Assessment of Deoxynivalenol in Wheat, Corn and Its Products and Estimation of Dietary Intake
Abstract
1. Introduction
- (1)
- Examining the amount of DON in wheat, corn and their products from summer and winter seasons;
- (2)
- Relating the concentrations of DON with EU recommended limits;
- (3)
- Estimating the dietary intake of DON in wheat and corn products.
2. Materials and Methods
2.1. Samples
2.2. Chemicals and Reagents
2.3. Extraction of DON and HPLC Conditions
2.4. Dietary Intake Evaluation
2.5. Method Validation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Method Validation
3.2. DON Incidence in Wheat and What Products
3.3. Corn and Products
3.4. Dietary Estimation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Awika, J.M. Major cereal grains production and use around the world. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; American Chemical Society: Washington, DC, USA, 2011; Volume 1089, pp. 1–13. [Google Scholar]
- Available online: http://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 4 June 2020).
- Iqbal, S.Z.; Paterson, R.R.M.; Bhatti, I.A.; Asi, M.R.; Sheikh, M.A.; Bhatti, H.N. Aflatoxin B1 in chilies from the Punjab region, Pakistan. Mycotoxin Res. 2010, 26, 205–209. [Google Scholar] [CrossRef]
- Villa, P.; Markaki, P. Aflatoxin B1 and ochratoxin A in breakfast cereals from Athens market: Occurrence and risk assessment. Food Control 2009, 20, 455–461. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Malik, S.; Asi, M.R.; Jinap, S.; Malik, N. Natural occurrence of patulin in different fruits, juices and smoothies and evaluation of dietary intake. Food Control 2018, 84, 370–374. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Hanif, U.; Zuber, M.; Jinap, S. The presence of aflatoxins and ochratoxin A in rice and rice products; and evaluation of dietary intake. Food Chem. 2016, 210, 135–140. [Google Scholar] [CrossRef]
- Palacios, S.A.; Erazo, J.G.; Ciasca, B.; Lattanzio, V.M.T.; Reynoso, M.M.; Farnochi, M.C.; Torres, A.M. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat from Argentina. Food Chem. 2017, 230, 728–734. [Google Scholar] [CrossRef]
- Masood, M.; Iqbal, S.Z.; Asi, M.R.; Malik, N. Natural occurrence of aflatoxins in dry fruits and edible nuts. Food Control 2015, 55, 62–65. [Google Scholar] [CrossRef]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Martins, M.; Almeida, I.; Marques, M.F.; Guerra, M.M. Fumonisins and deoxynivalenol in corn-based food products in Portugal. Food Chem. Toxicol. 2008, 46, 2585–2587. [Google Scholar] [CrossRef]
- Ji, X.; Yang, H.; Wang, J.; Li, R.; Zhao, H.; Xu, J.; Xiao, Y.; Tang, B.; Qian, M. Occurrence of deoxynivalenol (DON) in cereal-based food products marketed through e-commerce stores and an assessment of dietary exposure of Chinese consumers to DON. Food Control 2018, 92, 391–398. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health Part B 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. Eur. J. Union 2006, 364, 5–24. [Google Scholar]
- USDA. Pakistan: Grain and Feed Annual. 2017. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Grain%20and%20Feed%20Annual_Islamabad_Pakistan_4-3-2017.pdf (accessed on 7 July 2020).
