Prevalence of Undernutrition and Anemia among Santal Adivasi Children, Birbhum District, West Bengal, India
Abstract
1. Introduction
2. Materials and Methods
2.1. Village Selection, Household Survey and Medical Checkup
2.2. Target Group and Response Rate
2.3. Sample Size Calculation
2.4. Outcome Variables
2.5. Statistics
2.6. Predictor Analysis
2.7. Trial Registration
2.8. Ethics Approval and Consent to Participate
3. Analysis and Results
3.1. Anthropometric and Hematological Data
3.1.1. Anthropometric Measurements and Hemoglobin Levels in Study Children
3.1.2. The Composite Index of Anthropometric Failure (CIAF) Versus Conventional Indices for the Assessment of Undernutrition
3.1.3. Nutrition-Specific and Sensitive Drivers of Hemoglobin Levels ≥10 g/dL
3.1.4. Hemoglobin Levels by Maternal Educational Level or Household Wealth
3.2. Socio-Demographic Information of Caretakers of Study Children
3.2.1. Background Information
3.2.2. Household Characteristics
3.2.3. Aspects of Food Security
3.2.4. Hygiene Habits
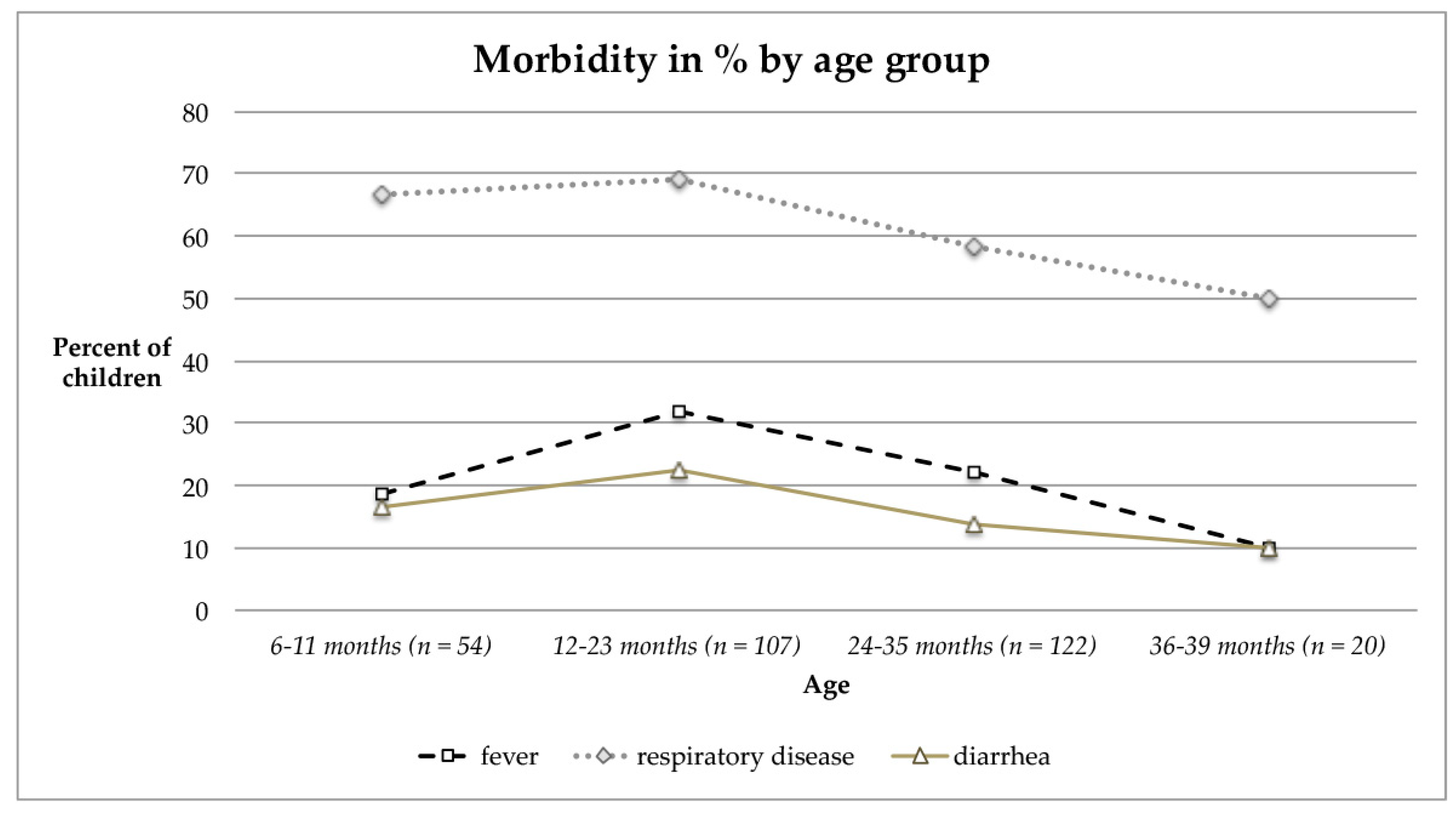
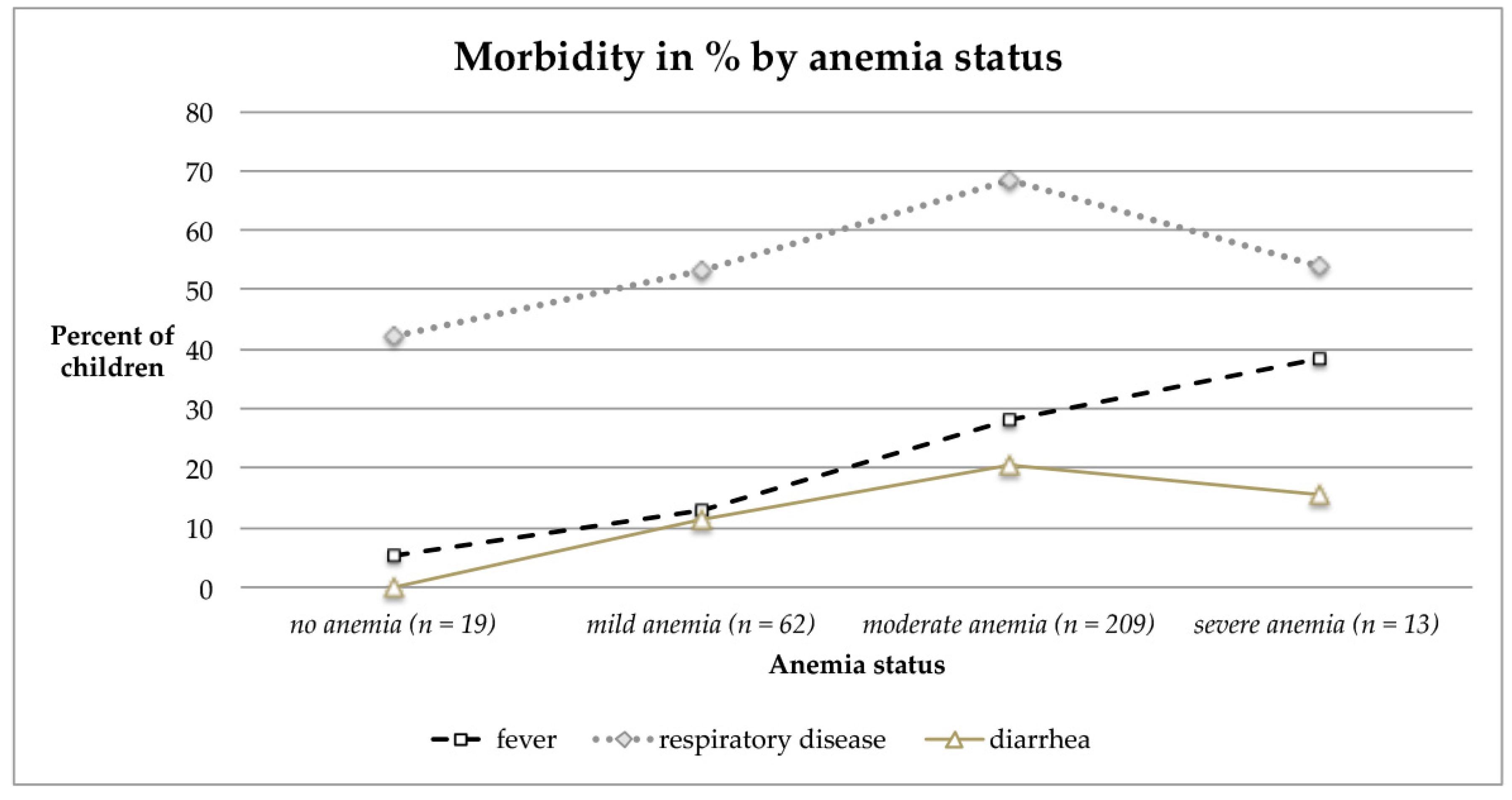
3.2.5. Morbidity Pattern and Visible Signs of Malnutrition of Children and Their Mothers
3.2.6. Health Access and Health Seeking Behavior
3.2.7. Access to Food-Home Production and Local Markets
3.2.8. Family Food
4. Discussion
4.1. Prevalence of Undernutrition
4.2. CIAF Versus Conventional Indices for the Assessment of Undernutrition
4.3. Use of Weight-for-Height and Mid-Upper Arm Circumference to Diagnose Acute Malnutrition
4.4. Predictors of Anemia
4.5. Household (HH) Characteristics and Aspects of Food Security
4.6. Morbidity Rates
4.7. Health Access and Health Seeking Behavior
4.8. Access to Food and Family Food
4.9. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NCSC. Chapter II: Special Constitutional Provisions for Protection and Development of the Scheduled Castes and the Scheduled Tribes; National Commission for Scheduled Castes (NCSC), Government of India: New Delhi, India; pp. 8–17. Available online: http://ncsc.nic.in/files/Chapter%202.pdf (accessed on 10 April 2019).
- Ministry of Home Affairs, Government of India. Scheduled Castes and Scheduled Tribes. Available online: http://censusindia.gov.in/Census_And_You/scheduled_castes_and_sceduled_tribes.aspx (accessed on 10 April 2019).
- Tribal people of India, Scheduled castes and scheduled tribes. In India, Minorities, castes and regions: Facts and Details. 2015. Available online: http://factsanddetails.com/india/Minorities_Castes_and_Regions_in_India/sub7_4h/entry-4216.html (accessed on 31 December 2019).
