Acute Effects of a Short Bout of Physical Activity on Cognitive Function in Sport Students
Abstract
:1. Introduction
2. Materials and Methods
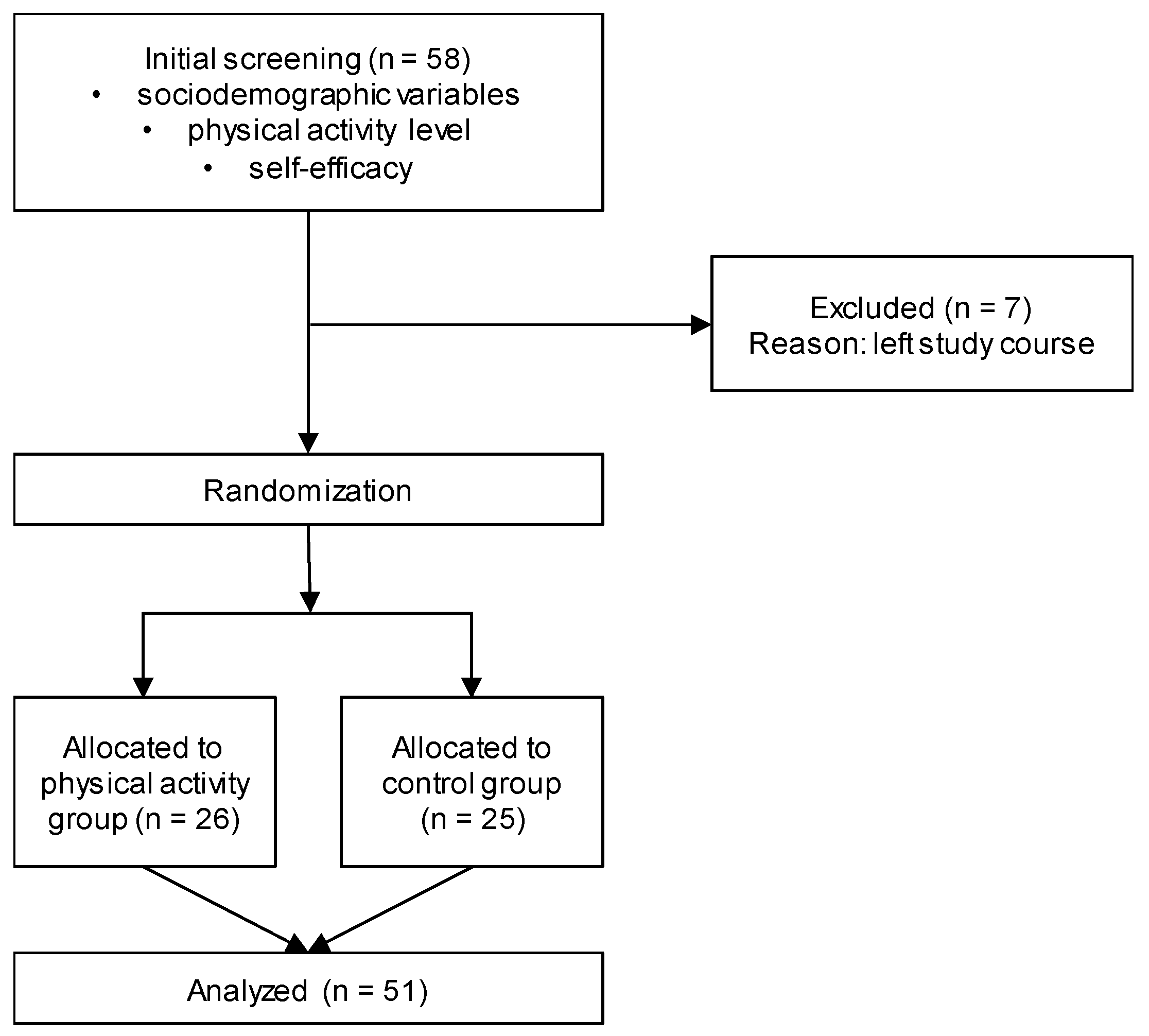
2.1. Design and Participants
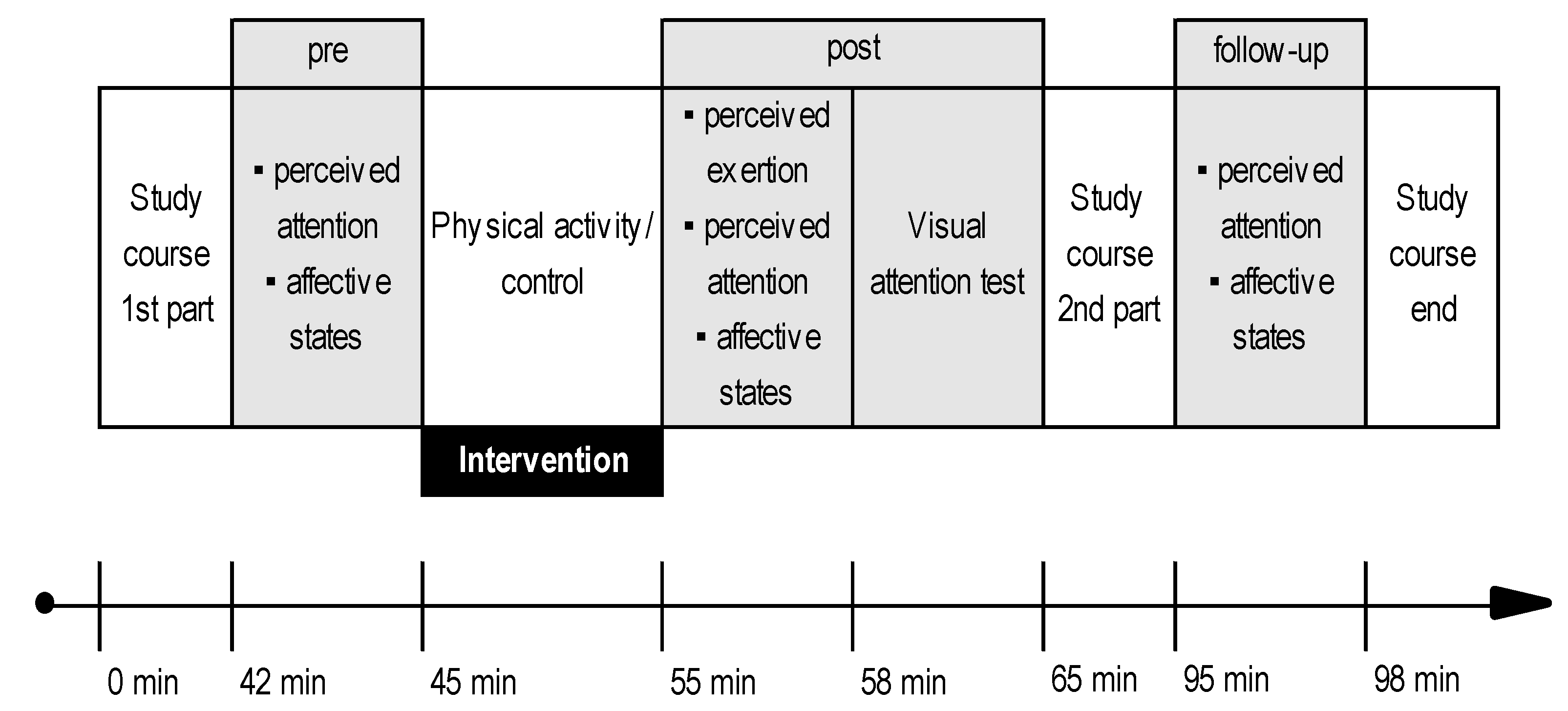
2.2. Procedure
2.3. Interventions
2.4. Measurements
2.4.1. Initial Screening
2.4.2. Visual Attention
2.4.3. Perceived Attention and Affective States
2.5. Statistical Analyses
3. Results
