Comparisons of Clinical Outcomes in Women with Advanced Ovarian Cancer Treated with Frontline Intraperitoneal versus Dose-Dense Platinum/Paclitaxel Chemotherapy without Bevacizumab
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alberts, D.S.; Liu, P.Y.; Hannigan, E.V.; O’Toole, R.; Williams, S.D.; Young, J.A.; Franklin, E.W.; Clarke-Pearson, D.L.; Malviya, V.K.; DuBeshter, B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N. Engl. J. Med. 1996, 335, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Markman, M.; Bundy, B.N.; Alberts, D.S.; Fowler, J.M.; Clark-Pearson, D.L.; Carson, L.F.; Wadler, S.; Sickel, J. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol. 2001, 19, 1001–1007. [Google Scholar] [PubMed]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A.; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Landrum, L.M.; Java, J.; Mathews, C.A.; Lanneau, G.S., Jr.; Copeland, L.J.; Armstrong, D.K.; Walker, J.L. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: A Gynecologic Oncology Group study. Gynecol. Oncol. 2013, 130, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, N.; Yasuda, M.; Takahashi, F.; Isonishi, S.; Jobo, T.; Aoki, D.; Tsuda, H.; Sugiyama, T.; Kodama, S.; Kimura, E.; et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: A phase 3, open-label, randomised controlled trial. Lancet 2009, 374, 1331–1338. [Google Scholar] [CrossRef]
- Katsumata, N.; Yasuda, M.; Isonishi, S.; Takahashi, F.; Michimae, H.; Kimura, E.; Aoki, D.; Jobo, T.; Kodama, S.; Terauchi, F.; et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 2013, 14, 1020–1026. [Google Scholar] [CrossRef]
- Walker, J.L.; Brady, M.F.; Wenzel, L.; Fleming, G.F.; Huang, H.Q.; DiSilvestro, P.A.; Fujiwara, K.; Alberts, D.S.; Zheng, W.; Tewari, K.S.; et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: An NRG Oncology/Gynecologic Oncology Group study. J. Clin. Oncol. 2019, 37, 1380–1390. [Google Scholar] [CrossRef]
- Rettenmaier, M.A.; Micha, J.P.; Bohart, R.; Goldstein, B.H. A retrospective study comparing the efficacy of dose-dense chemotherapy, intraperitoneal chemotherapy and dose-dense chemotherapy with hyperthermic intraperitoneal chemotherapy in the treatment of advanced stage ovarian carcinoma. Eur. J. Obstet. Gynecol. Reprod. Bio. 2020, 244, 101–105. [Google Scholar] [CrossRef]
- Calvert, A.H.; Newell, D.R.; Gumbrell, L.A.; O’Reilly, S.; Burnell, M.; Boxall, F.E.; Siddik, Z.H.; Judson, I.R.; Gore, M.E.; Wiltshaw, E. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin. Oncol. 1989, 7, 1748–1756. [Google Scholar] [CrossRef]