- Office of the Registrar General and Census Commissioner, India, West Bengal. Data Highlights: The Scheduled Tribes, Census of India; RGCCI: New Delhi, India, 2001. [Google Scholar]
- Das, M.B.; Metha, S.K. Issue Brief, Poverty and Social Exclusion in India: Adivasis; World Bank Group: Washington DC, USA, 2012. [Google Scholar]
- Barman, K. Socio-Economic Profile of Tribal Populations in Birbhum District of West Bengal. In Central India Journal of Historical and Archaeological Research; Socio-Economic Profile of Tribal Populations in Birbhum District of West. Bengal; Vidya Career Research Foundation: Panna, India, 2014; Volume III, pp. 187–192. [Google Scholar]
- Das, M.B.; Hall, G.; Kapoor, S.; Nikitin, D. Indigenous Peoples, India’s Adivasis. In India Country Brief No. 4; World Bank Group: Washington, DC, USA, 2011. [Google Scholar]
- IIPS National Family Health Survey (NFHS-4), 2015–2016: India. Available online: http://rchiips.org/nfhs/NFHS-4Reports/India.pdf (accessed on 18 June 2019).
- International Institute for Population Sciences. IIPS India Fact Sheet. In National Family Health Survey (NFHS-4) 2015-16; International Institute of Population Sciences: Mumbai, India, 2017. [Google Scholar]
- UNICEF. Strategy for Improved Nutrition of Children and Women in Developing Countries; UNICEF: New York, NY, USA, 1990. [Google Scholar]
- Ruel, M.T.; Alderman, H.; The Maternal and Child Nutrition Study Group. Maternal and Child Nutrition 3: Nutrition-sensitive interventions and programmes: How can they help to accelerate progress in improving maternal and child nutrition? Lancet 2013, 382, 536–551. [Google Scholar] [CrossRef]
- Gross, R.; Schoeneberger, H.; Pfeifer, H.; Preuss, H.J. The four dimensions of food and nutrition security: Definitions and concepts. Nutr. Food Secur. 2000, 20, 20–25. [Google Scholar]
- Lwanga, S.K.; Lemeshow, S.; World Health Organisation. Sample Size Determination in Health Studies: A Practical Manual; World Health Organization (WHO): Geneva, Switzerland, 1991; ISBN 92-4-154405-8. [Google Scholar]
- IIPS. National Family Health Survey (NFHS-4), India, 2015–2016: West Bengal; International Institute for Population Sciences (IIPS) and ICF: Mumbai, India, 2017. [Google Scholar]
- Green, S.B. How Many Subjects Does It Take To Do A Regression Analysis. Multivar. Behav. Res. 1991, 26, 499–510. [Google Scholar] [CrossRef] [PubMed]
- VanVoorhis, C.W.; Morgan, B.L. Understanding Power and Rules of Thumb for Determining Sample Size. Tutor. Quant. Methods Psychol. 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Child Growth Standards: WHO Anthro (version 3.2.2, January 2011) and Macros. Available online: http://www.who.int/childgrowth/software/en/ (accessed on 16 January 2019).
