment
3. Results and Discussion
3.1. Metals Concentrations in Soils and Different Vegetal Foodstuffs from Investigated Areas
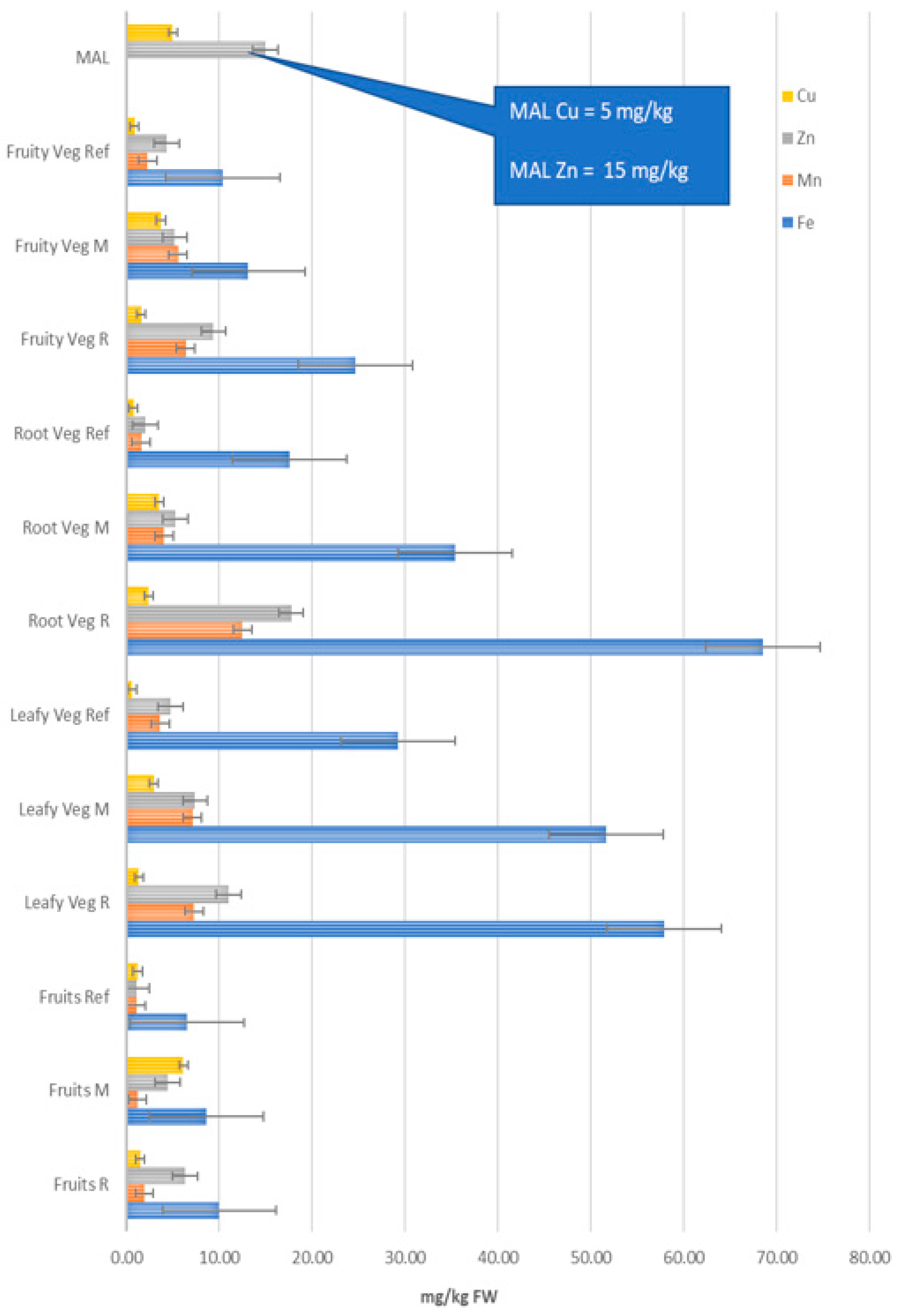
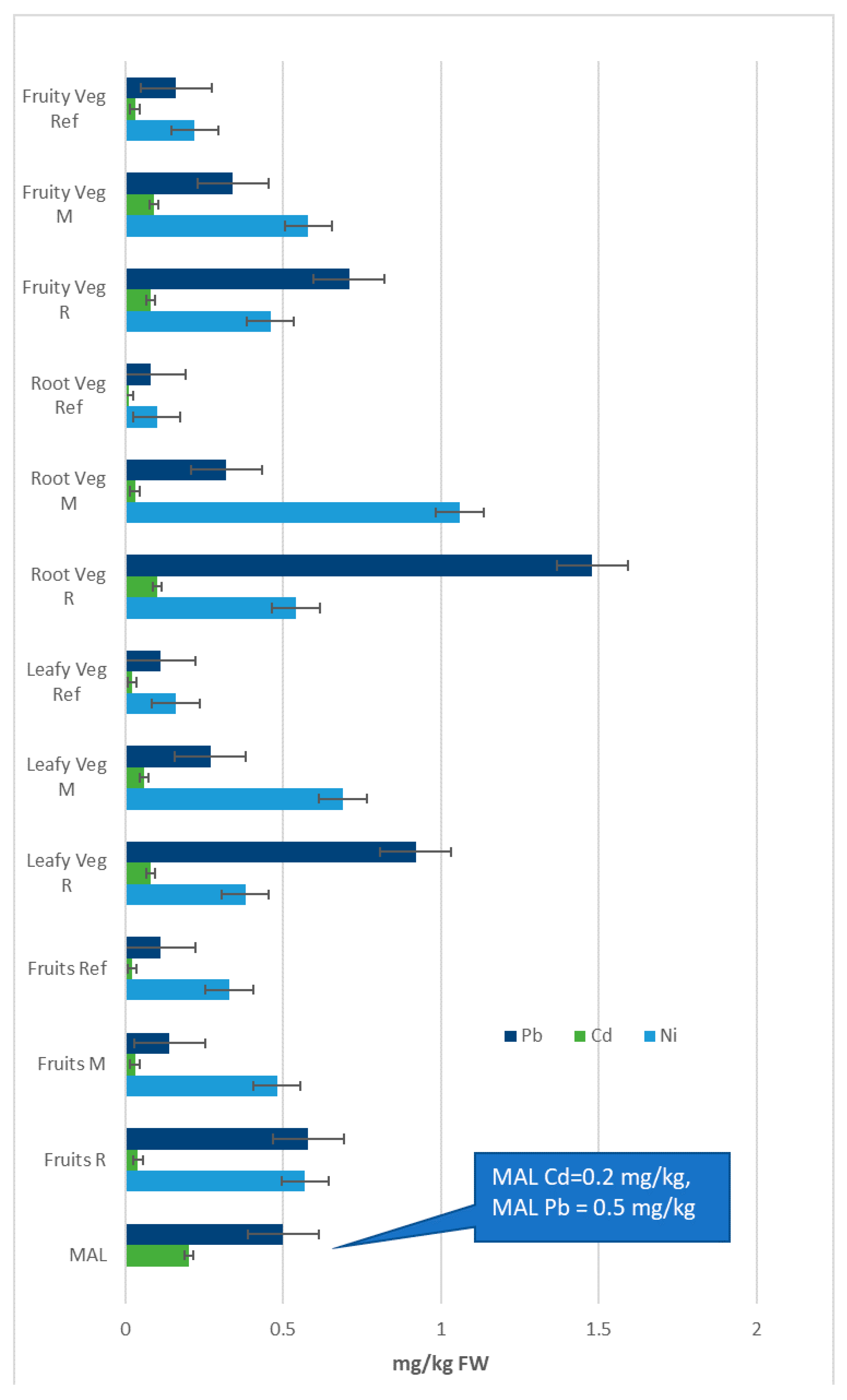
3.2. Concentration of Fe, Mn, Zn, Cu, Ni, Cd and Pb in Different Groups of Vegetables
3.3. Health Risk Assessment of Metals via Food Consumption
3.3.1. Metal Contribution of the Foodstuff Groups
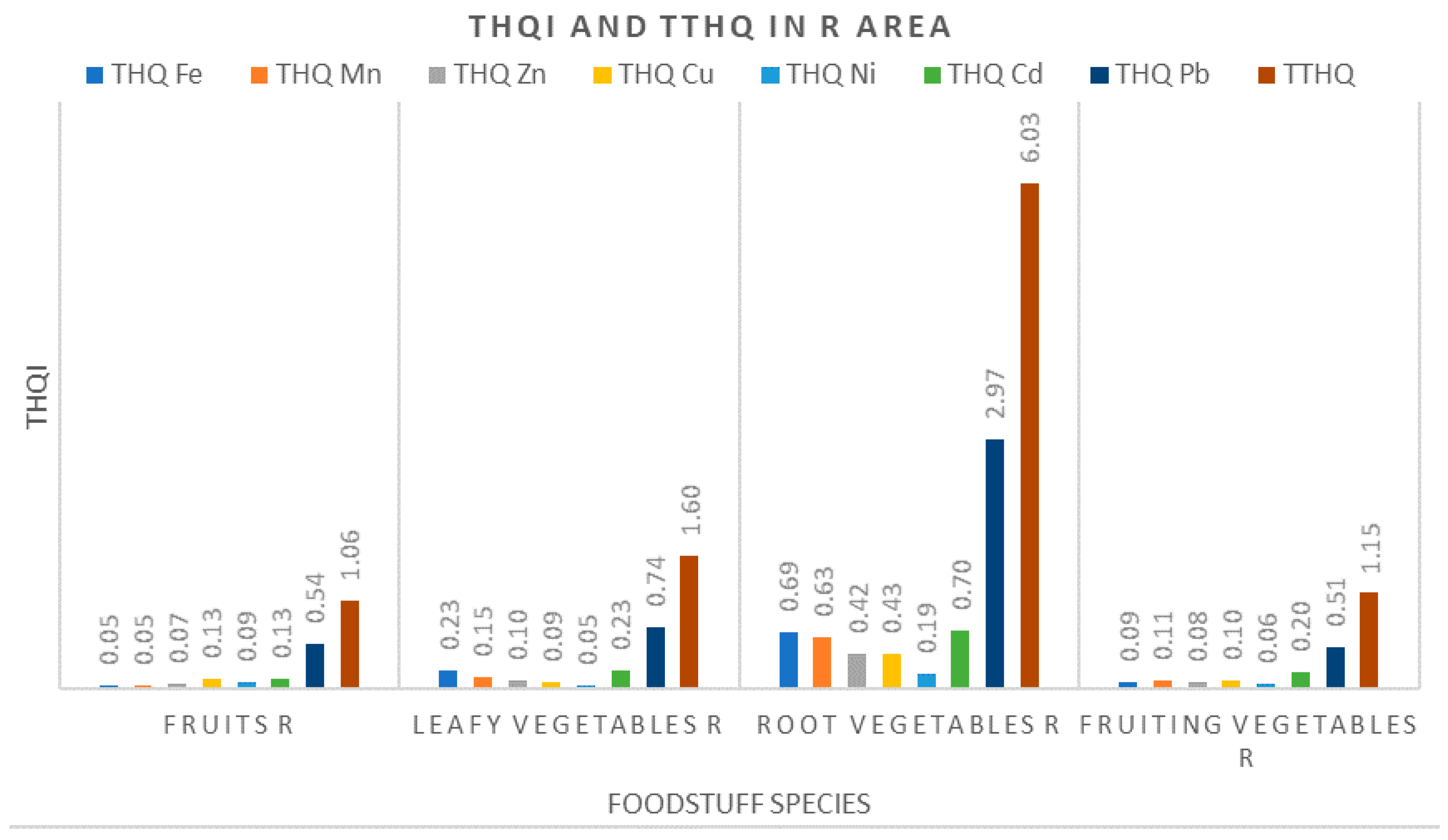
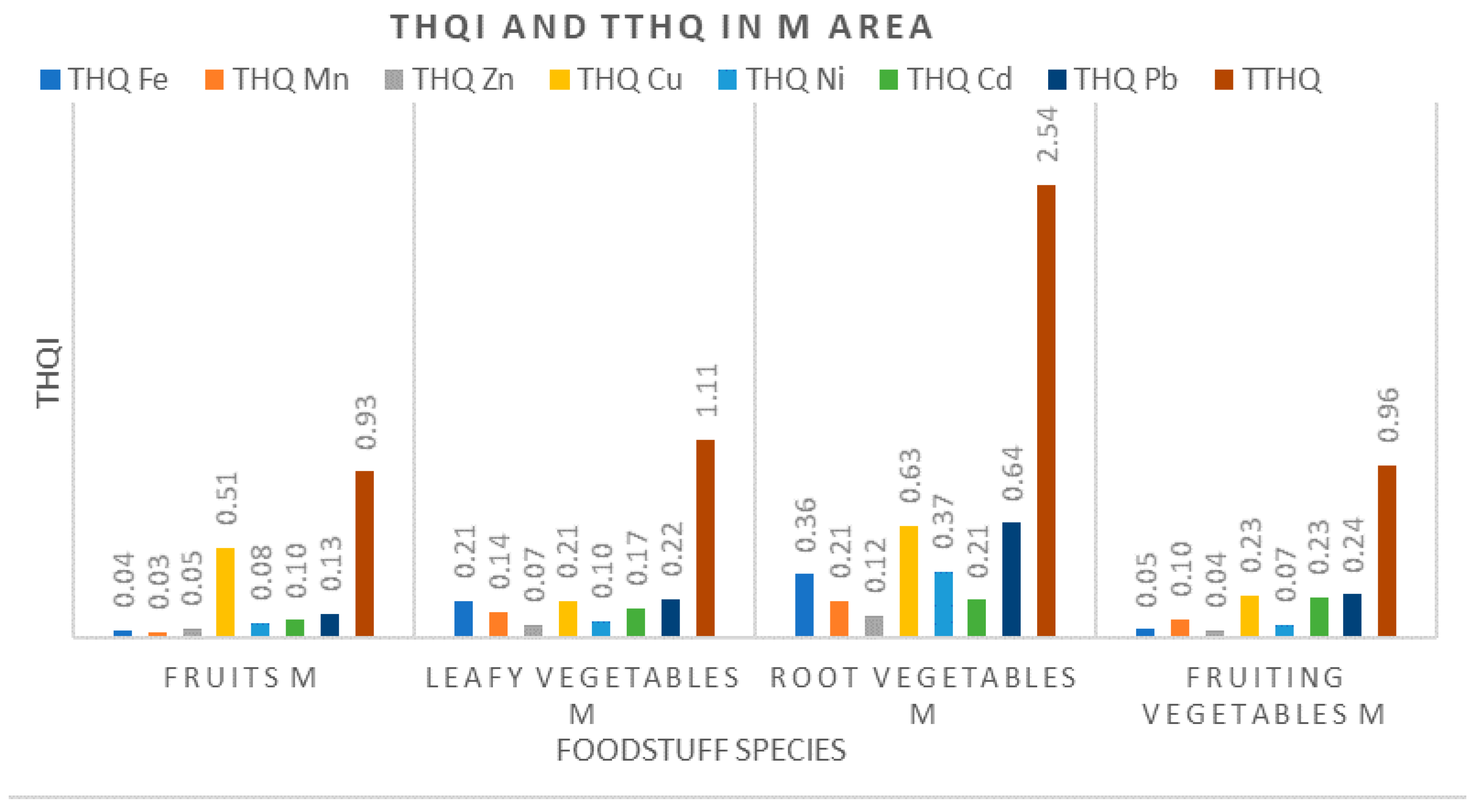
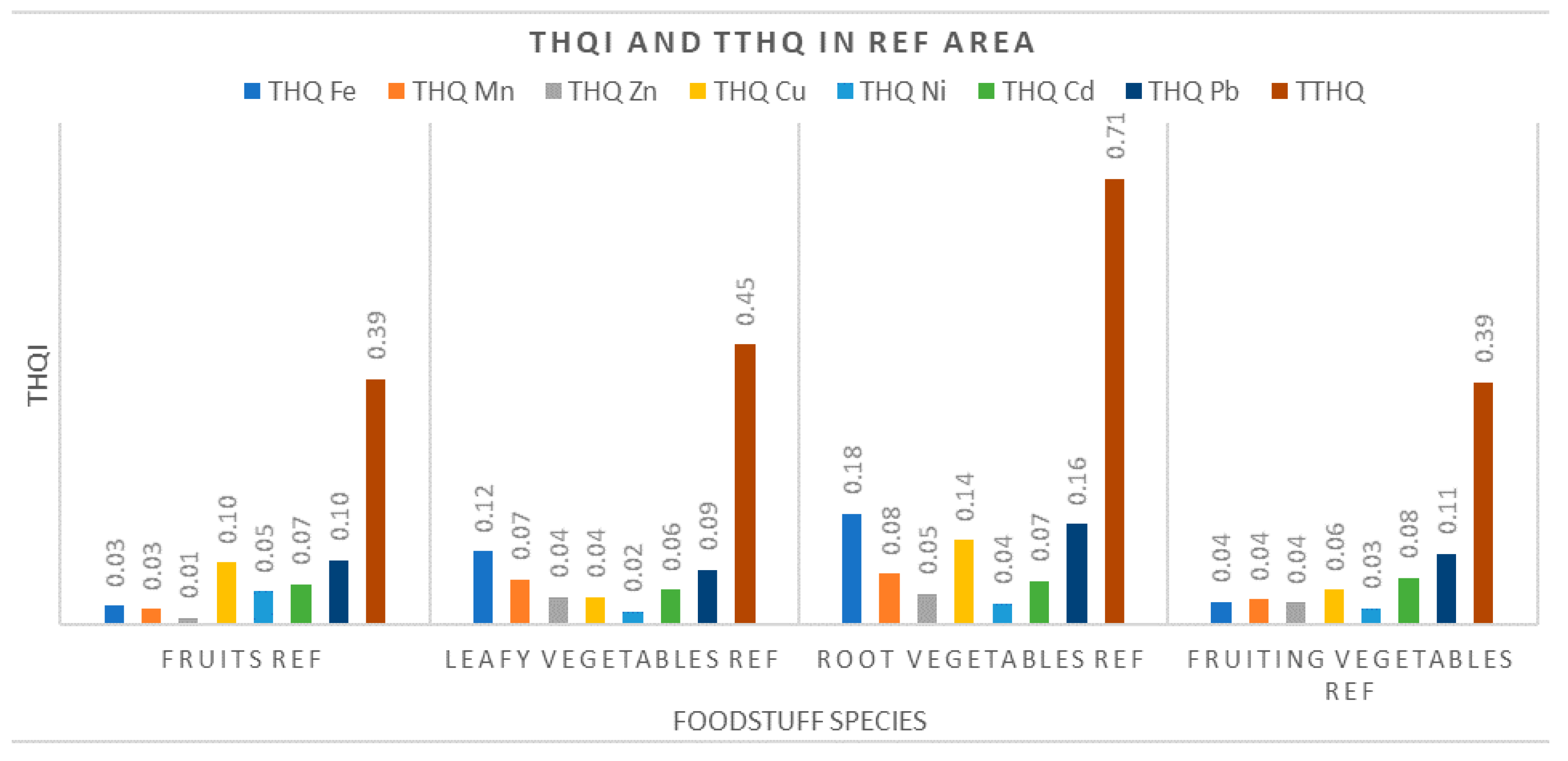
3.3.2. Target Hazard Quotients (THQ) and Total Target Hazard Quotients (TTHQ)
4. Conclusions
- Metal concentrations in the old mining areas of Moldova Veche (M) and Rusca Montana (R) were above the values measured in the nonpolluted reference area, irrespective of metal analyzed and sample type (soil, vegetal foodstuffs);
- Among metals investigated, Pb and Cu were predominant in the R area and in the M area, respectively. The measured values in soils for these metals at the two former mining sites exceeded the normal limits, reaching the Intervention threshold values for Romania (ITV). These metals were also found at high levels in vegetables and fruit collected from these areas, with root vegetables accumulating the highest levels of metals, followed by leafy vegetables and fruity vegetables;
- Assessment of noncarcinogenic risk for the health of local inhabitants was performed based on the current methodology (US EPA–WHO FAO) via calculating the weighted estimated daily intakes of metals (WEDIM), the target hazard quotients (THQ) and the total target hazard quotients (TTHQ). By using these indices, it was found that consumption of root vegetables (parsley, carrot, onion, potatoes) and leafy vegetables (parsley leaves, cabbage, lettuce) originating from either the M area or the R area is not free of health-related risks;
- Our results indicated that local residents from the R area and the M area can be exposed to hazardous metal levels, especially Pb and Cu, through consumption of vegetables and fruits grown in these areas. The population in the Ref area is not exposed to this risk, both parameters THQ and TTHQ having values well below 1;
