Assessment of Functional Activities in Individuals with Parkinson’s Disease Using a Simple and Reliable Smartphone-Based Procedure
Abstract
1. Introduction
2. Materials and Methods
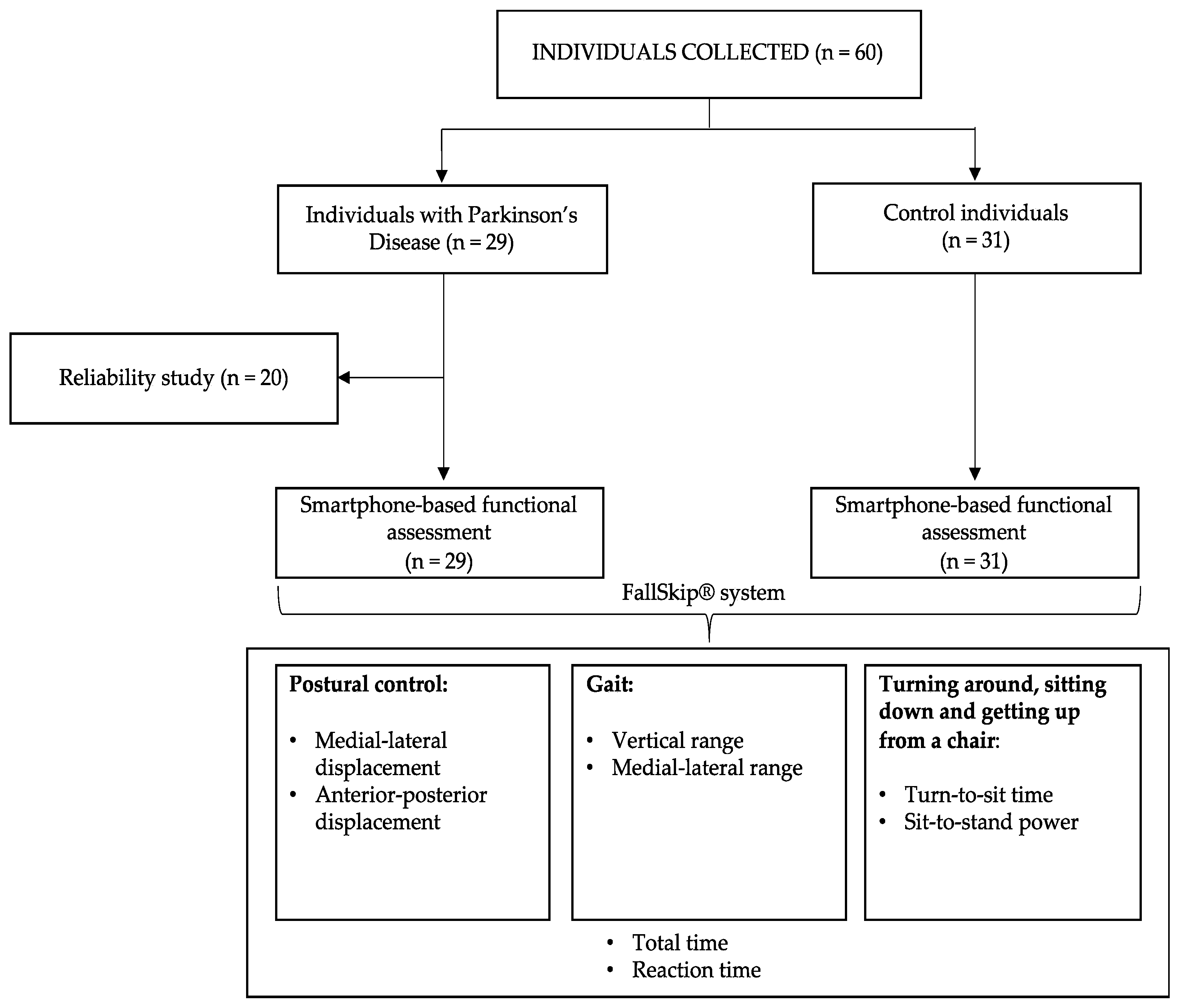
2.1. Participants
2.2. Sample Size Calculation
2.3. Reliability of the Assessment Protocol
2.4. Smartphone-Based Functional Assessment
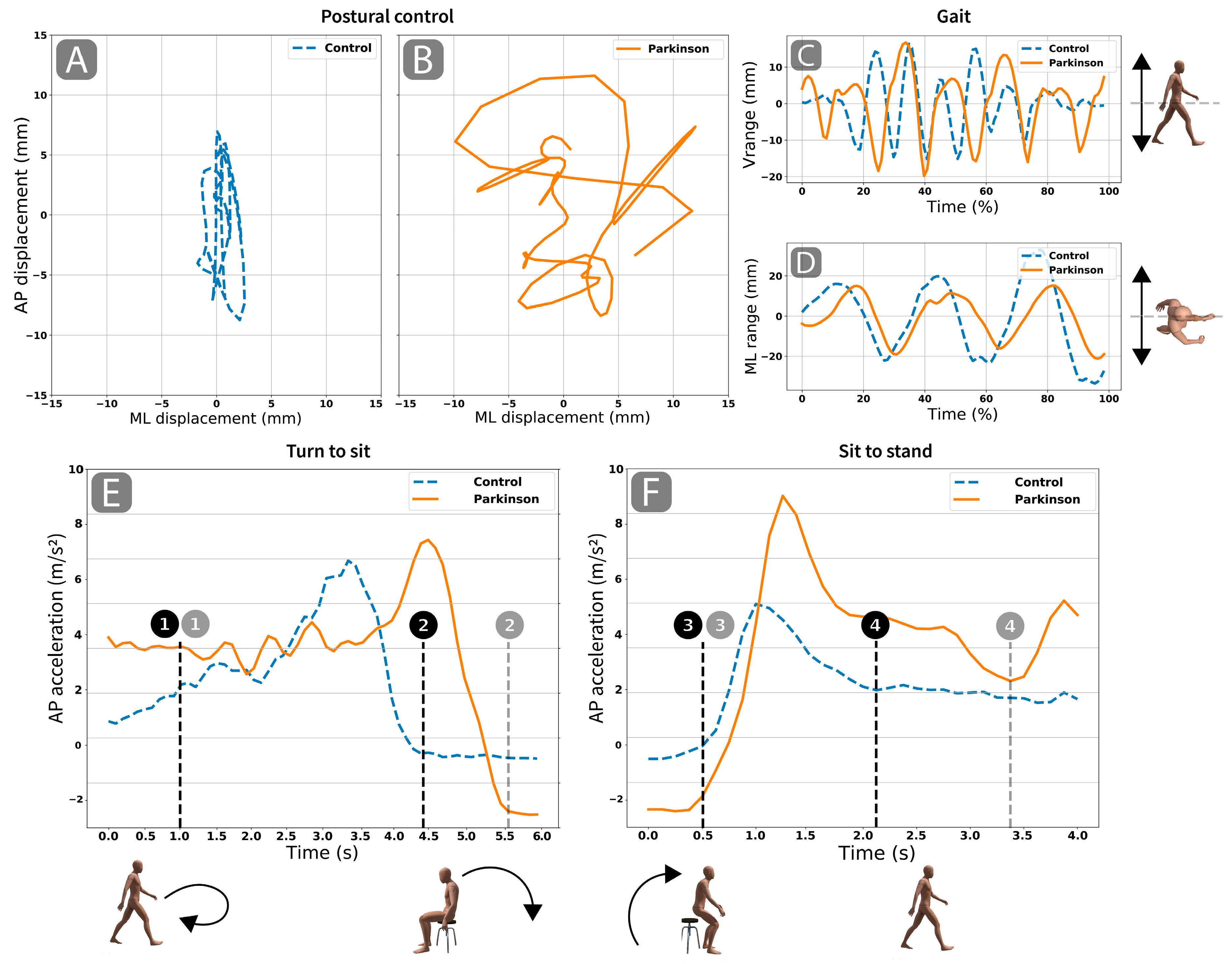
2.5. Smartphone Data Analysis and Outcomes
2.6. Statistics
3. Results
3.1. Participants
3.2. Reliability Study
3.3. Functional Assessment Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Soh, S.-E.; McGinley, J.L.; Watts, J.J.; Iansek, R.; Murphy, A.T.; Menz, H.B.; Huxham, F.; Morris, M.E. Determinants of health-related quality of life in people with Parkinson’s disease: A path analysis. Qual. Life Res. 2013, 22, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.K.; Wong-Yu, I.S. Exercise for Parkinson’s disease. Int. Rev. Neurobiol. 2019, 147, 1–44. [Google Scholar] [PubMed]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- King, L.A.; Wilhelm, J.; Chen, Y.; Blehm, R.; Nutt, J.; Chen, Z.; Serdar, A.; Horak, F.B. Effects of Group, Individual, and Home Exercise in Persons with Parkinson Disease: A Randomized Clinical Trial. J. Neurol. Phys. Ther. JNPT 2015, 39, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Haji Ghassemi, N.; Hannink, J.; Roth, N.; Gaßner, H.; Marxreiter, F.; Klucken, J.; Eskofier, B.M. Turning Analysis during Standardized Test Using On-Shoe Wearable Sensors in Parkinson’s Disease. Sensors 2019, 19, 3103. [Google Scholar] [CrossRef]
- Weiss, A.; Herman, T.; Mirelman, A.; Shiratzky, S.S.; Giladi, N.; Barnes, L.L.; Bennett, D.A.; Buchman, A.S.; Hausdorff, J.M. The transition between turning and sitting in patients with Parkinson’s disease: A wearable device detects an unexpected sequence of events. Gait Posture 2019, 67, 224–229. [Google Scholar] [CrossRef]
- Pham, M.H.; Warmerdam, E.; Elshehabi, M.; Schlenstedt, C.; Bergeest, L.-M.; Heller, M.; Haertner, L.; Ferreira, J.J.; Berg, D.; Schmidt, G. Validation of a Lower Back “Wearable”-Based Sit-to-Stand and Stand-to-Sit Algorithm for Patients with Parkinson’s Disease and Older Adults in a Home-Like Environment. Front. Neurol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Rojas, H.A.G.; Cuevas, P.C.; Figueras, E.E.Z.; Foix, S.C.; Egea, A.J.S. Time measurement characterization of stand-to-sit and sit-to-stand transitions by using a smartphone. Med. Biol. Eng. Comput. 2018, 56, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Del Din, S.; Godfrey, A.; Mazzà, C.; Lord, S.; Rochester, L. Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov. Disord. 2016, 31, 1293–1313. [Google Scholar] [CrossRef]