- Iqbal, S.Z.; Asi, M.R.; Jinap, S.; Rashid, U. Detection of aflatoxins and zearalenone contamination in wheat derived products. Food Control 2014, 35, 223–226. [Google Scholar] [CrossRef]
- Alim, M.; Iqbal, S.Z.; Mahmood, Z.; Asi, M.R.; Zikar, H.; Chanda, H.; Malik, N. Survey of mycotoxins in retail market cereals, derived products and dietary assessment. Food Control 2018, 84, 471–477. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Rehman, B.; Jinap, S.; Akram, N.; Ahmad, M.N.; Sanny, M. Assessment of fumonisin B1 concentrations in wheat and barley products in the Punjab region of Pakistan. J. Food Prot. 2020. [Google Scholar] [CrossRef]
- Khatoon, S.; Hanif, Q.N.; Tahira, I.; Sultana, N.; Sultana, K.; Ayub, N. Natural occurrence of aflatoxins, zearalenone and trichothecenes in maize grown in Pakistan. Pak. J. Bot. 2012, 44, 231–236. [Google Scholar]
- Sahar, N.; Ahmed, M.; Parveen, Z.; Ilyas, A.; Bhutto, A. Screening of mycotoxins in wheat, fruits and vegetables grown in Sindh, Pakistan. Pak. J. Bot. 2009, 41, 337–341. [Google Scholar]
- Golge, O.; Kabak, B. Occurrence of deoxynivalenol and zearalenone in cereals and cereal products from Turkey. Food Control 2020, 110, 106982. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Zhou, T.; Yang, D.; Wang, Q.; Zhou, Y. Occurrence of four mycotoxins in cereal and oil products in Yangtze Delta region of China and their food safety risks. Food Control 2014, 35, 117–122. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Zhang, Y.; Lu, M.; Sun, L.; Li, W.; Hu, X.; Wang, B. Retention of deoxynivalenol and its derivatives during storage of wheat grain and flour. Food Control 2016, 65, 177–181. [Google Scholar] [CrossRef]
- Rahmani, A.; Jinap, S.; Soleimany, F. Validation of the procedure for the simultaneous determination of aflatoxins ochratoxin A and zearalenone in cereals using HPLC-FLD. Food Addit. Contam. 2010, 27, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, 70, 19–23. [Google Scholar]
- Borras-Vallverdú, B.; Ramos, A.J.; Marín, S.; Sanchis, V.; Rodríguez-Bencomo, J.J. Deoxynivalenol degradation in wheat kernels by exposition to ammonia vapours: A tentative strategy for detoxification. Food Control 2020, 118, 107744. [Google Scholar] [CrossRef]