- Expansion of present research to other routes of metal exposure, e.g., consumption of other foods (cereals, meat, milk), drinking water and contact with air, is imperative for refining our understanding of populational risk associated with former mining areas.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fergusson, J.F. The Heavy Elements: Chemistry, Environmental Impact and Health Effects; Pergamon Press: Oxford, UK, 1990; p. 614. [Google Scholar]
- Gupta, U.C.; Gupta, S.C. Trace element toxicity relationships to crop production and livestock and human health: Implications for management. Commun. Soil Sci. Plant Anal. 1998, 29, 1491–1522. [Google Scholar] [CrossRef]
- Powers, K.; Smith-Weller, T.; Franklin, G.M.; Longstreth, W.T.; Swanson, P.D.; Checkoway, H. Parkinson’s disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology 2003, 60, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Caspi, A.; Belsky, D.W.; Broadbent, J.; Harrington, H.; Sugden, K.; Houts, R.M.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between child hood and adult hood. JAMA 2017, 317, 1244–1251. [Google Scholar] [CrossRef]
- Naser, H.M.; Sultana, S.; Gomes, R.; Noor, S. Heavy metal pollution of soil and vegetable grown near roadside at Gazipur, Bangladesh. J. Agric. Res. 2012, 37, 9–17. [Google Scholar]
- Ismail, A.; Riaz, M.; Akhtar, S.; Ismail, T.; Amir, M.; Zafar-ul-Hye, M. Heavy metals in vegetables and respective soils irrigated by canal, municipal waste and tube well waters. Food Addit. Contam. Part B Surveill. 2014, 7, 213–219. [Google Scholar] [CrossRef]
- Mahmood, A.; Malik, R.N. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab. J. Chem. 2014, 7, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Alghobar, M.A.; Suresha, S. Evaluation of metal accumulation in soil and tomatoes irrigated with sewage water from Mysore city, Karnataka. India. J. Saudi Soc. Agric. Sci. 2017, 16, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Onyedikachi, U.B.; Belonwu, D.C.; Wegwu, M.O. Human health risk assessment of heavy metals in soils and commonly consumed food crops from quarry sites located at Isiagwu, Ebonyi State. Ovidius Univ. Ann. Chem. 2018, 29, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Harmanescu, M.; Alda, L.M.; Bordean, D.M.; Gogoasa, I.; Gergen, I. Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chem. Cent. J. 2011, 5, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaoua, S.; Boussaa, S.; El Gharmali, A.; Boumezzough, A. Impact of irrigation with wastewater on accumulation of heavy metals in soil and crops in the region of Marrakech in Morocco. J. Saudi Soc. Agric. Sci. 2018, 18, 429–436. [Google Scholar] [CrossRef]
- Shaheen, N.N.; Irfan, M.; Khan, I.N.; Islam, S.; Islam, M.S.; Ahmed, M.K. Presence of heavy metals in fruits and vegetables: Health risk implications in Bangladesh. Chemosphere 2016, 152, 431–438. [Google Scholar] [CrossRef]
- Ferreira, K.S.; Gomes, J.C.; Chaves, J.B.P. Cooper content of commonly consumed food in Brazil. Food Chem. 2005, 92, 29–32. [Google Scholar] [CrossRef]
- Javed, M.; Usmani, N. Accumulation of heavy metals and human health risk assessment via the consumption of freshwater fish Mastacembelus armatus inhabiting, thermal power plant effluent loaded canal. Springer Plus 2016, 5, 776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, Z.; Lin, C.J.; Bi, X.; Liu, J.; Feng, X.; Zhang, H.; Chen, J.; Wu, T. Health risks of heavy metal exposure through vegetable consumption near a large-scale Pb/Zn smelter in central China. Ecotoxicol. Environ. Saf. 2018, 161, 99–110. [Google Scholar]
- Pipoyan, D.; Beglaryan, M.; Sireyan, L.; Merendino, N. Exposure assessment of potentially toxic trace elements via consumption of fruits and vegetables grown under the impact of Alaverdi’s mining complex. Hum. Ecol. Risk Assess. 2019, 25, 819–834. [Google Scholar] [CrossRef]
- Liu, C.; Lu, L.; Huang, T.; Huang, Y.; Ding, L.; Zhao, W. The distribution and health risk assessment of metals in soils in the vicinity of industrial sites in Dongguan, China. Int. J. Environ. Res. Public Health 2016, 13, 832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Wang, Y.; Wu, Z.; Hou, C.; Mao, H.; Li, T.; Nie, X. PM2.5-Bound Toxic Elements in an Urban City in East China: Concentrations, Sources, and Health Risks. Int. J. Environ. Res. Public Health 2019, 16, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepanosyan, G.; Sahakyan, L.; Maghakyan, N.; Saghatelyan, A. Combination of compositional data analysis and machine learning approaches to identify sources and geochemical associations of potentially toxic elements in soil and assess the associated human health risk in a mining city. Environ. Pollut. 2020, 261, 114210. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration). Dietary Supplements. Center for Food Safety and Applied Nutrition. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; Report of the Panel on Micronutrients; National Academy Press: Washington, DC, USA, 2001.
- Sharma, A.D. Low Nickel Diet in Dermatology. Indian J. Dermatol. 2013, 58, 240. [Google Scholar] [CrossRef] [PubMed]
- Llobet, J.M.; Falco, G.; Casas, C.; Teixido, A.; Domingo, J.L. Concentration of arsenic, cadmium, mercury, and lead in common foods and estimated daily intake by children, adolescents, adults, and seniors of Catalonia, Spain. J. Agric. Food Chem. 2003, 51, 838–842. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Lead exposure and oxidative stress—A life course approach in US adults. Toxics 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghaie, A.H.; Fereydoni, M. The potential risk of heavy metals on human health due to the daily consumption of vegetables. Environ. Health Eng. Manag. J. 2019, 6, 11–16. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, E.-H.; Park, H.; Ha, M.; Kim, Y.; Hong, Y.-C.; Kim, E.-J.; Kim, B.-N. Prenatal lead and cadmium co-exposure and infant neurodevelopment at 6 months of age: The Mothers and Children’s Environmental Health (MOCEH) study. Neurotoxicology 2013, 35, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Gong, W.; Li, B.; Zuo, J.; Pan, L.; Liu, X. Analysis of heavy metals in foodstuffs and an assessment of the health risks to the general public via consumption in Beijing, China. Int. J. Environ. Res. Public Health 2019, 16, 909. [Google Scholar] [CrossRef] [Green Version]
- Steblez, W.G. The Mineral Industry of ROMANIA. U.S. Geological Survey Minerals Yearbook—1994–1999. Available online: https://www.usgs.gov/centers/nmic/europe-and-central-eurasia#ro (accessed on 5 May 2020).