- Child Growth Standards: The WHO Child Growth Standards. Available online: http://www.who.int/childgrowth/standards/en/ (accessed on 14 January 2019).
- UNHCR; WFP. Guidelines for Selective Feeding: The Management of Malnutrition in Emergencies. Available online: https://www.ennonline.net/attachments/930/wfp-unhcr-sfp-guidelines.pdf (accessed on 10 October 2019).
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011; WHO reference number: WHO/NMH/NHD/MNM/11.1. [Google Scholar]
- Martinez, B.A.F.; Leotti, V.B.; de Silva, G.S.E.; Nunes, L.N.; Machado, G.; Corbellini, L.G. Odds Ratio or Prevalence Ratio? An Overview of Reported Statistical Methods and Appropriateness of Interpretations in Cross-sectional Studies with Dichotomous Outcomes in Veterinary Medicine. Front. Vet. Sci. 2017, 4, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, K.F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcome. JAMA 1998, 280. [Google Scholar] [CrossRef]
- Schmidt, C.O.; Kohlmann, T. When to use the odds ratio or the relative risk? Int. J. Public Health 2008, 53, 165–167. [Google Scholar] [CrossRef]
- Cummings, P. The relative merits of risk ratios and odds ratios. Arch. Pediatr. Adolesc. Med. 2009, 163, 438–445. [Google Scholar] [CrossRef]
- Cummings, P. Methods for Estimating Adjusted Risk Ratios. Stata J. 2009, 9, 175–196. [Google Scholar] [CrossRef]
- Kleinbaum, D.G.; Kupper, L.L.; Morgenstern, H. Epidemiologic Research: Principles and Quantitative Methods; John Wiley & Sons: Hoboke, NJ, USA, 1982. [Google Scholar]
- Cook, T.D. Advanced statistics: Up with odds ratios! A case for odds ratios when outcomes are common. Acad. Emerg. Med. 2002, 9, 1430–1434. [Google Scholar] [CrossRef]
- Mickey, R.M.; Greenland, S. The impact of confounder selection criteria on effect estimation. Am. J. Epidemiol. 1989, 129, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Model building strategy for logistic regression: Purposeful selection. Ann. Transl. Med. 2016, 4, 111. [Google Scholar] [CrossRef] [PubMed]
- Abera, S.F.; Kantelhardt, E.J.; Bezabih, A.M.; Gebru, A.A.; Ejeta, G.; Lauvai, J.; Wienke, A.; Scherbaum, V. Nutrition-specific and sensitive drivers of poor child nutrition in Kilte Awlaelo-Health and Demographic Surveillance Site, Tigray, Northern Ethiopia: Implications for public health nutrition in resource-poor settings. Glob. Health Action 2019, 12, 1556572. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F. Multivariate Data Analysis, 5th ed.; Pearson Education Limited: London, UK, 1998; ISBN 978-0-13-930587-0. [Google Scholar]
- World Health Organisation. Physical Status: The Use and Interpretation of Anthropometry. In Report of a WHO Expert Committee on Nutrition and Physical Status; Technical Report Series No. 854; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Savanur, M.S.; Ghugre, P.S. Magnitude of undernutrition in children aged 2 to 4 years using CIAF and conventional indices in the slums of Mumbai city. J. Health Popul. Nutr. 2015, 33, 7. [Google Scholar] [CrossRef] [PubMed]
- Nandy, S.; Irving, M.; Gordon, D.; Subramanian, S.V.; Smith, G.D. Poverty, child undernutrition and morbidity: New evidence from India. Bull. World Health Organ. 2005, 83, 210–216. [Google Scholar] [PubMed]
- O’Neill, S.M.; Fitzgerald, A.; Briend, A.; Van den Broeck, J. Child mortality as predicted by nutritional status and recent weight velocity in children under two in rural Africa. J. Nutr. 2012, 142, 520–525. [Google Scholar] [PubMed]
- Khara, T.; Mwangome, M.; Ngari, M.; Dolan, C. Children concurrently wasted and stunted: A meta-analysis of prevalence data of children 6-59 months from 84 countries. Matern. Child Nutr. 2018, 14, e12516. [Google Scholar] [CrossRef]
- Gupta, P.; Menon, P.S.N.; Ramji, S.; Lodha, R. PG Textbook of Pediatrics: Volume 1: General Pediatrics and Neonatology; Jaypee Brothers Medical Publishers: New Delhi, India, 2015; Volume 1, p. 841. ISBN 93-5152-725-5. [Google Scholar]
- World Health Organisation. Indicators for Assessing Infant and Young Child Feeding Practices; Part. 3, Country Profiles; World Health Organisation: Geneva, Switzerland, 2010; ISBN 978 92 4 159975 7. [Google Scholar]
- The World Bank: Understanding Poverty: Global Poverty Line Update. 2015. Available online: https://www.worldbank.org/en/topic/poverty/brief/global-poverty-line-faq (accessed on 30 December 2019).
- Ministry of Rural Development. Mahatma Gandhi National Rural Employment Gurantee Act., 2005. Master Circular—A Guide for Programme Implementation FY 2018-19; Ministry of Rural Development: New Delhi, India, 2018.