- Galán-Mercant, A.; Barón-López, F.J.; Labajos-Manzanares, M.T.; Cuesta-Vargas, A.I. Reliability and criterion-related validity with a smartphone used in timed-up-and-go test. Biomed. Eng. Online 2014, 13, 156. [Google Scholar] [CrossRef]
- López-Pascual, J.; Hurtado, A.J.; Inglés, M.; Espí-López, G.; Serra-Añó, P. P 151-Reliability of variables measured with an Android device during a modified timed up and go test in patients with Alzheimer’s disease. Gait Posture 2018, 65, 484. [Google Scholar] [CrossRef]
- Serra-Añó, P.; Pedrero-Sánchez, J.F.; Hurtado-Abellán, J.; Inglés, M.; Espí-López, G.V.; López-Pascual, J. Mobility assessment in people with Alzheimer disease using smartphone sensors. J. Neuroeng. Rehabil. 2019, 16, 103. [Google Scholar] [CrossRef]
- Yahalom, G.; Yekutieli, Z.; Israeli-Korn, S.; Elincx-Benizri, S.; Livneh, V.; Fay-Karmon, T.; Tchelet, K.; Rubel, Y.; Hassin-Baer, S. Smartphone Based Timed Up and Go Test Can Identify Postural Instability in Parkinson’s Disease. Isr. Med. Assoc. J. Imaj 2020, 22, 37–42. [Google Scholar] [PubMed]
- Kerr, G.K.; Worringham, C.J.; Cole, M.H.; Lacherez, P.F.; Wood, J.M.; Silburn, P.A. Predictors of future falls in Parkinson disease. Neurology 2010, 75, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Channa, A.; Popescu, N.; Ciobanu, V. Wearable Solutions for Patients with Parkinson’s Disease and Neurocognitive Disorder: A Systematic Review. Sensors 2020, 20, 2713. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 2001, 57, 11–26. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Dal Bello-Haas, V.; Klassen, L.; Sheppard, M.S.; Metcalfe, A. Psychometric properties of activity, self-efficacy, and quality-of-life measures in individuals with Parkinson disease. Physiother. Can. 2011, 63, 47–57. [Google Scholar] [CrossRef]
- Ribeiro, J.G.; De Castro, J.T.; Freire, J.L. Using the FFT-DDI method to measure displacements with piezoelectric, resistive and ICP accelerometers. In Proceedings of the Conference and exposition on structural dynamics; Citeseer: Rio de Janeiro, Brazil, 2003. [Google Scholar]
- Zijlstra, W.; Hof, A.L. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture 2003, 18, 1–10. [Google Scholar] [CrossRef]