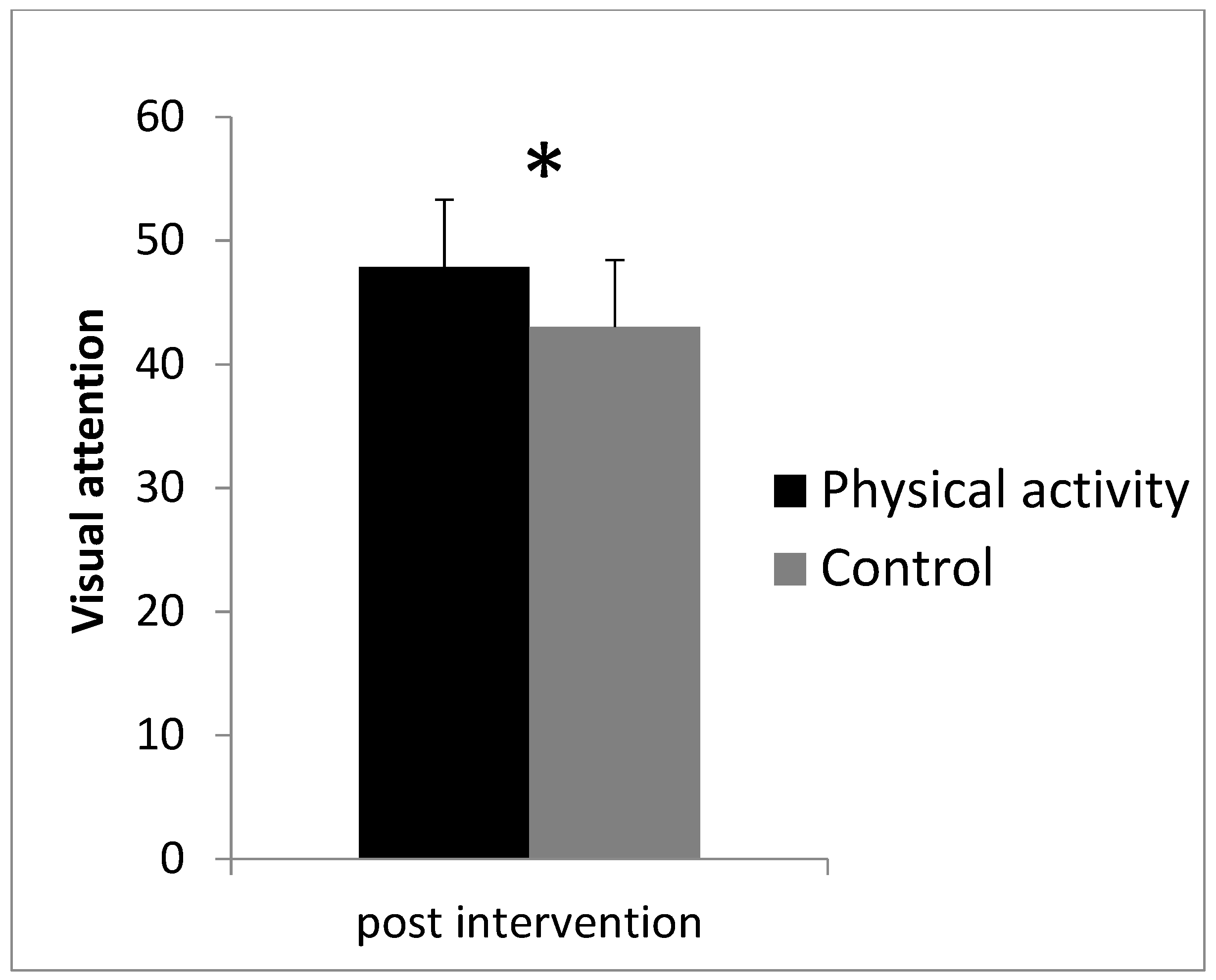
3.1. Visual Attention
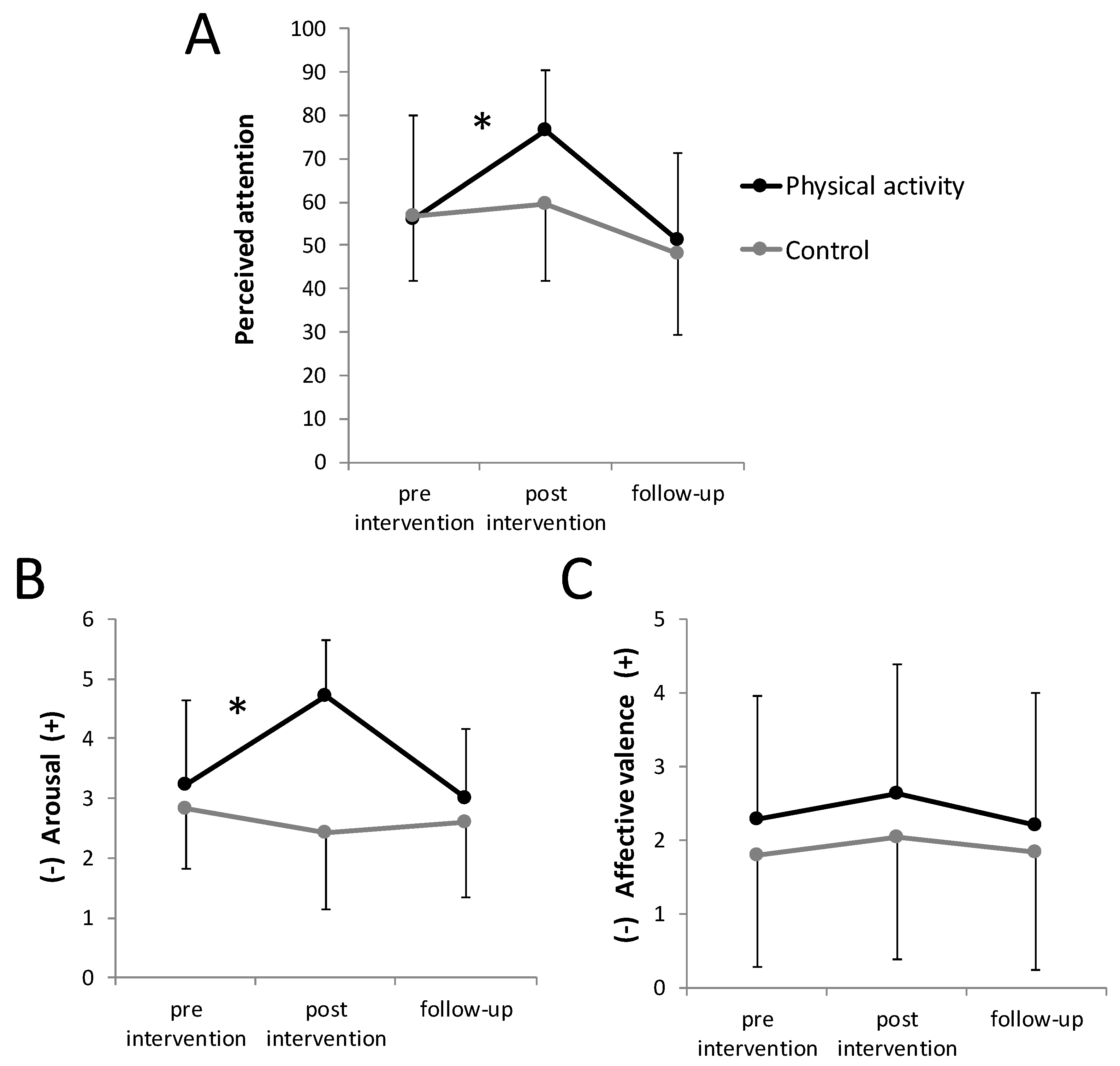
3.2. Perceived Attention and Affective States
3.3. Covariate-Adjusted Analyses
4. Discussion
4.1. Cognition Following Physical Activity
4.2. Affective States and Perceived Attention
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.M. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Katzmarzyk, P.T.; Church, T.S.; Craig, C.L.; Bouchard, C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med. Sci. Sports Exerc. 2009, 41, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Chastin, S.F.M.; Mandrichenko, O.; Helbostadt, J.L.; Skelton, D.A. Associations between objectively-measured sedentary behaviour and physical activity with bone mineral density in adults and older adults, the nhanes study. Bone 2014, 64, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Coombs, N.; Stamatakis, E. Associations between objectively assessed and self-reported sedentary time with mental health in adults: An analysis of data from the health survey for England. BMJ Open 2014, 4, e004580. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Austria—Physical Activity Factsheet. Available online: http://www.euro.who.int/en/countries/austria/data-and-statistics/austria (accessed on 28 January 2019).