- UNICEF. Under-Five Mortality Rates; UNICEF: New York, NY, USA, 2018. [Google Scholar]
- Werner, D.; Maxwell, J. Where There Is no Doctor: A Village Health Care Handbook, revised ed.; Hesperian Health Guides: Berkeley, CA, USA, 1992; pp. 140–141. ISBN 978-0-942364-15-6. [Google Scholar]
- Hunter, P.R.; MacDonald, A.M.; Carter, R.C. Water Supply and Health. PLOS Med. 2010, 7, e1000361. [Google Scholar] [CrossRef]
- Das, T. Quack: Their Role in Health Sector. SSRN Electron. J. 2008. [Google Scholar] [CrossRef]
- Das, S.; Mathew, M. Drive for Equal Access: Access and Participation of Women and Girls to Nutrition & Health, Education & Training, Science & Technology; Partridge Publishing: Bloomington, India, 2017; ISBN 978-1-4828-5760-3. [Google Scholar]
- World Health Organisation. Nutrition Landscape Information System (NLIS). Country Profile Indicators. Interpretation Guide; World Health Organisation: Geneva, Switzerland, 2010; ISBN 978 92 4 159995 5. [Google Scholar]
- SOWC; UNICEF. A Fair Chance for Every Child; UNICEF: New York, NY, USA, 2016. [Google Scholar]
- Bisai, S. Prevalence of Undernutrition among Santal tribal Preschool Children of Paschim Medinipur District, West Bengal, India. Int. J. Pediatrics 2014, 2, 347–354. [Google Scholar]
- Ghosh, J.R.; Sarkar, A. Prevalence of undernutrition among Santal children of Birbhum District, West. Bengal, India. Sri Lanka J. Child Health 2013, 42, 147–150. [Google Scholar] [CrossRef]
- Ghosh, J.; Ranjan Pati, R. Assessment of nutritional status among Santal-Munda tribal children in rural area of Amdanga block, North 24th Parganas District of West Bengal, India. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 810–814. [Google Scholar]
- Das, S.; Bose, K. Assessment of Nutritional Status by Anthropometric Indices in Santal Tribal Children. J. Life Sci. 2011, 3, 81–85. [Google Scholar] [CrossRef]
- Ruhul Kabir, M.; Rahman, M.; Abdullah Al Mamun, M.; Islam, H. Prevalence of malnutrition and associated factors affecting the nutritional status of Adivasi (tribal) children aged 24-59 months in Bangladesh. Asian J. Med. Biol. Res. 2018, 4, 178–185. [Google Scholar] [CrossRef]
- Bhadoria, A.S.; Kapil, U.; Bansal, R.; Pandey, R.M.; Pant, B.; Mohan, A. Prevalence of severe acute malnutrition and associated sociodemographic factors among children aged 6 months-5 years in rural population of Northern India: A population-based survey. J. Fam. Med. Prim. Care 2017, 6, 380–385. [Google Scholar]
- IIPS. District Fact Sheet, Birbhum, West. Bengal (NFHS-4); International Institute for Population Sciences: Mumbai, India, 2015. [Google Scholar]
- Victora, C.G.; de Onis, M.; Hallal, P.C.; Blossner, M.; Shrimpton, R. Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatrics 2010, 125, e473–e480. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Mandal, G.C.; Bose, K. Overall burden of under-nutrition measured by a Composite Index in rural pre-school children in Purba Medinipur, West Bengal, India. Anthropol. Rev. 2013, 76, 109–116. [Google Scholar] [CrossRef]
- Sen, J.; Dey, S.; Nitish, M. Conventional nutritional indices and Composite Index of Anthropometric Failure: Which seems more appropriate for assessing under-nutrition among children? A cross-sectional study among school children of the Bengalee Muslim Population of North. Bengal, India. J. Public Health 2011, 8, 172–184. [Google Scholar]
- Sinha, N.; Maiti, S. Prevalence of undernutrition among underprivileged preschool children (2–6 yrs) of Midnapore town, India. MJPCH 2012, 6, 18. [Google Scholar]
- Biswas, S.; Bose, K.; Mukhopadhyay, A.; Bhadra, M. Prevalence of undernutrition among pre-school children of Chapra, Nadia District, West Bengal, India, measured by composite index of anthropometric failure (CIAF). Anthropol. Anz. 2009, 67, 269–279. [Google Scholar] [CrossRef]
- Biswas, S.; Prasad Giri, S.; Bose, K. Assessment of nutritional status by composite index of anthropometric failure (CIAF): A study among preschool children of Sagar Block, South 24 Parganas District, West Bengal, India. Pol. Anthropol. Soc. 2018, 81, 269–277. [Google Scholar] [CrossRef][Green Version]
- Mukhopadhyay, D.K.; Biswas, A.B. Food security and anthropometric failure among tribal children in Bankura, West Bengal. Indian Pediatr. 2011, 48, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Chandra Mandal, G.; Bose, K. Assessment of Overall Prevalence of Undernutrition Using Composite Index of Anthropometric Failure (CIAF) Among Preschool Children of West Bengal, India. Iran. J. Pediatr 2009, 19, 237–243. [Google Scholar]
- World Health Organisation. Iron Deficiency Anaemia: Assessment, Prevention and Control: A Guide for Programme Managers; World Health Organisation: Geneva, Switzerland, 2001. [Google Scholar]
- Domellof, M.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Iron requirements of infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 119–129. [Google Scholar] [CrossRef]
- Vart, P.; Jaglan, A.; Shafique, K. Caste-based social inequalities and childhood anemia in India: Results from the National Family Health Survey (NFHS) 2005–2006. BMC Public Health 2015, 15, 537. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Black, J.; Muthayya, S.; Shet, A.; Bhat, V.; Nagaraj, S.; Prashanth, N.S.; Sudarshan, H.; Biggs, B.-A.; Shet, A.S. Determinants of anemia among young children in rural India. Pediatrics 2010, 126, e140–e149. [Google Scholar] [CrossRef]
- Kumar, T.; Taneja, S.; Yajnik, C.S.; Bhandari, N.; Strand, T.A. Prevalence and predictors of anemia in a population of North Indian children. Nutrition 2014, 30, 531–537. [Google Scholar] [CrossRef]
- Martins, V.J.B.; Toledo Florêncio, T.M.M.; Grillo, L.P.; do Carmo, P.; Franco, M.; Martins, P.A.; Clemente, A.P.G.; Santos, C.D.L.; de Fatima, A.; Vieira, M.; et al. Long-lasting effects of undernutrition. Int. J. Environ. Res. Public Health 2011, 8, 1817–1846. [Google Scholar] [CrossRef]
- Burke, R.M.; Leon, J.S.; Suchdev, P.S. Identification, prevention and treatment of iron deficiency during the first 1000 days. Nutrients 2014, 6, 4093–4114. [Google Scholar] [CrossRef]
- Chowdhury, S.D.; Ghosh, T. Undernutrition in Santal children: A biochemical and hematological study. Homo 2013, 64, 215–227. [Google Scholar] [CrossRef]
- Harding, K.L.; Aguayo, V.M.; Namirembe, G.; Webb, P. Determinants of anemia among women and children in Nepal and Pakistan: An analysis of recent national survey data. Matern. Child Nutr. 2018, 14 (Suppl. 4), e12478. [Google Scholar] [CrossRef]