- Miller, A.B.; Hoogstraten, B.; Staquet, M.; Winkler, A. Reporting results of cancer treatment. Cancer 1981, 47, 207–214. [Google Scholar] [CrossRef]
- Rustin, G.J.; Nelstrop, A.E.; McClean, P.; Brady, M.F.; McGuire, W.P.; Hoskins, W.J.; Mitchell, H.; Lambert, H.E. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J. Clin. Oncol. 1996, 14, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Rustin, G.J.; Eisenhauer, E.A.; Kristensen, G.B.; Pujade-Lauraine, E.; Parmar, M.K.; Friedlander, M.; Jakobsen, A.; Vermorken, J.B. Re: New guidelines to evaluate the response to treatment in solid tumors [ovarian cancer]. Gynecologic Cancer Intergroup. J. Natl. Cancer Inst. 2000, 92, 1534–1535. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.M.; Chen, C.A.; Lin, H.H.; Hsieh, C.Y.; Wei, L.H. Phase II trial of carboplatin and distearoylphosphatidylcholine pegylated liposomal doxorubicin (Lipo-Dox) in recurrent platinum-sensitive ovarian cancer following front-line therapy with paclitaxel and platinum. Gynecol. Oncol. 2009, 112, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.M. Letter to the Editor on “A retrospective study comparing the efficacy of dose-dense chemotherapy, intraperitoneal chemotherapy and dose-dense chemotherapy”. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 257. [Google Scholar] [CrossRef]
- Bixel, K.; Vetter, M.; Davidson, B.; Berchuck, A.; Cohn, D.; Copeland, L.; Fowler, J.M.; Havrilesky, L.; Lee, P.S.; O’Malley, D.M.; et al. Intraperioneal chemotherapy following neoadjuvant chemotherapy and optimal interval tumor reductive surgery for advanced ovarian cancer. Gynecol. Oncol. 2020, 156, 530–534. [Google Scholar] [CrossRef]
- Chan, J.K.; Brady, M.F.; Penson, R.T.; Huang, H.; Birrer, M.J.; Walker, J.L.; DiSilvestro, P.A.; Rubin, S.C.; Martin, L.P.; Davidson, S.A.; et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N. Engl. J. Med. 2016, 374, 738–748. [Google Scholar] [CrossRef]
- Marchetti, P.; Urien, S.; Cappellini, G.A.; Ronzino, G.; Ficorella, C. Weekly administration of paclitaxel: Theoretical and clinical basis. Crit. Rev. Oncol. Hematol. 2002, 44, S3–S13. [Google Scholar] [CrossRef]
- Pinato, D.J.; Graham, J.; Gabra, H.; Sharma, R. Evolving concepts in the management of drug resistant ovarian cancer: Dose dense chemotherapy and the reversal of clinical platinum resistance. Cancer Treat. Rev. 2013, 39, 153–160. [Google Scholar] [CrossRef]