- Damian, F.; Damian, G.; Lăcătuşu, R.; Iepure, G. Heavy metals concentration of the soils around Zlatna and Copsa Mica smelters Romania. Carpath. J. Earth Environ. Sci. 2008, 3, 65–82. [Google Scholar]
- Secu, C.V.; Iancu, O.G.; Buzgar, N. Lead, zinc and copper in the bioaccumulative horizon of soils from Iasi and the surrounding areas. Carpath. J. Earth Environ. Sci. 2008, 3, 131–144. [Google Scholar]
- Lăcătuşu, R.; Cîtu, G.; Aston, J.; Lungu, M.; Lăcătuşu, A.R. Heavy metals soil pollution state in relation to potential future mining activities in the Roşia Montană Area. Carpath. J. Earth Environ. Sci. 2009, 4, 39–50. [Google Scholar]
- Gogoasa, I.; Oprea, G.; Gergen, I.; Alexa, E.; Cozma, A.; Bordean, D.M.; Moigrădean, D.; Alda, L.M. Researches regarding the soil contamination with heavy metals in the Banat mountains. J. Agroaliment. Process. Technol. 2009, 15, 172–176. [Google Scholar]
- Damian, G.; Damian, F.; Năsui, D.; Pop, C.; Pricop, C. The soils quality from the southern–eastern part of Baia Mare zone affected by metallurgical industry. Carpath. J. Earth Environ. Sci. 2010, 5, 139–147. [Google Scholar]
- Environmental Protection Agency; Office of Water Regulations and Standard. Guidance Manual for Assessing Human Health Risks from Chemically Contaminated, Fish and Shellfish U.S.; EPA-503/8-89-002; Environmental Protection Agency: Washington, DC, USA, 1989.
- WHO. The World Health Report 2002: Reducing Risks, Promoting Healthy Life, World Health Organization. 2002. Available online: https://apps.who.int/iris/handle/10665/42510 (accessed on 5 May 2020).
- RAIS. Risk Exposure Models for Chemicals User’s Guide. 2020. Available online: http://rais.ornl.gov (accessed on 5 May 2020).
- Das, S.N.; Grewal, A.S.; Banerjee, M. A brief review: Heavy metal and their analysis. Int. J. Pharm. Sci. Rev. Res. 2011, 11, 13–18. [Google Scholar]
- Geographical Description. Available online: http://en.wikipedia.org/wiki/Banat (accessed on 6 May 2020).
- Ianoş, G.; Goian, M. Banat’s Soils-Evolution and Agrochemical Characterization, (Solurile Banatului-Evoluţie şi Caracterizare Agrochimică); Mirton: Timişoara, Romanian, 1995; pp. 100–150. [Google Scholar]
- Băjescu, I.; Chiriac, A. Microelements Distribution in the Soils of Romania; Agriculture Implications (Distribuţia Microelementelor în Solurile din Romania; Implicaţii în Agricultură); Ceres: Bucureşti, Romanian, 1984; p. 156. [Google Scholar]
- Wilson, B.; Braithwaite, A.; Pyatt, F.B. An evaluation of procedures for the digestion of soils and vegetation from areas with metalliferous pollution. Toxicol. Environ. Chem. 2005, 87, 335–344. [Google Scholar] [CrossRef]
- Birghilă, S.; Dobrinaş, S.; Matei, N.; Magearu, V.; Popescu, V.; Soceanu, A. Distribution of Cd, Zn and Ascorbic Acid in Different Stages of Tomato Plant Growing. Rev. Chim. 2004, 55, 683–685. [Google Scholar]
- Saulea, M.; Stoica, A.I.; Baiulescu, G.E.; Marinescu, D.; Ionică, M. Determination of Co in Food Samples. Rev. Chim. 2004, 55, 301–303. [Google Scholar]
- US EPA. Risk-Based Concentration Table; United States Environmental Protection Agency: Washington, DC, USA, 2000.
- Life Expectancy in Romania. Available online: https://tinyurl.com/ybou7ggo (accessed on 6 May 2020).
- Institutul National de Statistica Romania. Consumption Availability of the Romanian Population, for 2018.pdf. publ. 2020. Available online: https://insse.ro/cms/ro/tags/disponibilitatile-de-consum-ale-populatiei (accessed on 6 May 2020).
- Average Weight in Romania. Available online: https://adevarul.ro/sanatate/medicina/topul-celor-mai-grase-popoare-lume-situeaza-romanii-1_50aed8ac7c42d5a663a13699/index.html (accessed on 6 May 2020).
- Zheng, N.; Wang, Q.; Zhang, X.; Zheng, D.; Zhang, Z.; Zhang, S. Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city, China. Sci. Total Environ. 2007, 387, 96–104. [Google Scholar]
- Chen, C.; Qian, Y.Z.; Chen, Q.; Li, C.Y. Assessment of Daily Intake of Toxic Elements Due to Consumption of Vegetables, Fruits, Meat, and Seafood by Inhabitants of Xiamen, China. J. Food Sci. 2011, 76, 181–188. [Google Scholar]
- Tepanosyan, G.; Sahakyan, L.; Belyaeva, O.; Asmaryan, S.; Saghatelyan, A. Continuous impact of mining activities on soil heavy metals levels and human health. Sci. Total Environ. 2018, 639, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Ordinul Ministerului Apelor Pădurii şi Protecţiei Mediului nr. 756/1997 Valori de Referinţă Pentru urme de Elemente Chimice în sol (Excerpt from Romanian Ministry of Water, Wood and Environment; Ordinance 756/1997—Reference Values for Trace Elements in Soil). Available online: https://biosol.ro/wp-content/uploads/linkuri/ord-756-din-03-11-1997-pentru-aprobarea-Reglementarii-privind-evaluarea-poluarii-mediului.pdf (accessed on 20 May 2020).
- My Food Data. Fruits and Vegetables High in Iron. Available online: https://www.myfooddata.com/articles/fruits-and-vegetables-high-in-iron.php (accessed on 9 May 2020).
- Schümann, K. Safety aspects of iron in food. Ann. Nutr. Metab. 2001, 45, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Ordinul Ministerului Sănătăţii nr. 975/1998. (Excerpt from Ministry of Public Health, Ordinance no. 975/1998), Limite Maxime de Arsen şi Metale Grele în Alimente (Maximum Limits for Heavy Metals in Food). Available online: https://tinyurl.com/yaq8rky4 (accessed on 20 May 2020).