- Caulfield, L.E.; de Onis, M.; Blossner, M.; Black, R.E. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am. J. Clin. Nutr. 2004, 80, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Lenters, L.; Wazny, K.; Bhutta, Z.A. Chapter 11: Management of severe and moderate acute malnutrition in children. In Disease Control Priorities: Reproductive, Maternal, Newborn, and Child Health; The World Bank: Washington, DC, USA, 2016; Volume 2. [Google Scholar]
- Chwang, L.C.; Soemantri, A.G.; Pollitt, E. Iron supplementation and physical growth of rural Indonesian children. Am. J. Clin. Nutr. 1988, 47, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Darshan, D.; Frazer, D.M.; Wilkins, S.J.; Anderson, G.J. Severe Iron Deficiency Blunts the Response Of The Iron Regulatory Gene Hamp And Pro-Inflammatory Cytokines To Lipopolysaccharide. Haematologica 2010, 95, 1660. [Google Scholar] [CrossRef] [PubMed]
- Brabin, B.J.; Premji, Z.; Verhoeff, F. An analysis of anemia and child mortality. J. Nutr. 2001, 131, 636S–645S; discussion 646S–648S. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.P.; Chen-Edinboro, L.P.; Caulfield, L.E.; Murray-Kolb, L.E. The impact of anemia on child mortality: An updated review. Nutrients 2014, 6, 5915–5932. [Google Scholar] [CrossRef]
- Da Silva, L.L.S.; Fawzi, W.W.; Cardoso, M.A.; ENFAC Working Group. Factors associated with anemia in young children in Brazil. PLoS ONE 2018, 13, e0204504. [Google Scholar] [CrossRef]
- Turkay, S.; Tanzer, F.; Gultekin, A.; Bakici, M.Z. The influence of maternal iron deficiency anaemia on the haemoglobin concentration of the infant. J. Trop. Pediatr. 1995, 41, 369–371. [Google Scholar] [CrossRef]
- Emamghorashi, F.; Heidari, T. Iron status of babies born to iron-deficient anaemic mothers in an Iranian hospital. East. Mediterr. Health J. 2004, 10, 808–814. [Google Scholar]
- De Paiva, A.A.; Rondo, P.H.C.; Pagliusi, R.A.; do Latorre, M.R.D.O.; Cardoso, M.A.A.; Gondim, S.S.R. Relationship between the iron status of pregnant women and their newborns. Rev. Saude Publica 2007, 41, 321–327. [Google Scholar] [CrossRef]
- Kilbride, J.; Baker, T.G.; Parapia, L.A.; Khoury, S.A.; Shuqaidef, S.W.; Jerwood, D. Anaemia during pregnancy as a risk factor for iron-deficiency anaemia in infancy: A case-control study in Jordan. Int. J. Epidemiol. 1999, 28, 461–468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Da Silva Vieira, R.D.F.; de Taddei, J.A.A.C.; Konstantyner, T.; Marques, A.C.V.; Braga, J.A.P. Correlation between hemoglobin levels of mothers and children on exclusive breastfeeding in the first six months of life. J. Pediatr. (Rio J.) 2016, 92, 479–485. [Google Scholar]
- Malako, B.G.; Teshome, M.S.; Belachew, T. Anemia and associated factors among children aged 6–23 months in Damot Sore District, Wolaita Zone, South Ethiopia. BMC Hematol. 2018, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, R.J. Defining Iron-Deficiency Anemia in Public Health Terms: A Time for Reflection. J. Nutr. 2001, 131, 565S–567S. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Global Prevalence of Anaemia in 2011; World Health Organisation: Geneva, Switzerland, 2015; ISBN 978 92 4 156496 0. [Google Scholar]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Pena-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dhar, S.; Sengupta, B.; Sengupta, S.; Mazumder, L.; Chakarabarti, S. Coexistence of Haemoglobinopathies and Iron Deficiency in the Development of Anemias in the Tribal Population Eastern India. Studies Tribes Tribals 2011, 9, 111–121. [Google Scholar] [CrossRef]
- Houghton, L.A.; Trilok-Kumar, G.; McIntosh, D.; Haszard, J.J.; Harper, M.J.; Reid, M.; Erhardt, J.; Bailey, K.; Gibson, R.S. Multiple micronutrient status and predictors of anemia in young children aged 12-23 months living in New Delhi, India. PLoS ONE 2019, 14, e0209564. [Google Scholar] [CrossRef]
- Dey, S.; Goswami, S.; Dey, T. Identifying predictors of childhood anaemia in north-east India. J. Health Popul. Nutr. 2013, 31, 462–470. [Google Scholar] [CrossRef][Green Version]
- Engle-Stone, R.; Aaron, G.J.; Huang, J.; Wirth, J.P.; Namaste, S.M.; Williams, A.M.; Peerson, J.M.; Rohner, F.; Varadhan, R.; Addo, O.Y.; et al. Predictors of anemia in preschool children: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 402S–415S. [Google Scholar]
- Kejo, D.; Petrucka, P.M.; Martin, H.; Kimanya, M.E.; Mosha, T.C. Prevalence and predictors of anemia among children under 5 years of age in Arusha District, Tanzania. Pediatric Health Med. Ther. 2018, 9, 9–15. [Google Scholar] [CrossRef]
- Dey, S.; Bedi, A.S. The National Rural employment guarantee scheme in Birbhum. Commentary. Econ. Political Wkly. 2010, 41, 19–25. [Google Scholar]
- Divakar, S.; Balaji, P.; Poornima, S.; Varne, S.; Ali, S.; Puttaswamy, M. A comparative assessment of nutritional and health status between tribal and nontribal under five children of Mysore, India. Muller J. Med. Sci. Res. 2013, 4, 82–85. [Google Scholar]
- Srinivasan, T.; Muraleedharan, V.; Pratap, B. Morbidity in India since 1944. Indian Econ. Rev. 2017, 52, 3–35. [Google Scholar] [CrossRef][Green Version]
- Rousham, E.; Mascie-Taylor, C. Seasonality and child morbidity in rural Bangladesh. Am. J. Hum. Biol. 1995, 7, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Kapoor, S.; Nikitin, D. A Closer Look at Child Mortality among Adivasis in India; World Bank: Washington, DC, USA, 2010. [Google Scholar]
- Dutta-Bergmann, M. Poverty, structural barriers, and health: A Santali narrative of health communication. Qual. Health Res. 2004, 14, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- FAO What Is a Serving? Available online: http://www.fao.org/english/newsroom/focus/2003/fruitveg2.htm (accessed on 20 August 2019).
- WHO; FAO. Global Strategy on Diet, Physical Activity and Health: Promoting Fruit and Vegetable Consumption Around the World; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- National Institute of Nutrition (India). Dietary Guidelines for Indians: A Manual; National Institute of Nutrition: Secunderabad, India, 2011. [Google Scholar]
- Mittal, S. Can Horticulture Be a Success Story for India; ICRIER working papers; Indian Council for Research on International Economic Relations: New Delhi, India, 2007. [Google Scholar]
- Talukder, A.; Haselow, N.; Osei, A.; Villate, E.; Reario, D.; Kroeun, H.; SokHoing, L.; Uddin, A.; Dhungel, S.; Quinn, V.; et al. Homestead food production model contributes to improved household food security and nutrition status of young children and women in poor populations—Lessons learned from scaling-up programs in Asia (Bangladesh, Cambodia, Nepal and Philippines). Field Actions Sci. Rep. J. Field Actions 2010. Available online: http://journals.openedition.org/factsreports/404 (accessed on 10 September 2019).