- Şengül, Ü. Comparing determination methods of detection and quantification limits for aflatoxin analysis in Hazelnut. J. Food Drug Anal. 2016, 24, 56–62. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 mycotoxins in the grain of cereals cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Wang, L.; Chang, F.; Yang, L. Occurrence of deoxynivalenol in wheat, Hebei Province, China. Food Chem. 2016, 197, 1271–1274. [Google Scholar] [CrossRef]
- Ji, F.; Xu, J.; Liu, X.; Yin, X.; Shi, J. Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu province, China. Food Chem. 2014, 15, 393–397. [Google Scholar] [CrossRef]
- Obonyo, M.A.; Salano, E.N. Perennial and seasonal contamination of maize by aflatoxins in eastern Kenya. Int. J. Food Contam. 2018. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. How will climate change affect mycotoxins in food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Kamala, A.; Ortiz, J.; Kimanya, M.; Haesaert, G.; Donoso, S.; Tiisekwa, B.; Meulenaer, B.D. Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of Tanzania. Food Control 2015, 54, 208–215. [Google Scholar] [CrossRef]
- Abia, W.; Warth, A.; Sulyok, M.; Krska, R.; Tchana, A.N.; Njobeh, P.B. Determination of multi-mycotoxin occurrence in cereals, nuts and their products in Cameroon by liquid chromatography tandem mass spectrometry (LCMS/MS). Food Control 2013, 31, 438–453. [Google Scholar] [CrossRef]
- Cirillo, T.; Ritieni, A.; Galvano, F.; Cocchieri, R.A. Natural co-occurrence of deoxynivalenol and fumonisins B1 and B2 in Italian marketed foodstuffs. Food Addit. Contam. 2003, 20, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Sokolović, M.; Perši, N.; Zadravec, M.; Jaki, V.; Vulić, A. Contamination of maize with deoxynivalenol and zearalenone in Croatia. Food Control 2012, 28, 94–98. [Google Scholar] [CrossRef]
- Setyabudi, F.M.C.S.; Nuryono, N.; Wedhastri, S.; Mayer, H.K.; Razzazi-Fazeli, E. Limited survey of deoxynivalenol occurrence in maize kernels and maizeproducts collected from Indonesian retail market. Food Control 2012, 24, 123–127. [Google Scholar] [CrossRef]