- Buckworth, J.; Nigg, C. Physical activity, exercise, and sedentary behavior in college students. J. Am. Coll. Health 2004, 53, 28–34. [Google Scholar] [CrossRef]
- Buckley, J.P.; Hedge, A.; Yates, T.; Copeland, R.J.; Loosemore, M.; Hamer, M.; Bradley, G.; Dunstan, D.W. The sedentary office: An expert statement on the growing case for change towards better health and productivity. Br. J. Sports Med. 2015, 49, 1357–1362. [Google Scholar] [CrossRef]
- Chau, J.Y.; van der Ploeg, H.P.; Merom, D.; Chey, T.; Bauman, A.E. Cross-sectional associations between occupational and leisure-time sitting, physical activity and obesity in working adults. Prev. Med. 2012, 54, 195–200. [Google Scholar] [CrossRef]
- Mullane, S.L.; Buman, M.P.; Zeigler, Z.S.; Crespo, N.C.; Gaesser, G.A. Acute effects on cognitive performance following bouts of standing and light-intensity physical activity in a simulated workplace environment. J. Sci. Med. Sport 2017, 20, 489–493. [Google Scholar] [CrossRef]
- Baker, R.; Coenen, P.; Howie, E.; Williamson, A.; Straker, L. The short term musculoskeletal and cognitive effects of prolonged sitting during office computer work. Int. J. Environ. Res. Public Health 2018, 15, 1678. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Lambourne, K.; Tomporowski, P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Res. 2010, 1341, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.B.; McGowan, A.L.; Chandler, M.C.; Gwizdala, K.L.; Parks, A.C.; Fenn, K.; Kamijo, K. A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychol. Sport Exerc. 2019, 40, 1–22. [Google Scholar] [CrossRef]
- Fishbein, M.; Ajzen, I. Predicting and Changing Behavior: The Reasoned Action Approach; Psychology Press: New York, NY, USA, 2010. [Google Scholar]
- Ekkekakis, P.; Petruzzello, S.J. Analysis of the affect measurement conundrum in exercise psychology: IV. A conceptual case for the affect circumplex. Psychol. Sport Exerc. 2002, 3, 35–63. [Google Scholar] [CrossRef]
- Williams, D.M. Exercise, affect, and adherence: An integrated model and a case for self-paced exercise. J. Sport Exerc. Psychol. 2008, 30, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, R.E.; Kates, A. Can the affective response to exercise predict future motives and physical activity behavior? A systematic review of published evidence. Ann. Behav. Med. 2015, 49, 715–731. [Google Scholar] [CrossRef]
- University of Innsbruck. Bachelor’s Programme Sport Science. Available online: https://www.uibk.ac.at/studium/angebot/ba-sportwissenschaft/ (accessed on 6 September 2019).
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott, Williams, & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Borg, G. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 377–381. [Google Scholar] [CrossRef]
- Noble, B.J.; Robertson, R.J. Perceived Exertion; Human Kinetics: Champaign, IL, USA, 1996. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Schwarzer, R.; Jerusalem, M. Skalen zur Erfassung von Lehrer- und Schülermerkmalen. Dokumentation der Psychometrischen Verfahren im Rahmen der wissenschaftlichen Begleitung des Modellversuchs Selbstwirksame Schulen; Freie Universität Berlin: Berlin, Germany, 1999. [Google Scholar]
- Schwarzer, R.; Mueller, J.; Greenglass, E. Assessment of perceived general self-efficacy on the internet: Data collection in cyberspace. Anxiety Stress Coping 1999, 12, 145–161. [Google Scholar] [CrossRef]
- Bandura, A. Perceived self-efficacy in cognitive development and functioning. Educ. Psychol. 1993, 28, 117–148. [Google Scholar] [CrossRef]
- Oswald, W. Zahlen-Verbindungs-Test ZVT, 3rd ed.; Hogrefe: Göttingen, Germany, 2016. [Google Scholar]
- Tombaugh, T. Trail making Test a and b: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Russell, J.A. A circumplex model of affect. J. Person. Soc. Psychol. 1980, 39, 1161–1178. [Google Scholar] [CrossRef]
- Hardy, C.J.; Rejeski, W.J. Not what, but how one feels: The measurement of affect during exercise. J. Sport Exerc. Psychol. 1989, 11, 304–317. [Google Scholar] [CrossRef]
- Maibach, M.; Niedermeier, M.; Sudeck, G.; Kopp, M. Erfassung unmittelbarer affektiver Reaktionen auf körperliche Aktivität. Zeitschr. Sportpsychol. 2020, 27, 4–12. (In German) [Google Scholar]
- Svebak, S.; Murgatroyd, S. Metamotivational dominance: A multimethod validation of reversal theory constructs. J. Person. Soc. Psychol. 1985, 48, 107. [Google Scholar] [CrossRef]