- Olney, D.; Dillon, A.; Ruel, M.; Nielsen, J. Chapter 6: Lessons Learned from the Evaluation of Helen Keller International’s Enhanced Homestead Food Production Program (EHFP). In Achieving a Nutrition Revolution for Africa: The road to Healthier Diets and Optimal Nutrition; Namukolo, C., Sheryl, L.H., Eds.; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2016; pp. 67–81. [Google Scholar]
- Gabrysch, S. Food and Agricultural Approaches to Reducing Malnutrition (FAARM); Heidelberg University: Heidelberg, Germany, 2015. [Google Scholar]
- De-Regil, L.M.; Suchdev, P.S.; Vist, G.E.; Walleser, S.; Pena-Rosas, J.P. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Hirve, S.; Martini, E.; Juvekar, S.K.; Agarwal, D.; Bavdekar, A.; Sari, M.; Molwane, M.; Janes, S.; Haselow, N.; Yeung, D.L.; et al. Delivering Sprinkles Plus through the Integrated Child Development Services (ICDS) to reduce anemia in pre-school children in India. Indian J. Pediatr. 2013, 80, 990–995. [Google Scholar] [CrossRef]
- Unicef The State of the World’s Children 2016. Reaching Every Child: The Promise of Equity 2016. Available online: https://www.unicef.org/publications/files/UNICEF_SOWC_2016.pdf (accessed on 2 January 2020).
- Marriott, B.P.; White, A.; Hadden, L.; Davies, J.C.; Wallingford, J.C. World Health Organization (WHO) infant and young child feeding indicators: Associations with growth measures in 14 low-income countries. Matern. Child Nutr. 2012, 8, 354–370. [Google Scholar] [CrossRef]
| Mild and Adequate 2 > z-score ≥ −2 MUAC ≥ 12.5 cm | Moderate −2 > z-score ≥ −3 11.5 cm ≤ MUAC < 12.5 cm | Severe −3 > z-score 11.5 cm > MUAC | Total Malnourished | ||||
|---|---|---|---|---|---|---|---|
| Total | n | Mean ± SD/Median, Min/Max | n (%) | n (%) | n (%) | n (%) | |
| Sex ratio, (n = 307) | 307 | 0.872 (872 girls/1000 boys) | |||||
| Age (months) NN *** | 307 | 22.5 ± 9.5/23.0, 6/39 | |||||
| Weight (kg) NN * | 307 | 9.1 ± 1.7/9.0, 5.5/14.1 | |||||
| Height/Length (cm) NN ** | 306 | 78.1 ± 7.5/78.0, 61.5/96.8 | |||||
| HAZ | 306 | −2.03 ± 1.11, −5.75/2.00 | 146 (47.7) | 109 (35.6) | 50 (16.3) | 159 (51.9) | |
| WAZ | 307 | −1.95 ± 0.98, −4.36/1.44 | 156 (50.8) | 110 (35.8) | 41 (13.4) | 151 (49.2) | |
| WHZ | 306 | −1.19 ± 0.93, −3.84/2.51 | 247 (80.7) | 48 (15.7) | 10 (3.3) | 58 (19.0) | |
| BAZ | 306 | −0.96 ± 0.96, −3.75/2.87 | 264 (86.3) | 37 (12.1) | 4 (1.3) | 41 (13.4) | |
| MUAC z-score NN * | 307 | −1.09 ± 0.88/−1.02, −3.52/1.69 | 260 (84.7) | 39 (12.7) | 8 (2.6) | 47 (15.3) | |
| MUAC NN ** (cm) | 307 | 13.8 ± 1.0/13.8, 11.0/16.8 | 276 (89.9) | 27 (8.8) | 4 (1.3) | 31 (10.1) | |
| by Gender | n | p-value | Mean ± SD, Min/Max | n (%) | n (%) | n (%) | n (%) |
| Age (months) Boys | 164 | 0.491 | 22.1 ± 10.0, 6/39 | ||||
| Age (months) Girls | 143 | 22.9 ± 8.9, 6/38 | |||||
| Weight (kg) Boys | 164 | 0.005 ** | 9.4 ± 1.9, 5.5/14.1 | ||||
| Weight (kg) Girls | 143 | 8.8 ± 1.5, 5.6/12.9 | |||||
| Height/Length (cm) Boys | 163 | 0.164 | 78.6 ± 8.1, 61.5/96.8 | ||||
| Height/Length (cm) Girls | 143 | 77.4 ± 6.8, 61.5/92.8 | |||||
| HAZ Boys | 163 | 0.391 | −1.98 ± 1.09, −4.50/1.02 | 85 (52.1) | 52 (31.9) | 26 (16.0) | 78 (47.9) |
| HAZ Girls | 143 | −2.09 ± 1.14, −5.75/2.00 | 61 (42.7) | 57 (39.9) | 24 (16.8) | 81 (56.7) | |
| WAZ Boys | 164 | 0.395 | −1.91 ± 0.94, −4.27/1.44 | 84 (51.2) | 61 (37.2) | 19 (11.6) | 80 (48.8) |
| WAZ Girls | 143 | −2.00 ± 1.02, −4.36/0.31 | 72 (50.3) | 49 (34.3) | 22 (15.4) | 71 (49.7) | |
| WHZ Boys | 163 | 0.866 | −1.19 ± 0.96, −3.84/2.51 | 133 (81.6) | 23 (14.1) | 6 (3.7) | 29 (17.8) |
| WHZ Girls | 143 | −1.18 ± 0.89, −3.21/1.18 | 114 (79.7) | 25 (17.5) | 4 (2.8) | 29 (20.3) | |
| BAZ Boys | 163 | 0.880 | −0.96 ± 1.00, −3.75/2.87 | 138 (84.7) | 20 (12.3) | 4 (2.5) | 24 (14.8) |
| BAZ Girls | 143 | −0.95 ± 0.91, −2.97/1.33 | 126 (88.1) | 17 (11.9) | 0 (0) | 17 (11.9) | |
| MUAC (cm) Boys | 164 | 0.005 ** | 13.9 ± 1.0, 11.0/16.7 | 151 (92.1) | 10 (6.1) | 3 (1.8) | 13 (7.9) |
| MUAC (cm) Girls | 143 | 13.6 ± 1.0, 11.0/16.8 | 125 (87.4) | 17 (11.9) | 1 (0.7) | 18 (12.6) | |
| MUAC z-score Boys | 164 | 0.784 | −1.08 ± 0.90, −3.40/1.69 | 138 (84.1) | 21 (12.8) | 5 (3.0) | 26 (15.8) |
| MUAC z-score Girls | 143 | −1.10 ± 0.86, −3.52/1.20 | 122 (85.3) | 18 (12.6) | 3 (2.1) | 21 (14.7) | |