- Dedrick, R.L.; Flessner, M.F. Pharmacokinetic Problems in Peritoneal Drug Administration: Tissue Penetration and Surface Exposure. J. Natl. Cancer Inst. 1997, 89, 480–487. [Google Scholar] [CrossRef]
- Fujiwara, K.; Aotani, E.; Hamano, T.; Nagao, S.; Yoshikawa, H.; Sugiyama, T.; Kigawa, J.; Aoki, D.; Katsumata, N.; Takeuchi, M.; et al. A randomized Phase II/III trial of 3 weekly intraperitoneal versus intravenous carboplatin in combination with intravenous weekly dose-dense paclitaxel for newly diagnosed ovarian, fallopian tube and primary peritoneal cancer. Jpn. J. Clin. Oncol. 2011, 41, 278–282. [Google Scholar] [CrossRef]
- Chang, C.L.; Hsu, Y.T.; Wu, C.C.; Lai, Y.Z.; Wang, C.; Yang, Y.C.; Wu, T.C.; Hung, C.F. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res. 2013, 73, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Zunino, B.; Rubio-Patiño, C.; Villa, E.; Meynet, O.; Proics, E.; Cornille, A.; Pommier, S.; Mondragón, L.; Chiche, J.; Bereder, J.M.; et al. Hyperthermic intraperitoneal chemotherapy leads to an anticancer immune response via exposure of cell surface heat shock protein 90. Oncogene 2016, 35, 261–268. [Google Scholar] [CrossRef]
- Yen, M.S.; Twu, N.F.; Lai, C.R.; Horng, H.C.; Chao, K.C.; Juang, C.M. Importance of delivered cycles and nomogram for intraperitoneal chemotherapy in ovarian cancer. Gynecol. Oncol. 2009, 114, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.S.; Juang, C.M.; Lai, C.R.; Chao, G.C.; Ng, H.T.; Yuan, C.C. Intraperitoneal cisplatin-based chemotherapy vs. intravenous cisplatin-based chemotherapy for stage III optimally cytoreduced epithelial ovarian cancer. Int. J. Gynaecol. Obstet. 2001, 72, 55–60. [Google Scholar] [CrossRef]
- Walker, J.L.; Armstrong, D.K.; Huang, H.Q.; Fowler, J.; Webster, K.; Burger, R.A.; Clarke-Pearson, D. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2006, 100, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Nagao, S.; Fujiwara, K.; Ohishi, R.; Nakanishi, Y.; Iwasa, N.; Shimizu, M.; Goto, T.; Shimoya, K. Combination chemotherapy of intraperitoneal carboplatin and intravenous paclitaxel in suboptimally debulked epithelial ovarian cancer. Int. J. Gynecol. Cancer 2008, 18, 1210–1214. [Google Scholar] [CrossRef]

| Variable | IP (n = 22) | Dose-Dense (n = 28) | †p |
|---|---|---|---|
| Age (years) | 52.6 ± 7.4 | 54.2 ± 8.7 | 0.59 |
| Body mass index (kg/m2) | 25.4 ± 3.5 | 24.2 ± 4.4 | 0.20 |
| Baseline CA-125 (U/mL) | 2120 ± 2665 | 1180 ± 3330 | 0.53 |
| Site | |||
| Ovary | 19 (86) | 28 (100) | 0.08 |
| Fallopian tube | 1 (5) | 0 (0) | |