- Lăcătuşu, R.; Lăcătuşu, A.R. Vegetable and fruits quality within heavy metals polluted areas in Romania. Carpth. J. Earth Environ. Sci. 2008, 3, 115–129. [Google Scholar]
- Kachenko, A.G.; Singh, B. Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut. 2006, 169, 101–123. [Google Scholar] [CrossRef]
- Micic, R.; Mitic, S.; Arsic, B.; Jokic, A.; Mitic, M.; Kostic, D.; Pavlovic, A.; Cekerevac, M.; Nikolic-Bujanovic, L.; Spalevic, Z. Statistical characteristics of selected elements in vegetables from Kosovo. Environ. Monit. Assess. 2015, 187, 389. [Google Scholar]
- Schäfer, U. Manganese In: Elements and their Compounds in the Environment, 2nd ed.; Merian, E., Anke, M., Ihnat, M., Stoepler, M., Eds.; Wiley-VCH: Weinheim, Germany, 2004; pp. 901–930. [Google Scholar]
- Secer, M.; Bodur, A.; Elmaci, O.L.; Delibacak, S.; Iqbal, N. Trace element and heavy metal concentrations in fruits and vegetables of the Gediz River region. Int. J. Water 2002, 2, 196–211. [Google Scholar] [CrossRef]
- Guerra, F.; Trevizam, A.R.; Muraoka, T.; Marcante, N.C.; Canniatti-Brazaca, S.G. Heavy metals in vegetables and potential risk for human health. Sci. Agric. 2012, 69, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Gebeyehu, H.R.; Bayissa, L.D. Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia. PLoS ONE 2020, 15, e0227883. [Google Scholar] [CrossRef] [Green Version]
- Soceanu, A. Presence of heavy metals in fruits from Prunus genera. Ovidius Univ. Ann. Chem. 2009, 20, 108–110. [Google Scholar]
- The Risk Assessment Information System. RAIS Toxicity Values and Physical Parameters Search. Available online: https://rais.ornl.gov/cgi-bin/tools/TOX_search (accessed on 11 May 2020).
| Area/Metal Contents | Fe | Mn | Zn | Cu | Ni | Cd | Pb |
|---|---|---|---|---|---|---|---|
| R Area | 56,445.00 | 5622.33 | 359.33 | 49.33 | 15.00 | 2.08 | 136.00 |
| SD | 1698.51 | 375.58 | 21.23 | 4.19 | 0.82 | 0.18 | 4.55 |
| M Area | 44,548.33 | 1860.67 | 203.67 | 231.00 | 18.67 | 0.39 | 23.00 |
| SD | 413.67 | 53.61 | 16.13 | 16.57 | 2.05 | 0.07 | 1.63 |
| Ref Area | 32,563.33 | 2060.33 | 130.67 | 24.67 | 10.00 | 0.17 | 14.77 |
| SD | 385.81 | 153.23 | 11.44 | 2.49 | 0.82 | 0.02 | 0.71 |
| Normal contents for | 900.00 | 100.00 | 20.00 | 20.00 | 1.00 | 20.00 | |
| Romania (NC) | |||||||
| Alert threshold values | 1500.00 | 300.00 | 100.00 | 75.00 | 3.00 | 50.00 | |
| for Romania (ATV) | |||||||
| Intervention threshold | 2500.00 | 600.00 | 200.00 | 150.00 | 5.00 | 100.00 | |
| values for Romania (ITV) |
| Vegetables | Fe | ±SD | Mn | ±SD | Zn | ±SD | Cu | ±SD | Ni | ±SD | Cd | ±SD | Pb | ±SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAL | 15.00 | 5.00 | 0.20 | 0.50 | ||||||||||
| Leafy vegetables | ||||||||||||||
| Carrot leaf R # | 31.59 * | 5.62 | 3.13 * | 0.64 | 3.77 | 0.81 | 0.4 * | 0.08 | 0.17 * | 0.02 | 0.01 * | 0.00 | 0.20 | 0.07 |
| Carrot leaf M # | 51.45 | 7.48 | 5.57 | 0.40 | 5.61 | 0.40 | 2.12 | 0.18 | 0.38 | 0.09 | 0.04 | 0.00 | 0.12 * | 0.00 |
| Carrot leaf Ref # | 14.25 | 1.75 | 1.38 | 0.09 | 1.36 | 0.36 | 0.29 | 0.02 | 0.11 | 0.02 | 0.01 | 0.00 | 0.03 | 0.00 |
| Parsley leaf R # | 104.03 | 26.52 | 7.32 | 1.56 | 9.39 * | 1.21 | 1.77 * | 0.11 | 0.38 * | 0.02 | 0.09 | 0.01 | 1.97 | 0.20 |
| Parsley leaf M # | 106.75 | 13.63 | 9.72 | 1.55 | 10.44 * | 2.16 | 4.79 | 0.75 | 1.87 | 0.19 | 0.05 | 0.01 | 0.50 | 0.08 |
| Parsley leaf Ref # | 73.17 | 5.51 | 5.88 | 0.65 | 9.13 | 2.45 | 1.03 | 0.27 | 0.30 | 0.11 | 0.03 | 0.01 | 0.28 | 0.07 |
| Cabbage R # | 60.11 | 6.34 | 10.47 | 1.46 | 16.30 | 3.74 | 1.36 | 0.15 | 0.70 | 0.11 | 0.12 | 0.02 | 0.90 | 0.30 |
| Cabbage M # | 31.53 * | 15.54 | 9.15 | 4.24 | 8.51 | 4.55 | 2.77 | 0.90 | 0.33 | 0.18 | 0.06 | 0.03 | 0.25 | 0.16 |
| Cabbage Ref # | 16.06 | 2.95 | 3.85 | 0.80 | 3.28 | 0.63 | 0.45 | 0.10 | 0.13 | 0.03 | 0.01 | 0.00 | 0.05 | 0.01 |
| Lettuce R # | 35.88 | 4.36 | 8.33 | 1.32 | 14.46 | 2.77 | 1.86 | 0.27 | 0.28 | 0.04 | 0.09 | 0.01 | 0.62 | 0.12 |
| Lettuce M # | 16.9 * | 2.55 | 4.12 * | 0.60 | 5.14 * | 0.80 | 2.22 | 0.40 | 0.18 * | 0.03 | 0.09 | 0.01 | 0.21 | 0.11 |
| Lettuce Ref # | 13.60 | 1.40 | 3.46 | 0.55 | 5.32 | 0.68 | 0.76 | 0.15 | 0.10 | 0.03 | 0.02 | 0.00 | 0.08 | 0.02 |