- Prieto, T.E.; Myklebust, J.B.; Hoffmann, R.G.; Lovett, E.G.; Myklebust, B.M. Measures of postural steadiness: Differences between healthy young and elderly adults. IEEE Trans. Biomed. Eng. 1996, 43, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Esser, P.; Dawes, H.; Collett, J.; Howells, K. IMU: Inertial sensing of vertical CoM movement. J. Biomech. 2009, 42, 1578–1581. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.E.; Ferris, D.P.; Kuo, A.D. Metabolic and mechanical energy costs of reducing vertical center of mass movement during gait. Arch. Phys. Med. Rehabil. 2009, 90, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, U.; Claus, H.; Stuber, M.; Augat, P.; Muche, R.; Nikolaus, T.; Becker, C. Measuring power during the sit-to-stand transfer. Eur. J. Appl. Physiol. 2003, 89, 466–470. [Google Scholar] [CrossRef]
- Ansai, J.H.; de Andrade, L.P.; Rossi, P.G.; Nakagawa, T.H.; Vale, F.A.C.; Rebelatto, J.R. Differences in Timed Up and Go Subtasks Between Older People with Mild Cognitive Impairment and Mild Alzheimer’s Disease. Motor Control 2018, 20, 1–12. [Google Scholar] [CrossRef]
- Beauchet, O.; Annweiler, C.; Callisaya, M.L.; De Cock, A.-M.; Helbostad, J.L.; Kressig, R.W.; Srikanth, V.; Steinmetz, J.-P.; Blumen, H.M.; Verghese, J. Poor gait performance and prediction of dementia: Results from a meta-analysis. J. Am. Med. Dir. Assoc. 2016, 17, 482–490. [Google Scholar] [CrossRef]
- Delval, A.; Tard, C.; Defebvre, L. Why we should study gait initiation in Parkinson’s disease. Neurophysiol. Clin. Neurophysiol. 2014, 44, 69–76. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420. [Google Scholar] [CrossRef]
- Cicchetti, D.V.; Sparrow, S.A. Developing criteria for establishing interrater reliability of specific items: Applications to assessment of adaptive behavior. Am. J. Ment. Defic. Am. J. Ment. Defic. 1981, 127–137. [Google Scholar]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
- de Carvalho, A.O.; Sá Filho, A.S.; Murillo-Rodriguez, E.; Rocha, N.B.; Carta, M.G.; Machado, S. Physical exercise for parkinson’s disease: Clinical and experimental evidence. Clin. Pract. Epidemiol. Ment. Health CP EMH 2018, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, C.L.; Patel, S.; Meek, C.; Herd, C.P.; Clarke, C.E.; Stowe, R.; Shah, L.; Sackley, C.M.; Deane, K.H.; Wheatley, K. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst. Rev. 2013, 11, 1–96. [Google Scholar] [CrossRef] [PubMed]
- Keus, S.; Munneke, M.; Graziano, M.; Paltamaa, J.; Pelosin, E.; Domingos, J.; Brühlmann, S.; Ramaswamy, B.; Prins, J.; Struiksma, C. European physiotherapy guideline for Parkinson’s disease. Neth. KNGFParkinsonNet 2014, 1–19. [Google Scholar]
- Shen, X.; Wong-Yu, I.S.; Mak, M.K. Effects of exercise on falls, balance, and gait ability in Parkinson’s disease: A meta-analysis. Neurorehabil. Neural Repair 2016, 30, 512–527. [Google Scholar] [CrossRef]