- Martos, P.; Thompson, W.; Diaz, G. Multiresidue mycotoxin analysis in wheat, barley, oats, rye and maize grain by high-performance liquid chromatography tandem mass spectrometry. World Mycotox J. 2010, 3, 205–223. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T. Approaches to the risk analysis of mycotoxins in the food supply. In Proceedings of the Food and Nutrition div. Eng Joint FAO/WHO/UNEP International Conference on Mycotoxins, Tunis, Tunisia, 3–6 March 1999; Volume 23, pp. 10–16. [Google Scholar]
- Raad, F.; Nasreddine, L.; Hilan, C.; Bartosik, M.; Parent-Massin, D. Dietary exposure to aflatoxins, ochratoxin A and deoxynivalenol from a total diet study in an adult urban Lebanese population. Food Chem. Toxicol. 2014, 73, 35–43. [Google Scholar] [CrossRef]
- Cano-Sancho, G.; Ramos, V.; Marín, S.; Sanchis, V. Micotoxinas. Estudio de dieta Total en Cataluña 2008–2009; Generalitat de Cataluña: Barcelona, Spain, 2012; pp. 135–140. [Google Scholar]
- Ostry, V.; Dofkova, M.; Blahova, J.; Malir, F.; Kavrik, R.; Rehurkova, I.; Ruprich, J. Dietary exposure assessment of sum deoxynivalenol forms, sum T-2/HT-2 toxins and zearalenone from cereal-based foods and beer. Food Chem. Toxicol. 2020, 139, 111280. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, O.; Juan, C.; Miere, D.; Berrada, H.; Loghin, F.; Mañes, J. First study on trichothecene and zearalenone exposure of the Romanian population through wheat based products consumption. Food Chem. Toxicol. 2018, 121, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Assunção, R.; Martins, C.; Vasco, E.; Jager, A.; Oliveira, C.; Cunha, S.C.; Fernandes, J.O.; Nunes, B.; Loureiro, S.; Alvito, P. Portuguese children dietary exposure to multiple mycotoxins—An overview of risk assessment under MYCOMIX project. Food Chem. Toxicol. 2018, 118, 399–408. [Google Scholar] [CrossRef] [PubMed]
| Don Level | Wheat | Wheat Flour | Corn | Corn Flour | ||||
|---|---|---|---|---|---|---|---|---|
| µg/Kg | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) |