| Peritoneum | 2 (9) | 0 (0) | |
| FIGO stage | |||
| 2 | 2 (9) | 2 (7) | 1.00 |
| 3 | 14 (64) | 17 (61) | |
| 4 | 6 (27) | 9 (32) | |
| Histologic subtype | |||
| Serous | 14 (61) | 18 (66) | 1.00 |
| Endometrioid | 2 (13) | 3 (10) | |
| Clear cell | 3 (13) | 3 (10) | |
| Mucinous | 0 (0) | 1 (3) | |
| Others | 3 (13) | 3 (7) | |
| Debulking surgery | |||
| ‡ Optimal | 15 (68) | 10 (36) | 0.10 |
| ‡ Suboptimal | 7 (32) | 13 (46) | |
| ECOG Score | |||
| 0 | 2 (9) | 0 (0) | 0.09 |
| 1 | 17 (77) | 18 (64) | |
| 2 | 3 (14) | 10 (36) | |
| Lymph node metastasis | 13 (59) | 19 (68) | 0.52 |
| Neoadjuvant chemotherapy | 1 (4) | 1 (3) | 1.00 |
| Number of chemotherapy cycles | 5.5 ± 1.6 | 5.7 ± 1.3 | 0.59 |
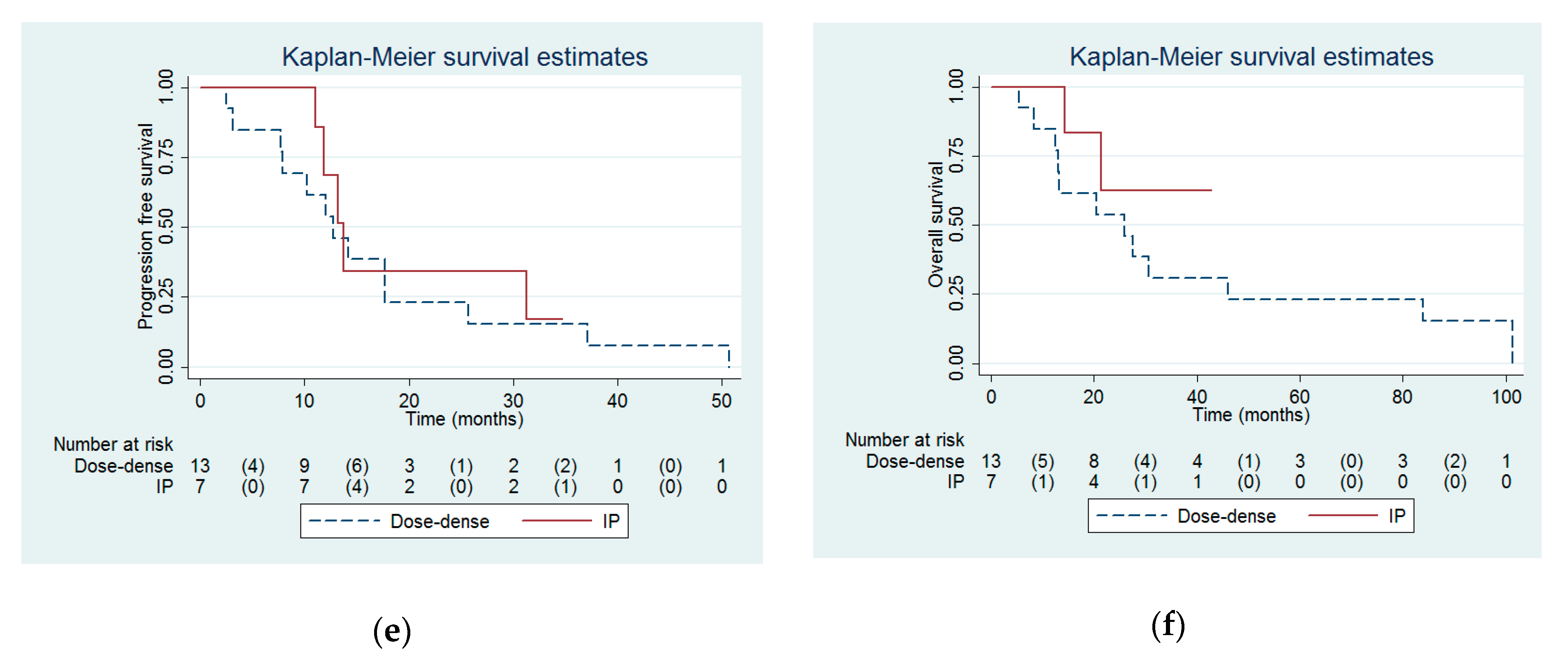
| Progression free interval (months) | 32.6 (13.8 to infinity) | 14.2 (10 to 19.4) | 0.03 |
| Overall survival (months) | Not reached (54.3 to infinity) | 30.7 (15.4 to 46) | 0.03 |
| Clinical response | |||
| CR | 19 (86) | 18 (64) | 0.31 |
| PR | 1 (5) | 5 (18) | |
| SD | 0 (0) | 1 (4) | |
| PD | 2 (9) | 4 (14) | |
| Follow-up interval (months) | 33.4 ± 18.3 | 48.3 ± 30.9 | 0.60 |
| Progression or recurrence | 12 (55) | 26 (93) | 0.002 |
| Subsequent therapy | 8 (36) | 21 (75) | 0.006 |
| Subsequent therapy lines | 1.4 ± 0.9 | 2.4 ± 1.4 | 0.04 |
| Variable | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | †p | Hazard Ratio | 95% CI | ‡p | |
| Regimen (IP = 1 vs. dose-dense = 0) | 0.47 | 0.24 to 0.94 | 0.03 | 0.38 | 0.16 to 0.90 | 0.03 |
| Age (years) | 1.02 | 0.98 to 1.07 | 0.27 | 1.01 | 0.97 to 1.06 | 0.60 |
| FIGO stage | 1.92 | 1.08 to 3.39 | 0.03 | 0.97 | 0.41 to 2.27 | 0.95 |
| ECOG score | 1.76 | 0.96 to 3.23 | 0.07 | 1.19 | 0.49 to 2.93 | 0.70 |
| Suboptimal debulking | 2.18 | 1.09 to 4.32 | 0.03 | 1.53 | 0.65 to 3.67 | 0.34 |
| Number of IP or dose-dense chemotherapy cycles | 0.91 | 0.73 to 1.14 | 0.41 | 0.78 | 0.59 to 1.04 | 0.09 |
| Variable | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | †p | Hazard Ratio | 95% CI | ‡p | |
| Regimen (IP = 1 vs. dose-dense = 0) | 0.35 | 0.13 to 0.93 | 0.04 | 0.23 | 0.07 to 0.79 | 0.02 |
| Age (years) | 1.04 | 0.99 to 1.10 | 0.10 | 1.04 | 0.99 to 1.10 | 0.13 |
| FIGO stage | 1.34 | 0.70 to 2.55 | 0.37 | 0.57 | 0.22 to 1.49 | 0.26 |
| ECOG score | 1.55 | 0.76 to 3.14 | 0.23 | 1.33 | 0.49 to 3.57 | 0.57 |
| Suboptimal debulking | 1.96 | 0.85 to 4.53 | 0.12 | 1.53 | 0.57 to 4.14 | 0.40 |
| Number of IP or dose-dense chemotherapy cycles | 0.84 | 0.66 to 1.09 | 0.19 | 0.66 | 0.47 to 0.94 | 0.02 |