- Post, B.; Muslimovic, D.; van Geloven, N.; Speelman, J.D.; Schmand, B.; de Haan, R.J.; CARPA-study group. Progression and prognostic factors of motor impairment, disability and quality of life in newly diagnosed Parkinson’s disease. Mov. Disord. 2011, 26, 449–456. [Google Scholar] [CrossRef]
- Leddy, A.L.; Crowner, B.E.; Earhart, G.M. Functional gait assessment and balance evaluation system test: Reliability, validity, sensitivity, and specificity for identifying individuals with Parkinson disease who fall. Phys. Ther. 2011, 91, 102–113. [Google Scholar] [CrossRef]
- Park, J.-H.; Kang, Y.-J.; Horak, F.B. What is wrong with balance in Parkinson’s disease? J. Mov. Disord. 2015, 8, 109. [Google Scholar] [CrossRef]
- Frenklach, A.; Louie, S.; Koop, M.M.; Bronte-Stewart, H. Excessive postural sway and the risk of falls at different stages of Parkinson’s disease. Mov. Disord. 2009, 24, 377–385. [Google Scholar] [CrossRef]
- Doná, F.; Aquino, C.C.; Gazzola, J.M.; Borges, V.; Silva, S.C.A.; Ganança, F.F.; Caovilla, H.H.; Ferraz, H.B. Changes in postural control in patients with Parkinson’s disease: A posturographic study. Physiotherapy 2016, 102, 272–279. [Google Scholar] [CrossRef]
- Takakusaki, K.; Habaguchi, T.; Ohtinata-Sugimoto, J.; Saitoh, K.; Sakamoto, T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: A new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience 2003, 119, 293–308. [Google Scholar] [CrossRef]
- Blaszczyk, J.W.; Orawiec, R.; Duda-K\lodowska, D.; Opala, G. Assessment of postural instability in patients with Parkinson’s disease. Exp. Brain Res. 2007, 183, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, G.A.; Margaria, R. Mechanics of walking. J. Appl. Physiol. 1966, 21, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Lebel, K.; Boissy, P.; Bogard, S.; Goubault, E.; Duval, C. Auto detection and segmentation of daily living activities during a Timed Up and Go task in people with Parkinson’s disease using multiple inertial sensors. J. Neuroengineering Rehabil. 2017, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.E.; Chou, L.-S. Can motion of individual body segments identify dynamic instability in the elderly? Clin. Biomech. 2003, 18, 737–744. [Google Scholar] [CrossRef]
- Donelan, J.M.; Shipman, D.W.; Kram, R.; Kuo, A.D. Mechanical and metabolic requirements for active lateral stabilization in human walking. J. Biomech. 2004, 37, 827–835. [Google Scholar] [CrossRef]
- Howell, D.R.; Osternig, L.R.; Chou, L.-S. Dual-task effect on gait balance control in adolescents with concussion. Arch. Phys. Med. Rehabil. 2013, 94, 1513–1520. [Google Scholar] [CrossRef]