- Van Landuyt, L.M.; Ekkekakis, P.; Hall, E.E.; Petruzzello, S.J. Throwing the mountains into the lakes: On the perils of nomothetic conceptions of the exercise-affect relationship. J. Sport Exerc. Psychol. 2000, 22, 208–234. [Google Scholar] [CrossRef]
- Falck, R.S.; Davis, J.C.; Liu-Ambrose, T. What is the association between sedentary behaviour and cognitive function? A systematic review. Br. J. Sports Med. 2017, 51, 800–811. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, MI, USA, 1988. [Google Scholar]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: Orlando, FL, USA, 2014. [Google Scholar]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [Green Version]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [Green Version]
- McMorris, T. History of research into the acute exercise-cognition interaction: A cognitive psychology approach. In Exercise-Cognition Interaction: Neuroscience Perspectives; McMorris, T., Ed.; Academic Press: London, UK, 2016; pp. 1–22. [Google Scholar]
- Ekkekakis, P.; Thome, J.; Petruzzello, S.J.; Hall, E.E. The preference for and tolerance of the intensity of exercise questionnaire: A psychometric evaluation among college women. J. Sports Sci. 2008, 26, 499–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelfo, F.; Mandolesi, L.; Serra, L.; Sorrentino, G.; Caltagirone, C. The neuroprotective effects of experience on cognitive functions: Evidence from animal studies on the neurobiological bases of brain reserve. Neuroscience 2018, 370, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of physical exercise on cognitive functioning and wellbeing: Biological and psychological benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Logan, N.E.; Shigeta, T.T. A review of acute physical activity effects on brain and cognition in children. Transl. J. Am. Coll. Sports Med. 2019, 4, 132–136. [Google Scholar]
- Backhouse, S.H.; Ekkekakis, P.; Bidle, S.J.; Foskett, A.; Williams, C. Exercise makes people feel better but people are inactive: Paradox or artifact? J. Sport Exerc. Psychol. 2007, 29, 498–517. [Google Scholar] [CrossRef] [Green Version]
- Ekkekakis, P. The dual-mode theory of affective responses to exercise in metatheoretical context: I. Initial impetus, basic postulates, and philosophical framework. Int. Rev. Sport Exerc. Psychol. 2009, 2, 73–94. [Google Scholar] [CrossRef]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; CUP Archive: Cambridge, UK, 1989. [Google Scholar]
- Niedermeier, M.; Einwanger, J.; Hartl, A.; Kopp, M. Affective responses in mountain hiking-a randomized crossover trial focusing on differences between indoor and outdoor activity. PLoS ONE 2017, 12, e0177719. [Google Scholar] [CrossRef]
| Variable | Physical Activity Group (n = 26) | Control Group (n = 25) | ||
|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | |
| Age (years) a | 22.8 | (2.0) | 21.8 | (2.0) |
| Height (cm) a | 176.8 | (9.5) | 174.4 | (7.5) |
| Body Mass (kg) a | 71.0 | (9.8) | 69.0 | (10.4) |
| Body Mass Index (kg/m2) a | 22.7 | (1.9) | 22.6 | (2.0) |
| Physical Activity Level (MET min/week) b | 7770 | (4691) | 4461 | (2352) |
| Self-efficacy (10: low, 40: high) a | 31 | (4) | 31 | (4) |
| % | (n) | % | (n) | |
| Sex, Female a | 34.6 | (9) | 33.3 | (8) |
| Study a | ||||
| Sports Science | 46.2 | (12) | 37.5 | (9) |
| Sports Management | 38.5 | (10) | 33.3 | (8) |
| Physical Education | 15.4 | (4) | 29.2 | (7) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedermeier, M.; Weiss, E.M.; Steidl-Müller, L.; Burtscher, M.; Kopp, M. Acute Effects of a Short Bout of Physical Activity on Cognitive Function in Sport Students. Int. J. Environ. Res. Public Health 2020, 17, 3678. https://doi.org/10.3390/ijerph17103678
Niedermeier M, Weiss EM, Steidl-Müller L, Burtscher M, Kopp M. Acute Effects of a Short Bout of Physical Activity on Cognitive Function in Sport Students. International Journal of Environmental Research and Public Health. 2020; 17(10):3678. https://doi.org/10.3390/ijerph17103678
Chicago/Turabian StyleNiedermeier, Martin, Elisabeth M. Weiss, Lisa Steidl-Müller, Martin Burtscher, and Martin Kopp. 2020. "Acute Effects of a Short Bout of Physical Activity on Cognitive Function in Sport Students" International Journal of Environmental Research and Public Health 17, no. 10: 3678. https://doi.org/10.3390/ijerph17103678