| Vegetables | Fe | ±SD | Mn | ±SD | Zn | ±SD | Cu | ±SD | Ni | ±SD | Cd | ±SD | Pb | ±SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAL | 15.00 | 5.00 | 0.20 | 0.50 | ||||||||||
| Root and bulb vegetables | ||||||||||||||
| Carrot root R # | 29.97 | 1.57 | 3.07 | 0.78 | 4.93 | 0.55 | 1.53 | 0.09 | 0.18 | 0.02 | 0.08 | 0.02 | 2.11 | 0.31 |
| Carrot root M # | 31.90 | 2.05 | 2.23 * | 0.69 | 3.17 | 0.34 | 1.77 | 0.11 | 0.08 * | 0.02 | 0.03 | 0.00 | 0.09 | 0.01 |
| Carrot root Ref # | 17.30 | 0.25 | 1.43 | 0.18 | 2.07 | 0.27 | 0.73 | 0.07 | 0.04 | 0.02 | 0.01 | 0.00 | 0.04 | 0.00 |
| Parsley root R # | 221.80 | 35.12 | 37.83 | 4.65 | 45.83 | 3.10 | 5.67 | 0.97 | 1.28 | 0.14 | 0.20 | 0.00 | 2.45 | 1.96 |
| Parsley root M # | 99.00 | 5.23 | 8.10 | 0.65 | 7.73 | 0.69 | 6.87 | 0.61 | 3.82 | 0.64 | 0.04 | 0.01 | 0.66 | 0.07 |
| Parsley root Ref # | 48.77 | 6.52 | 2.97 | 0.36 | 3.50 | 0.30 | 1.23 | 0.19 | 0.19 | 0.03 | 0.01 | 0.00 | 0.08 | 0.07 |
| Onion R # | 15.27 | 2.23 | 4.07 | 0.26 | 10.90 | 1.10 | 0.43 * | 0.05 | 0.21 | 0.02 | 0.06 | 0.01 | 0.50 | 0.04 |
| Onion M # | 4.67 | 0.31 | 1.33 | 0.11 | 2.00 | 0.19 | 1.37 | 0.15 | 0.01 | 0.00 | 0.01 | 0.00 | 0.13 | 0.01 |
| Onion Ref # | 1.57 | 0.33 | 0.33 | 0.04 | 0.77 | 0.07 | 0.23 | 0.05 | 0.03 | 0.00 | 0.01 | 0.00 | 0.04 | 0.00 |
| Potato R | 12.75 | 0.27 | 4.92 | 0.74 | 6.02 | 0.39 | 2.8 * | 0.76 | 0.63 | 0.08 | 0.05 | 0.01 | 1.06 | 0.08 |
| Potato M | 10.42 | 0.77 | 3.92 | 0.18 | 4.14 | 0.14 | 7.36 | 0.89 | 0.68 | 0.03 | 0.04 | 0.00 | 0.45 | 0.08 |
| Potato Ref | 8.21 | 0.66 | 1.99 | 0.15 | 2.35 | 0.35 | 1.94 | 0.15 | 0.22 | 0.03 | 0.02 | 0.00 | 0.25 | 0.04 |
| Vegetables | Fe | ±SD | Mn | ±SD | Zn | ±SD | Cu | ±SD | Ni | ±SD | Cd | ±SD | Pb | ±SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruiting vegetables | ||||||||||||||
| Cucumber R # | 2.40 | 0.12 | 6.73 | 0.81 | 8.97 | 1.12 | 0.97 | 0.06 | 0.28 * | 0.03 | 0.13 | 0.02 | 0.72 | 0.01 |
| Cucumber M # | 2.40 | 0.31 | 6.00 | 0.67 | 1.40 * | 0.16 | 2.40 | 0.21 | 0.54 | 0.06 | 0.15 | 0.02 | 0.37 | 0.04 |
| Cucumber Ref # | 1.70 | 0.11 | 0.47 | 0.05 | 0.93 | 1.00 | 0.47 | 0.04 | 0.23 | 0.03 | 0.03 | 0.01 | 0.16 | 0.03 |
| Green bean # | 58.87 | 11.29 | 7.07 | 0.66 | 13.23 | 1.08 | 1.07 | 0.15 | 0.49 | 0.05 | 0.06 | 0.03 | 0.35 | 0.07 |
| Green bean M # | 26.7 * | 5.16 | 6.77 | 0.51 | 10.17 * | 1.01 | 1.43 | 0.14 | 0.52 | 0.05 | 0.07 | 0.03 | 0.19 | 0.02 |
| Green bean Ref # | 21.20 | 2.19 | 4.43 | 0.41 | 9.67 | 1.06 | 0.30 | 0.08 | 0.22 | 0.04 | 0.03 | 0.01 | 0.07 | 0.02 |
| Tomato R | 7.22 | 0.24 | 5.10 | 0.27 | 9.34 | 0.58 | 2.06 * | 0.76 | 0.48 | 0.06 | 0.05 | 0.00 | 0.85 | 0.08 |
| Tomato M | 6.15 | 0.82 | 4.70 | 0.35 | 8.33 | 0.80 | 4.22 | 0.43 | 0.32 | 0.05 | 0.05 | 0.02 | 0.41 | 0.02 |
| Tomato Ref | 2.87 | 0.59 | 1.78 | 0.20 | 2.09 | 0.73 | 0.89 | 0.16 | 0.13 | 0.04 | 0.02 | 0.01 | 0.18 | 0.01 |
| Fruits Spec | Fe | ±SD | Mn | ±SD | Zn | ±SD | Cu | ±SD | Ni | ±SD | Cd | ±SD | Pb | ±SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAL | 15.00 | 5.00 | 0.20 | 0.50 | ||||||||||
| Apricot R | 11.49 | 1.01 | 2.53 | 0.43 | 8.14 | 0.16 | 2.09 * | 0.14 | 0.94 | 0.12 | 0.05 | 0.01 | 0.74 | 0.05 |
| Apricot M | 9.49 * | 0.96 | 1.81 * | 0.16 | 6.04 | 0.14 | 8.02 | 0.53 | 0.81 | 0.06 | 0.04 | 0.01 | 0.15 | 0.01 |
| Apricot Ref | 8.12 | 0.97 | 1.59 | 0.13 | 1.15 | 0.16 | 1.88 | 0.16 | 0.45 | 0.04 | 0.02 | 0.01 | 0.12 | 0.01 |
| Sw cher R | 7.13 * | 0.91 | 2.20 | 0.45 | 5.84 | 0.23 | 1.27 * | 0.09 | 0.45 | 0.05 | 0.04 | 0.01 | 0.46 | 0.04 |
| Sw cher M | 6.59 * | 1.04 | 1.14 * | 0.15 | 4.08 | 0.45 | 5.22 | 0.21 | 0.29 | 0.04 | 0.03 | 0.03 | 0.15 | 0.01 |
| Sw cher Ref | 5.62 | 0.51 | 0.93 | 0.16 | 1.17 | 0.32 | 1.33 | 0.16 | 0.14 | 0.01 | 0.02 | 0.01 | 0.11 | 0.02 |
| Peach R | 12.27 | 1.29 | 2.90 * | 0.56 | 8.09 | 0.89 | 1.97 | 0.14 | 0.94 * | 0.20 | 0.04 | 0.01 | 0.77 | 0.06 |
| Peach M | 10.24 * | 1.09 | 2.26 * | 0.25 | 6.16 | 0.66 | 8.61 | 0.48 | 0.84 * | 0.07 | 0.04 | 0.01 | 0.16 | 0.01 |
| Peach Ref | 9.16 | 1.07 | 2.12 | 0.38 | 1.07 | 0.21 | 1.44 | 0.13 | 0.72 | 0.07 | 0.02 | 0.01 | 0.12 | 0.01 |
| Plum R | 9.20 | 1.09 | 1.83 | 0.38 | 6.08 | 0.55 | 0.88 | 0.12 | 0.48 | 0.07 | 0.03 * | 0.01 | 0.49 | 0.02 |
| Plum M | 7.16 | 0.85 | 0.9 * | 0.10 | 4.39 | 0.53 | 4.68 | 0.28 | 0.39 * | 0.06 | 0.02 * | 0.01 | 0.15 | 0.01 |