- Akram, S.; Frank, J.S.; Jog, M. Parkinson’s disease and segmental coordination during turning: I. Standing turns. Can. J. Neurol. Sci. 2013, 40, 512–519. [Google Scholar] [CrossRef]
- Verheyden, G.; Willems, A.-M.; Ooms, L.; Nieuwboer, A. Validity of the trunk impairment scale as a measure of trunk performance in people with Parkinson’s disease. Arch. Phys. Med. Rehabil. 2007, 88, 1304–1308. [Google Scholar] [CrossRef]
- Mak, M.K.; Hui-Chan, C.W. The speed of sit-to-stand can be modulated in Parkinson’s disease. Clin. Neurophysiol. 2005, 116, 780–789. [Google Scholar] [CrossRef]
- Inkster, L.M.; Eng, J.J. Postural control during a sit-to-stand task in individuals with mild Parkinson’s disease. Exp. Brain Res. 2004, 154, 33–38. [Google Scholar] [CrossRef]
- Mak, M.K.; Levin, O.; Mizrahi, J.; Hui-Chan, C.W. Joint torques during sit-to-stand in healthy subjects and people with Parkinson’s disease. Clin. Biomech. 2003, 18, 197–206. [Google Scholar] [CrossRef]
- Trujillo, J.P.; Gerrits, N.J.; Vriend, C.; Berendse, H.W.; van den Heuvel, O.A.; van der Werf, Y.D. Impaired planning in P arkinson’s disease is reflected by reduced brain activation and connectivity. Hum. Brain Mapp. 2015, 36, 3703–3715. [Google Scholar] [CrossRef] [PubMed]
- Nocera, J.R.; Stegemöller, E.L.; Malaty, I.A.; Okun, M.S.; Marsiske, M.; Hass, C.J.; Investigators, N.P.F.Q.I.I. Using the Timed Up & Go test in a clinical setting to predict falling in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2013, 94, 1300–1305. [Google Scholar] [PubMed]
- Byl, N.; Henry, R.; Rizzo, R. Is the timed up and go (TUG) sensitive to differentiating patients with mild to moderate PD compared to age matched controls: A descriptive pilot study. Int. Phys. Med. Rehab. J. 2018, 3, 157–165. [Google Scholar] [CrossRef][Green Version]
- Barry, E.; Galvin, R.; Keogh, C.; Horgan, F.; Fahey, T. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta-analysis. BMC Geriatr. 2014, 14, 14. [Google Scholar] [CrossRef]
- Cohen, R.G.; Nutt, J.G.; Horak, F.B. Recovery from multiple APAs delays gait initiation in Parkinson’s disease. Front. Hum. Neurosci. 2017, 11, 60. [Google Scholar] [CrossRef]
- Bloem, B.R.; Hausdorff, J.M.; Visser, J.E.; Giladi, N. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. 2004, 19, 871–884. [Google Scholar] [CrossRef]
| Observer 1 | Observer 2 | ICC | |
|---|---|---|---|
| Medial-lateral displacement (mm) | 13.16 (9.33) | 16.11 (12.57) | 0.71 |
| Anterior-posterior displacement (mm) | 31.62 (13.26) | 34.15 (20.77) | 0.62 |