| Grade | Dose-Dense (n = 28) | IP (n = 22) | †p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||
| Hematological | |||||||||
| Leukopenia | 3 (11) | 12 (43) | 12 (43) | 1 (4) | 1 (5) | 8 (36) | 8 (36) | 2 (9) | 0.31 |
| Thrombocytopenia | 5 (18) | 6 (21) | 2 (7) | 1 (4) | 6 (27) | 7 (32) | 1 (5) | 0 (0) | 0.73 |
| Anemia | 2 (7) | 17 (61) | 9 (32) | 0 (0) | 2 (9) | 15 (68) | 4 (18) | 0 (0) | 0.56 |
| Non-hematological | |||||||||
| Nausea/vomiting | 17 (61) | 4 (14) | 1 (4) | 0 (0) | 8 (36) | 10 (45) | 4 (18) | 0 (0) | 0.02 |
| Diarrhea | 6 (21) | 0 (0) | 0 (0) | 0 (0) | 3 (14) | 2 (9) | 0 (0) | 0 (0) | 0.29 |
| Nephrotoxicity | 8 (29) | 0 (0) | 0 (0) | 0 (0) | 3 (14) | 12 (55) | 0 (0) | 0 (0) | <0.0001 |
| Hepatotoxicity | 10 (36) | 2 (7) | 0 (0) | 1 (4) | 8 (36) | 0 (0) | 2 (9) | 0 (0) | 0.43 |
| Neurotoxicity | 12 (43) | 4 (14) | 0 (0) | 0 (0) | 6 (27) | 10 (45) | 0 (0) | 0 (0) | 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ting, W.-H.; Hsiao, C.-H.; Chen, H.-H.; Wei, M.-C.; Lin, H.-H.; Hsiao, S.-M. Comparisons of Clinical Outcomes in Women with Advanced Ovarian Cancer Treated with Frontline Intraperitoneal versus Dose-Dense Platinum/Paclitaxel Chemotherapy without Bevacizumab. Int. J. Environ. Res. Public Health 2020, 17, 3603. https://doi.org/10.3390/ijerph17103603
Ting W-H, Hsiao C-H, Chen H-H, Wei M-C, Lin H-H, Hsiao S-M. Comparisons of Clinical Outcomes in Women with Advanced Ovarian Cancer Treated with Frontline Intraperitoneal versus Dose-Dense Platinum/Paclitaxel Chemotherapy without Bevacizumab. International Journal of Environmental Research and Public Health. 2020; 17(10):3603. https://doi.org/10.3390/ijerph17103603
Chicago/Turabian StyleTing, Wan-Hua, Chi-Huang Hsiao, Hui-Hua Chen, Ming-Chow Wei, Ho-Hsiung Lin, and Sheng-Mou Hsiao. 2020. "Comparisons of Clinical Outcomes in Women with Advanced Ovarian Cancer Treated with Frontline Intraperitoneal versus Dose-Dense Platinum/Paclitaxel Chemotherapy without Bevacizumab" International Journal of Environmental Research and Public Health 17, no. 10: 3603. https://doi.org/10.3390/ijerph17103603
APA StyleTing, W.-H., Hsiao, C.-H., Chen, H.-H., Wei, M.-C., Lin, H.-H., & Hsiao, S.-M. (2020). Comparisons of Clinical Outcomes in Women with Advanced Ovarian Cancer Treated with Frontline Intraperitoneal versus Dose-Dense Platinum/Paclitaxel Chemotherapy without Bevacizumab. International Journal of Environmental Research and Public Health, 17(10), 3603. https://doi.org/10.3390/ijerph17103603