| Mild and Adequate 2 > z-score ≥ −2 MUAC ≥ 12.5 cm | Moderate −2 > z-score ≥ −3 11.5 cm ≤ MUAC < 12.5 cm | Severe −3 > z-score 11.5 cm > MUAC | Total Mal-Nourished | ||||
|---|---|---|---|---|---|---|---|
| Total Age-Related | n | p-Value | Mean ± SD, Min/Max | n (%) | n (%) | n (%) | n (%) |
| HAZ 6–11 m | 54 | 0.000 *** (a, d, f) | −1.37 ± 1.17, −4.38/2.00 | 38 (70.4) | 12 (22.2) | 3 (5.6) | 15 (27.8) |
| HAZ 12–23 m | 109 | −2.01 ± 1.03, −4.38/1.02 | 56 (51.4) | 35 (32.1) | 18 (16.5) | 53 (48.6) | |
| HAZ 24–35 m | 123 | −2.30 ± 1.05, −5.75/0.76 | 46 (37.4) | 50 (40.7) | 27 (22.0) | 77 (62.7) | |
| HAZ 36–39 m | 20 | −2.25 ± 1.09, −4.24/−0.10 | 6 (30.0) | 12 (60.0) | 2 (10.0) | 14 (70.0) | |
| WAZ 6–11 m | 54 | 0.055 | −1.71 ± 1.00, −4.09/0.60 | 29 (53.7) | 21 (38.9) | 4 (7.4) | 25 (46.3) |
| WAZ 12–23 m | 109 | −1.87 ± 0.98, −4.09/1.44 | 61 (56.0) | 37 (33.9) | 11 (10.1) | 48 (44.0) | |
| WAZ 24–35 m | 124 | −2.10 ± 0.95, −4.36/0.31 | 58 (46.8) | 44 (35.5) | 22 (17.7) | 66 (53.2) | |
| WAZ 36–39 m | 20 | −2.11 ± 0.94, −3.69/−0.37 | 8 (40.0) | 8 (40.0) | 4 (20.0) | 12 (60.0) | |
| WHZ 6–11 m | 54 | 0.964 | −1.23 ± 0.99, −3.10/1.18 | 39 (72.2) | 13 (24.1) | 2 (3.7) | 15 (27.8) |
| WHZ 12–23 m | 109 | −1.20 ± 0.96, −3.84/2.51 | 93 (85.3) | 11 (10.1) | 4 (3.7) | 15 (13.8) | |
| WHZ 24–35 m | 123 | −1.16 ± 0.89, −3.21/1.01 | 99 (80.5) | 21 (17.1) | 3 (2.4) | 24 (19.5) | |
| WHZ 36–39 m | 20 | −1.15 ± 0.87, −3.07/0.46 | 16 (80.0) | 3 (15.0) | 1 (5.0) | 4 (20.0) | |
| BAZ 6–11 m | 54 | 0.085 | −1.26 ± 0.99, −3.2/1.15 | 40 (74.1) | 12 (22.2) | 2 (3.7) | 14 (25.9) |
| BAZ 12–23 m | 109 | −0.89 ± 0.97, −3.75/2.87 | 100 (91.7) | 6 (5.5) | 2 (1.8) | 8 (7.3) | |
| BAZ 24–35 m | 123 | −0.89 ± 0.93, −2.89/1.18 | 107 (87.0) | 16 (13.0) | 0 (0) | 16 (13.0) | |
| BAZ 36–39 m | 20 | −0.90 ± 0.93, −2.97/0.87 | 17 (85.0) | 3 (15.0) | 0 (0) | 3 (15.0) | |
| MUAC 6–11 m | 54 | 0.000 *** b: p = 0.000352 ** Bonfer. d: p = 0.001409 ** Bonfer. e: p = 0.004542 * Bonfer. f: p = 0.000015 *** Bonfer. | 13.3 ± 1.0, 11.0/15.4 | 45 (83.3) | 7 (13.0) | 2 (3.7) | 9 (16.7) |
| MUAC 12–23 m | 109 | 13.6 ± 0.9, 11.0/16.7 | 98 (89.9) | 9 (8.3) | 2 (1.8) | 11 (10.1) | |
| MUAC 24–35 m | 124 | 14.0 ± 1.0, 12.0/16.8 | 115 (92.7) | 9 (7.3) | 0 (0.0) | 9 (7.3) | |
| MUAC 36–39 m | 20 | 14.3 ± 1.2, 11.7/15.8 | 18 (90.0) | 2 (10.0) | 0 (0.0) | 2 (10.0) | |
| MUACz-score 6–11 m | 54 | 0.285 | −0.97 ± 0.91, −3.34/0.97 | 48 (88.9) | 4 (7.4) | 2 (3.7) | 6 (11.1) |
| MUACz-score 12–23 m | 109 | −1.00 ± 0.83, −3.36/1.69 | 96 (88.1) | 11 (10.1) | 2 (1.8) | 13 (11.9) | |
| MUACz-score 24–35 m | 124 | −1.19 ± 0.88, −3.40/1.20 | 101 (81.5) | 20 (16.1) | 3 (2.4) | 23 (18.5) | |
| MUACz-score 36–39 m | 20 | −1.28 ± 1.01, −3.52/−0.01 | 15 (75.0) | 4 (20.0) | 1 (5.0) | 5 (25.0) |
| No Anemia Hb ≥ 11.0 g/dL | Mild Hb 10.0–10.9 g/dL | Moderate Hb 7.0–9.9 g/dL | Severe Hb < 7.0 g/dL | Total Anemia | ||||
|---|---|---|---|---|---|---|---|---|
| n | p-Value | Mean ± SD, Min/Max (g/dL) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Hb Total | 307 | - | 9.1 ± 1.3, 5.0/12.7 | 19 (6.2) | 63 (20.5) | 211 (68.7) | 14 (4.6) | 288 (93.8) |
| Hb Boys | 164 | 0.915 | 9.1 ± 1.3, 5.6/12.7 | 10 (6.1) | 35 (21.3) | 112 (68.3) | 7 (4.3) | 154 (93.9) |
| Hb Girls | 143 | 9.1 ± 1.2, 5.0/11.8 | 9 (6.3) | 28 (19.6) | 99 (69.2) | 7 (4.9) | 134 (93.7) | |
| Hb 6–11 m | 54 | 0.000 *** (b, c, d, e, f) | 8.8 ± 1.1, 5.8/11.9 | 2 (3.7) | 5 (9.3) | 44 (81.5) | 3 (5.6) | 52 (96.4) |
| Hb 12–23 m | 109 | 8.7 ± 1.2, 5.0/12.0 | 2 (1.8) | 14 (12.8) | 88 (80.7) | 5 (4.6) | 107 (98.1) | |
| Hb 24–35 m | 124 | 9.4 ± 1.3, 5.0/12.7 | 11 (8.9) | 35 (28.2) | 73 (58.9) | 5 (4.0) | 113 (91.1) | |
| Hb 36–39 m | 20 | 10.0 ± 1.3, 6.6/11.8 | 4 (20.0) | 9 (45.0) | 6 (30.0) | 1 (5.0) | 16 (80.0) |
| Group | Description of the Group | Total n = 307 n (%) | Sex | Age | ||||
|---|---|---|---|---|---|---|---|---|
| Boys (n = 164) n (%) | Girls (n = 143) n (%) | 6–11 m (n = 54) n (%) | 12–23 m (n = 109) n (%) | 24–35 m (n = 124) n (%) | 36–47 m (n = 20) n (%) | |||
| A | No anthropometric failure | 118 (38.4) | 65 (39.6) | 53 (37.1) | 26 (48.1) | 48 (44.0) | 39 (31.5) | 5 (25.0) |
| B | Wasting only | 2 (0.7) | 2 (1.2) | 0 (0) | 1 (1.9) | 1 (0.9) | 0 (0) | 0 (0) |