| Vertical range (mm) | 24.13 (8.00) | 22.58 (7.28) | 0.92 |
| Medial-lateral range (mm) | 25.63 (13.23) | 25.28 (16.11) | 0.89 |
| Turn-to-sit time (s) | 76.92 (25.66) | 86.67 (30.43) | 0.89 |
| Sit-to-stand power (W) | 4.98 (1.69) | 4.42 (1.48) | 0.82 |
| Total time (s) | 14.69 (3.13) | 14.32 (3.33) | 0.94 |
| Reaction time (s) | 1.08 (0.40) | 0.93 (0.31) | 0.58 |
| CG (n = 31) | PG (n = 29) | p-Value | Cohens’ d | |
|---|---|---|---|---|
| Medial-lateral displacement (mm) | 8.44 (4.61) | 11.38 (5.14) * | 0.02 | 0.58 |
| Anterior-posterior displacement (mm) | 17.57 (6.34) | 27.6 (13.15) * | <0.01 | 0.88 |
| Vertical range (mm) | 20.43 (4.5) | 22.89 (7.32) | 0.12 | - |
| Medial-lateral range (mm) | 39.67 (25.72) | 27.85 (17.01) * | 0.04 | 0.52 |
| Turn-to-sit time (s) | 4.17 (1.01) | 5.21 (1.74) * | <0.01 | 0.70 |
| Sit-to-stand power (W) | 210.83 (67.11) | 206.42 (66.06) | 0.80 | - |
| Total time (s) | 15.15 (2.87) | 15.2 (3.04) | 0.95 | - |
| Reaction time (s) | 1.04 (0.36) | 1.07 (0.39) | 0.74 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra-Añó, P.; Pedrero-Sánchez, J.F.; Inglés, M.; Aguilar-Rodríguez, M.; Vargas-Villanueva, I.; López-Pascual, J. Assessment of Functional Activities in Individuals with Parkinson’s Disease Using a Simple and Reliable Smartphone-Based Procedure. Int. J. Environ. Res. Public Health 2020, 17, 4123. https://doi.org/10.3390/ijerph17114123
Serra-Añó P, Pedrero-Sánchez JF, Inglés M, Aguilar-Rodríguez M, Vargas-Villanueva I, López-Pascual J. Assessment of Functional Activities in Individuals with Parkinson’s Disease Using a Simple and Reliable Smartphone-Based Procedure. International Journal of Environmental Research and Public Health. 2020; 17(11):4123. https://doi.org/10.3390/ijerph17114123
Chicago/Turabian StyleSerra-Añó, Pilar, José Francisco Pedrero-Sánchez, Marta Inglés, Marta Aguilar-Rodríguez, Ismael Vargas-Villanueva, and Juan López-Pascual. 2020. "Assessment of Functional Activities in Individuals with Parkinson’s Disease Using a Simple and Reliable Smartphone-Based Procedure" International Journal of Environmental Research and Public Health 17, no. 11: 4123. https://doi.org/10.3390/ijerph17114123
APA StyleSerra-Añó, P., Pedrero-Sánchez, J. F., Inglés, M., Aguilar-Rodríguez, M., Vargas-Villanueva, I., & López-Pascual, J. (2020). Assessment of Functional Activities in Individuals with Parkinson’s Disease Using a Simple and Reliable Smartphone-Based Procedure. International Journal of Environmental Research and Public Health, 17(11), 4123. https://doi.org/10.3390/ijerph17114123