| 100 | 82.5 | 14 | 80.8 | 9 | 87.4 | 10 | 88.9 | 13 |
| 200 | 87.8 | 18 | 85.4 | 12 | 89.8 | 16 | 90.0 | 15 |
| 400 | 89.0 | 19 | 84.7 | 19 | 92.6 | 20 | 86.7 | 10 |
| 800 | 90.0 | 10 | 88.9 | 10 | 88.9 | 25 | 85.6 | 18 |
| 1600 | 87.8 | 23 | 84.1 | 19 | 86.7 | 25 | 86.7 | 20 |
| 2000 | 89.0 | 26 | 82.0 | 21 | 89.5 | 27 | 83.5 | 27 |
| Samples Type | Summer | Winter | ||||||
|---|---|---|---|---|---|---|---|---|
| Samples | Positive | Mean | Range | Samples | Positive | Mean | Range | |
| n | n (%) | µg/kg ± S.D | µg/kg | n | n (%) | µg/kg ± S.D | µg/kg | |
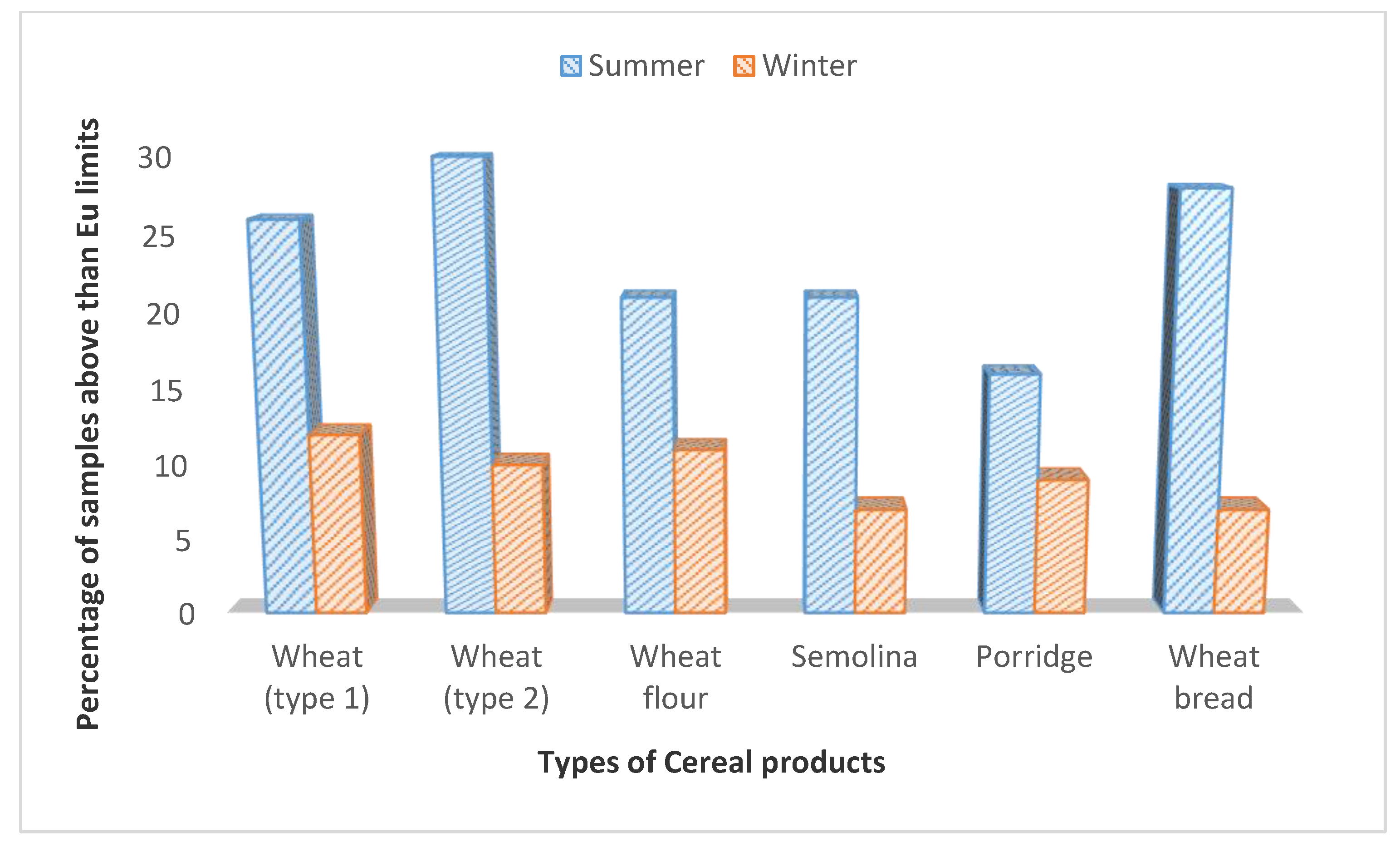
| Wheat (type 1) | 34 | 13 (38.2) | 1083.5 ± 15.2 a | LOD-2145 | 25 | 11 (44.0) | 1020.8 ± 21.6 a | LOD-1945 |
| Wheat (type 2) | 40 | 21 (52.5) | 945.1 ± 18.9 b | LOD-2021 | 40 | 15 (37.5) | 790 ± 20.9 c | LOD-2050 |
| Wheat flour | 38 | 21 (55.2) | 1325.5 ± 24.7 c | LOD-1890 | 38 | 15 (39.5) | 1080.7 ± 30.5 a | LOD-1500 |
| Semolina | 42 | 18 (42.8) | 1026.4 ± 17.3 a | LOD-1459 | 41 | 18 (43.9) | 750. 7 ± 25.6 b | LOD-1130 |
| Wheat porridge | 49 | 20 (40.8) | 934.1 ±19.2 b | LOD-980 | 46 | 19 (41.3) | 690. 5 ± 19.6 c | LOD-950 |
| Wheat bread | 29 | 11 (37.9) | 990.4± 21.8 b | LOD-1120 | 27 | 13 (48.1) | 705.6 ± 23.7 c | LOD-1080 |
| Total | 232 | 104 (44.8) | LOD-2145 | 217 | 91 (41.9) | L0D-2050 | ||
| Winter | Summer | |||||||
|---|---|---|---|---|---|---|---|---|
| Samples Type | Samples | Positive | Mean ± S.D | Range | Samples | Positive | Mean ± S.D | Range |
| n | n (%) | µg/kg | µg/kg | n | n (%) | µg/kg | µg/kg | |
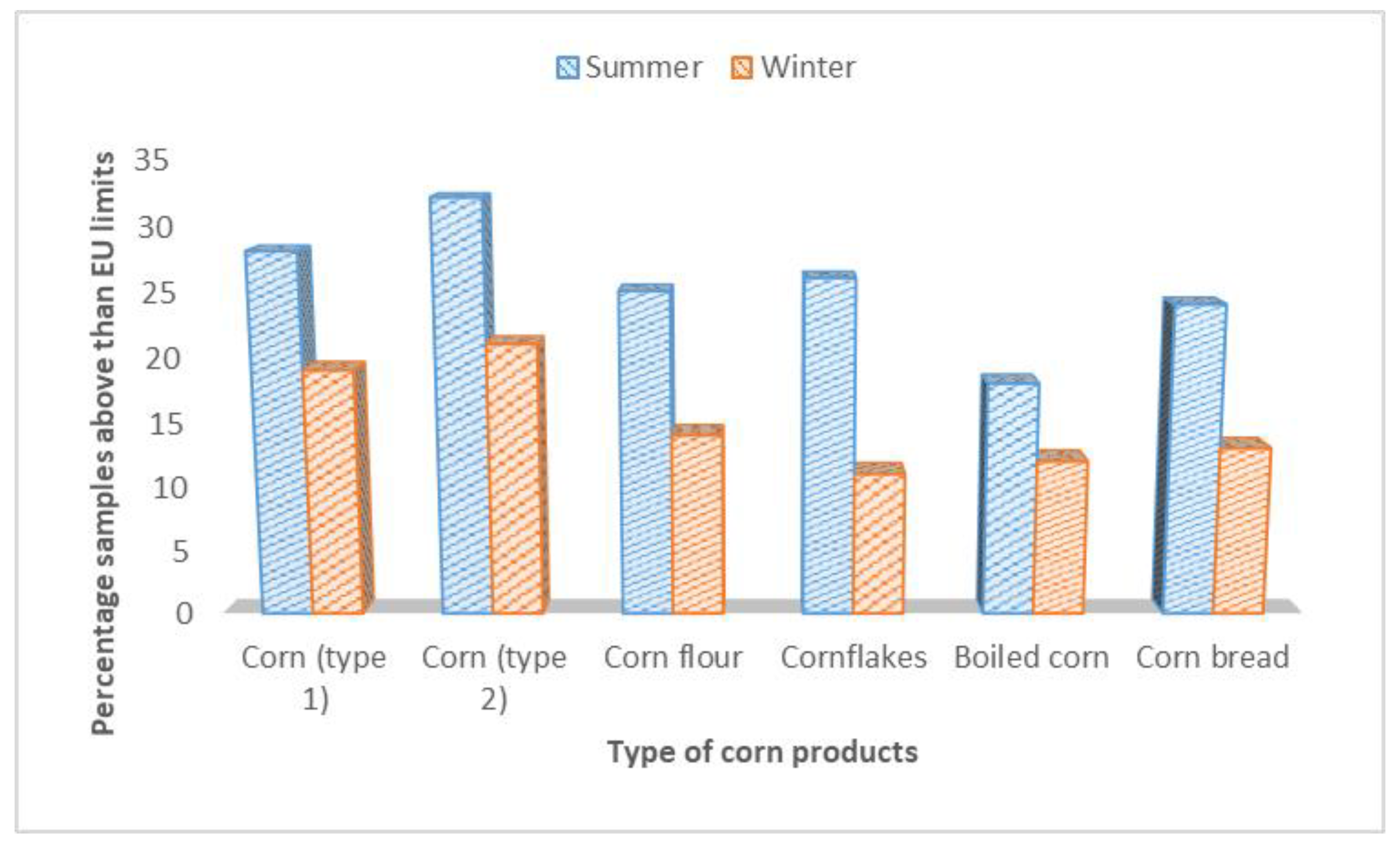
| Corn (type 1) | 25 | 14 (56.0) | 1345.7 ± 22.3 a | LOD-2967 | 20 | 9 (45.5) | 978. 7 ± 15.6 a | LOD-2490 |
| Corn (type 2) | 26 | 16 (61.5) | 998.2 ± 11.2 b | LOD-2234 | 25 | 11(44.0) | 680.6 ± 11.8 c | LOD-2650 |
| Corn flour | 25 | 18 (72.0) | 1434.8 ± 25.5 a | LOD-2434 | 26 | 12 (46.1) | 860.7 ± 13.9 b | LOD-1460 |
| Cornflakes | 24 | 12 (50.0) | 1245.9 ± 18.5 a | LOD-1534 | 22 | 10 (45.4) | 910.4 ± 23.7 b | LOD-1090 |
| Boiled corn | 22 | 13 (59.5) | 1067.5 ±14.1 b | LOD-920 | 20 | 9 (45.0) | 850.9 ± 15.7 d | LOD-980 |