| C | Wasting and underweight | 18 (5.9) | 12 (7.4) | 6 (4.2) | 11 (20.4) | 3 (2.8) | 3 (2.4) | 1 (5.0) |
| D | Wasting, underweight, stunting | 38 (12.4) | 15 (9.2) | 23 (16.1) | 3 (5.6) | 11 (10.1) | 21 (17.1) | 3 (15.0) |
| E | Stunting and underweight | 85 (27.8) | 46 (28.2) | 39 (27.3) | 10 (18.5) | 30 (27.5) | 37 (30.1) | 8 (40.0) |
| F | Stunting only | 36 (11.8) | 17 (10.4) | 19 (13.3) | 2 (3.7) | 12 (11.0) | 19 (15.4) | 3 (15.0) |
| Y | Underweight only | 9 (2.9) | 6 (3.7) | 3 (2.1) | 1 (1.9) | 4 (3.7) | 4 (3.3) | 0 (0) |
| Total anthropometric failure | 189 (61.6) | 99 (60.4) | 90 (62.9) | 28 (51.9) | 61 (56.0) | 85 (68.5) | 15 (75.0) | |
| CIAF Classification | MUAC Categories n = 307 | |||
|---|---|---|---|---|
| Adequate (n = 276) MUAC ≥ 12.5 cm n (%) | Moderate (n = 27) 11.5 cm ≤ MUAC < 12.5 cm n (%) | Severe (n = 4) 11.5 cm > MUAC n (%) | ||
| A | No anthropometric failure | 116 (42.0) | 2 (7.4) | 0 (0) |
| B | Wasting only | 2 (0.7) | 0 (0) | 0 (0) |
| C | Wasting and underweight | 15 (5.5) | 2 (7.4) | 1 (25.0) |
| D | Wasting, underweight and stunting | 22 (8.0) | 14 (51.9) | 2 (50.0) |
| E | Stunting and underweight | 77 (28.0) | 7 (25.9) | 1 (25.0) |
| F | Stunting only | 36 (13.1) | 0 (0) | 0 (0) |
| Y | Underweight only | 7 (2.5) | 2 (7.4) | 0 (0) |
| Total anthropometric failure | 160 (58.0) | 25 (92.6) | 4 (100.0) | |
| Final Model: Forward Wald | Beta | S.E. | Odds Ratio Exp(B) | 95% CI | p-Value |
|---|---|---|---|---|---|
| Age two categories, 1 = ≥24 months | 1.613 | 0.424 | 5.019 | 2.185, 11.527 | 0.000 *** |
| WAZ | 0.517 | 0.209 | 1.677 | 1.113, 2.526 | 0.013 * |
| Morbidity history, 1 = no morbidity | 0.866 | 0.398 | 2.376 | 1.089, 5.188 | 0.030 * |
| Maternal Hb level | 0.335 | 0.163 | 1.398 | 1.016, 1.923 | 0.040 * |
| Count of food groups consumed during previous 24 h | 0.497 | 0.209 | 1.644 | 1.091, 2.476 | 0.017 * |

| Visible Signs of Malnutrition at Medical Checkup | n Children | n (%) Children | n Mothers | n (%) Mothers |
| Dry eyes/eyeball with wavy structure | 275 | 110 (40.0%) | 288 | 69 (24.0%) |
| Night blindness | 303 | 0 (0.0%) | 288 | 12 (4.2%) |
| Bitot’s spot | 275 | 3 (1.2%) | 288 | 22 (7.6%) |
| Perlèche | 303 | 10 (3.3%) | 288 | 3 (1.0%) |
| Pale fingernails/paleness of skin | 302 | 72 (23.8%) | 287 | 60 (20.9%) |
| Skin lesion, rough skin, pigmentation | 304 | 4 (1.3%) | 288 | 74 (25.7%) |
| Dental disorders/caries | 303 | 29 (9.6%) | 288 | 39 (13.5%) |
| Morbidity Pattern at Medical Checkup or during Previous Week | n Children | n (%) Children | n Mothers | n (%) Mothers |
| Fever | 303 | 73 (24.1%) | 288 | 23 (8.0%) |
| Respiratory infections (cold/cough) | 303 | 191 (63.0%) | 288 | 47 (16.3%) |
| Intestinal infections (diarrhea) | 303 | 52 (17.2%) | 288 | 8 (2.8%) |
| Referred to hospital | 304 | 39 (12.8%) | 288 | 32 (11.1%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiller, C.K.; Golembiewski, S.K.E.; Golembiewski, M.; Mondal, S.; Biesalski, H.-K.; Scherbaum, V. Prevalence of Undernutrition and Anemia among Santal Adivasi Children, Birbhum District, West Bengal, India. Int. J. Environ. Res. Public Health 2020, 17, 342. https://doi.org/10.3390/ijerph17010342
Stiller CK, Golembiewski SKE, Golembiewski M, Mondal S, Biesalski H-K, Scherbaum V. Prevalence of Undernutrition and Anemia among Santal Adivasi Children, Birbhum District, West Bengal, India. International Journal of Environmental Research and Public Health. 2020; 17(1):342. https://doi.org/10.3390/ijerph17010342
Chicago/Turabian StyleStiller, Caroline Katharina, Silvia Konstanze Ellen Golembiewski, Monika Golembiewski, Srikanta Mondal, Hans-Konrad Biesalski, and Veronika Scherbaum. 2020. "Prevalence of Undernutrition and Anemia among Santal Adivasi Children, Birbhum District, West Bengal, India" International Journal of Environmental Research and Public Health 17, no. 1: 342. https://doi.org/10.3390/ijerph17010342
APA StyleStiller, C. K., Golembiewski, S. K. E., Golembiewski, M., Mondal, S., Biesalski, H.-K., & Scherbaum, V. (2020). Prevalence of Undernutrition and Anemia among Santal Adivasi Children, Birbhum District, West Bengal, India. International Journal of Environmental Research and Public Health, 17(1), 342. https://doi.org/10.3390/ijerph17010342