| Plum Ref | 4.35 | 0.59 | 0.78 | 0.06 | 1.19 | 0.14 | 0.48 | 0.12 | 0.29 | 0.04 | 0.02 | 0.01 | 0.10 | 0.01 |
| S cherry R | 10.70 | 1.16 | 2.00 | 0.18 | 6.84 | 0.74 | 2.12 | 0.14 | 0.49 | 0.08 | 0.05 | 0.01 | 0.47 | 0.06 |
| S cherry M | 9.99 | 0.98 | 1.01 * | 0.10 | 4.25 | 0.23 | 5.26 | 0.18 | 0.34 | 0.05 | 0.03 * | 0.01 | 0.14 | 0.01 |
| S cherry Ref | 7.35 | 0.69 | 0.92 | 0.10 | 1.26 | 0.15 | 1.45 | 0.20 | 0.17 | 0.02 | 0.02 | 0.01 | 0.10 | 0.01 |
| Apple R | 10.11 | 1.05 | 0.65 | 0.07 | 4.34 | 0.28 | 0.99 | 0.09 | 0.36 * | 0.06 | 0.04 | 0.01 | 0.56 | 0.05 |
| Apple M | 9.16 | 1.03 | 0.46 * | 0.04 | 2.93 | 0.41 | 4.91 | 0.48 | 0.28 * | 0.04 | 0.02 * | 0.01 | 0.13 * | 0.01 |
| Apple Ref | 6.51 | 0.51 | 0.37 | 0.08 | 1.17 | 0.19 | 0.82 | 0.04 | 0.24 | 0.04 | 0.02 | 0.01 | 0.11 | 0.01 |
| Pear R | 8.19 | 0.99 | 0.55 | 0.05 | 4.08 | 0.08 | 0.95 * | 0.07 | 0.32 * | 0.08 | 0.03 * | 0.01 | 0.56 | 0.05 |
| Pear M | 6.34 | 1.07 | 0.35 | 0.04 | 2.79 | 0.27 | 4.87 | 0.44 | 0.30 * | 0.06 | 0.02 * | 0.01 | 0.11 * | 0.01 |
| Pear Ref | 3.64 | 0.57 | 0.25 | 0.04 | 0.97 | 0.04 | 0.87 | 0.04 | 0.25 | 0.05 | 0.02 | 0.01 | 0.11 | 0.01 |
| Grape R | 11.15 | 1.08 | 3.05 | 0.22 | 7.11 | 0.30 | 1.99 | 0.10 | 0.58 | 0.08 | 0.03 | 0.01 | 0.61 | 0.11 |
| Grape M | 10.08 | 1.01 | 1.88 | 0.08 | 4.81 | 0.33 | 7.71 | 0.23 | 0.55 | 0.05 | 0.03 | 0.01 | 0.16 | 0.01 |
| Grape Ref | 7.87 | 0.86 | 1.88 | 0.12 | 0.98 | 0.11 | 1.50 | 0.08 | 0.35 | 0.05 | 0.02 | 0.01 | 0.12 | 0.01 |
| Foodstuff | WEDIM Fe | WEDIM Mn | WEDIM Zn | WEDIM Cu | WEDIM Ni | WEDIM Cd | WEDIM Pb |
|---|---|---|---|---|---|---|---|
| UL (mg/day/person) | 45 | 11 | 40 (25) | 10 | 1 (0.2) | 0.064 | 0.24 |
| Fruits R | 2.11 | 0.41 | 1.33 | 0.32 | 0.12 | 0.01 | 0.12 |
| Leafy vegetables R | 10.42 | 1.32 | 1.98 | 0.24 | 0.07 | 0.01 | 0.17 |
| Root vegetables R | 30.85 | 5.63 | 7.99 | 1.09 | 0.24 | 0.05 | 0.67 |
| Fruiting vegetables R | 3.95 | 1.03 | 1.51 | 0.26 | 0.07 | 0.01 | 0.11 |
| SUM R area | 47.33 | 8.39 | 12.80 | 1.91 | 0.50 | 0.08 | 1.07 |
| Fruit M | 1.81 | 0.26 | 0.93 | 1.29 | 0.10 | 0.01 | 0.03 |
| Leafy vegetables M | 9.30 | 1.29 | 1.34 | 0.54 | 0.12 | 0.01 | 0.05 |
| Root vegetables M | 15.94 | 1.84 | 2.39 | 1.60 | 0.48 | 0.01 | 0.14 |
| Fruiting vegetables M | 2.11 | 0.89 | 0.84 | 0.60 | 0.09 | 0.01 | 0.05 |
| SUM M area | 29.16 | 4.27 | 5.50 | 4.03 | 0.79 | 0.05 | 0.28 |
| Fruit Ref | 1.38 | 0.23 | 0.24 | 0.26 | 0.07 | 0.00 | 0.02 |
| Leafy vegetables Ref | 5.27 | 0.66 | 0.86 | 0.11 | 0.03 | 0.00 | 0.02 |
| Root vegetables Ref | 7.93 | 0.73 | 0.95 | 0.35 | 0.05 | 0.00 | 0.04 |
| Fruiting vegetables Ref | 1.66 | 0.37 | 0.69 | 0.14 | 0.04 | 0.00 | 0.03 |
| SUM Ref area | 16.24 | 1.99 | 2.73 | 0.86 | 0.18 | 0.02 | 0.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manea, D.N.; Ienciu, A.A.; Ştef, R.; Şmuleac, I.L.; Gergen, I.I.; Nica, D.V. Health Risk Assessment of Dietary Heavy Metals Intake from Fruits and Vegetables Grown in Selected Old Mining Areas—A Case Study: The Banat Area of Southern Carpathians. Int. J. Environ. Res. Public Health 2020, 17, 5172. https://doi.org/10.3390/ijerph17145172
Manea DN, Ienciu AA, Ştef R, Şmuleac IL, Gergen II, Nica DV. Health Risk Assessment of Dietary Heavy Metals Intake from Fruits and Vegetables Grown in Selected Old Mining Areas—A Case Study: The Banat Area of Southern Carpathians. International Journal of Environmental Research and Public Health. 2020; 17(14):5172. https://doi.org/10.3390/ijerph17145172
Chicago/Turabian StyleManea, Dan Nicolae, Anişoara Aurelia Ienciu, Ramona Ştef, Iosefina Laura Şmuleac, Iosif Ion Gergen, and Dragos Vasile Nica. 2020. "Health Risk Assessment of Dietary Heavy Metals Intake from Fruits and Vegetables Grown in Selected Old Mining Areas—A Case Study: The Banat Area of Southern Carpathians" International Journal of Environmental Research and Public Health 17, no. 14: 5172. https://doi.org/10.3390/ijerph17145172
APA StyleManea, D. N., Ienciu, A. A., Ştef, R., Şmuleac, I. L., Gergen, I. I., & Nica, D. V. (2020). Health Risk Assessment of Dietary Heavy Metals Intake from Fruits and Vegetables Grown in Selected Old Mining Areas—A Case Study: The Banat Area of Southern Carpathians. International Journal of Environmental Research and Public Health, 17(14), 5172. https://doi.org/10.3390/ijerph17145172