| Corn bread | 20 | 14 (70.0) | 640.5± 11.3 c | LOD-1320 | 15 | 6 (40.0) | 620.8 ± 17.8 0c | LOD-1250 |
| Total | 142 | 87 (61.2) | LOD-2967 | 128 | 57 (44.5) | LOD-2490 | ||
| Summer | Winter | ||||
|---|---|---|---|---|---|
| Cereal Product | Per Capita Consumption | DON Mean Level | Dietary Intake | DON Mean Level | Dietary Intake |
| g day−1 | ng g−1 | µg day−1 kg−1 Bodyweight | (ng g−1) | µg day−1 kg−1 Bodyweight | |
| Wheat flour | 400 | 1325.5 | 8.84 | 1080.7 | 7.21 |
| Bread | 400 | 990.4 | 6.61 | 705.6 | 4.71 |
| Semolina | 80 | 1026.4 | 1.37 | 750.7 | 1.01 |
| Porridge | 90 | 934.1 | 1.41 | 690.5 | 1.04 |
| Corn flour | 200 | 1434.8 | 4.78 | 860.7 | 2.87 |
| Bread | 200 | 640.5 | 2.14 | 620.8 | 2.07 |
| Cornflakes | 80 | 1245.9 | 1.66 | 910.4 | 1.24 |
| Boiled corn | 200 | 1067.5 | 3.56 | 850.9 | 2.84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, S.Z.; Usman, S.; Razis, A.F.A.; Basheir Ali, N.; Saif, T.; Asi, M.R. Assessment of Deoxynivalenol in Wheat, Corn and Its Products and Estimation of Dietary Intake. Int. J. Environ. Res. Public Health 2020, 17, 5602. https://doi.org/10.3390/ijerph17155602
Iqbal SZ, Usman S, Razis AFA, Basheir Ali N, Saif T, Asi MR. Assessment of Deoxynivalenol in Wheat, Corn and Its Products and Estimation of Dietary Intake. International Journal of Environmental Research and Public Health. 2020; 17(15):5602. https://doi.org/10.3390/ijerph17155602
Chicago/Turabian StyleIqbal, Shahzad Zafar, Sunusi Usman, Ahmad Faizal Abdull Razis, Nada Basheir Ali, Tahmina Saif, and Muhammad Rafique Asi. 2020. "Assessment of Deoxynivalenol in Wheat, Corn and Its Products and Estimation of Dietary Intake" International Journal of Environmental Research and Public Health 17, no. 15: 5602. https://doi.org/10.3390/ijerph17155602
APA StyleIqbal, S. Z., Usman, S., Razis, A. F. A., Basheir Ali, N., Saif, T., & Asi, M. R. (2020). Assessment of Deoxynivalenol in Wheat, Corn and Its Products and Estimation of Dietary Intake. International Journal of Environmental Research and Public Health, 17(15), 5602. https://doi.org/10.3390/ijerph17155602










