Kinetics and Thermodynamics of Uranium (VI) Adsorption onto Humic Acid Derived from Leonardite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation and Characterization of HA
2.3. Adsorption Experiments and Data Processing
3. Models
3.1. Adsorption Kinetics Models
3.2. Adsorption Isotherm Models
3.3. Thermodynamic Parameters
4. Results and Discussion
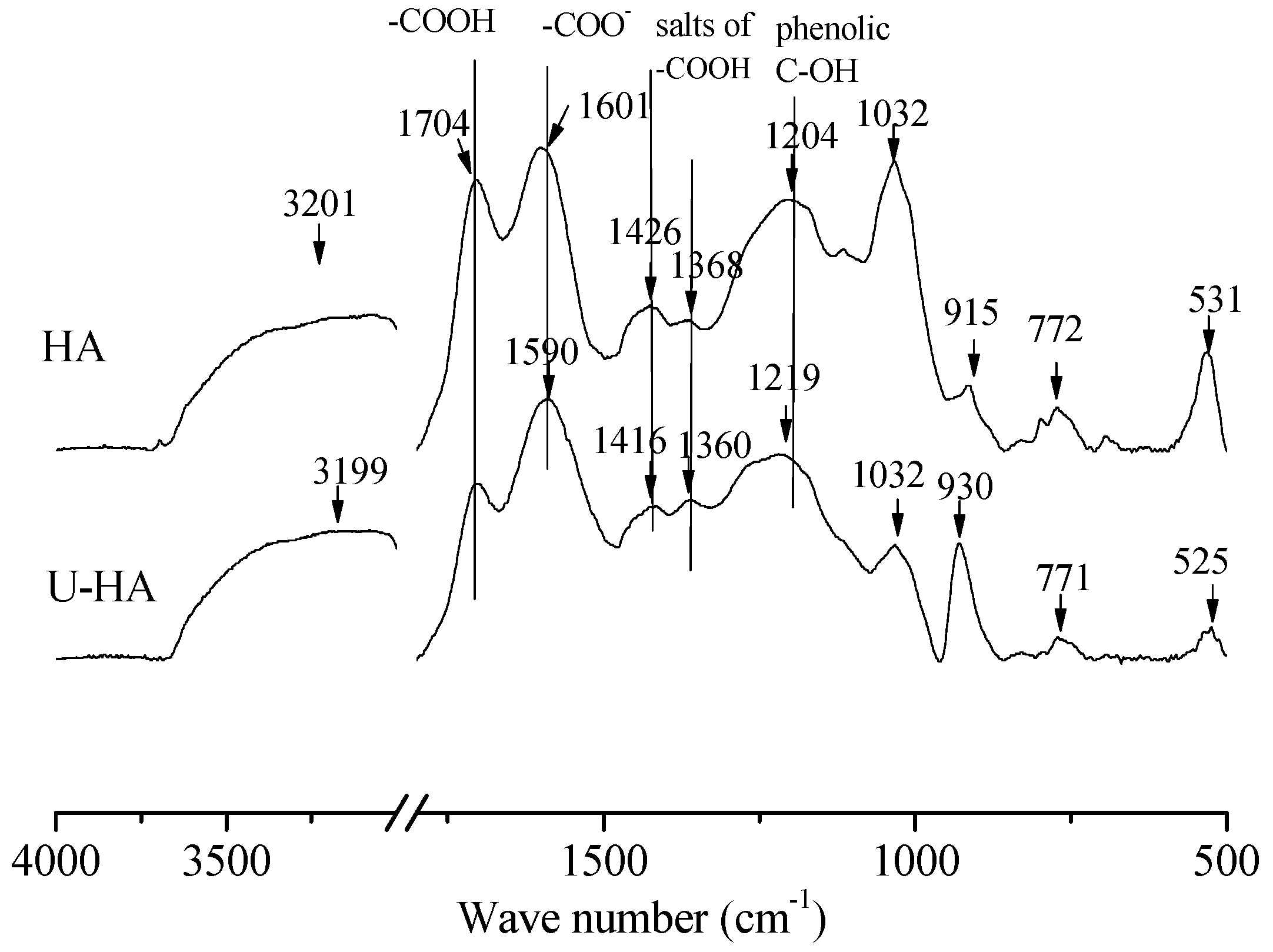
4.1. Properties of Adsorbents
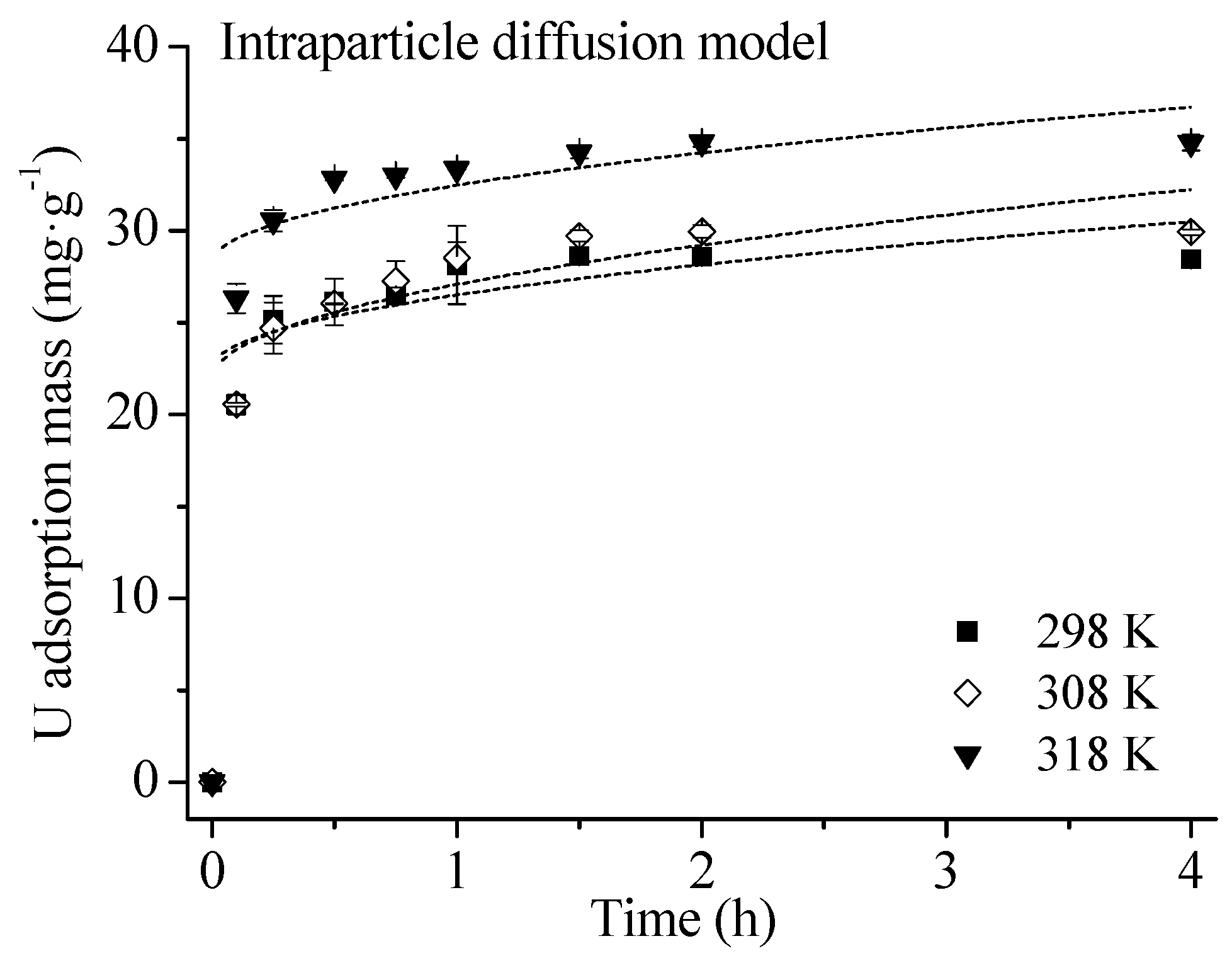
4.2. Adsorption Kinetics
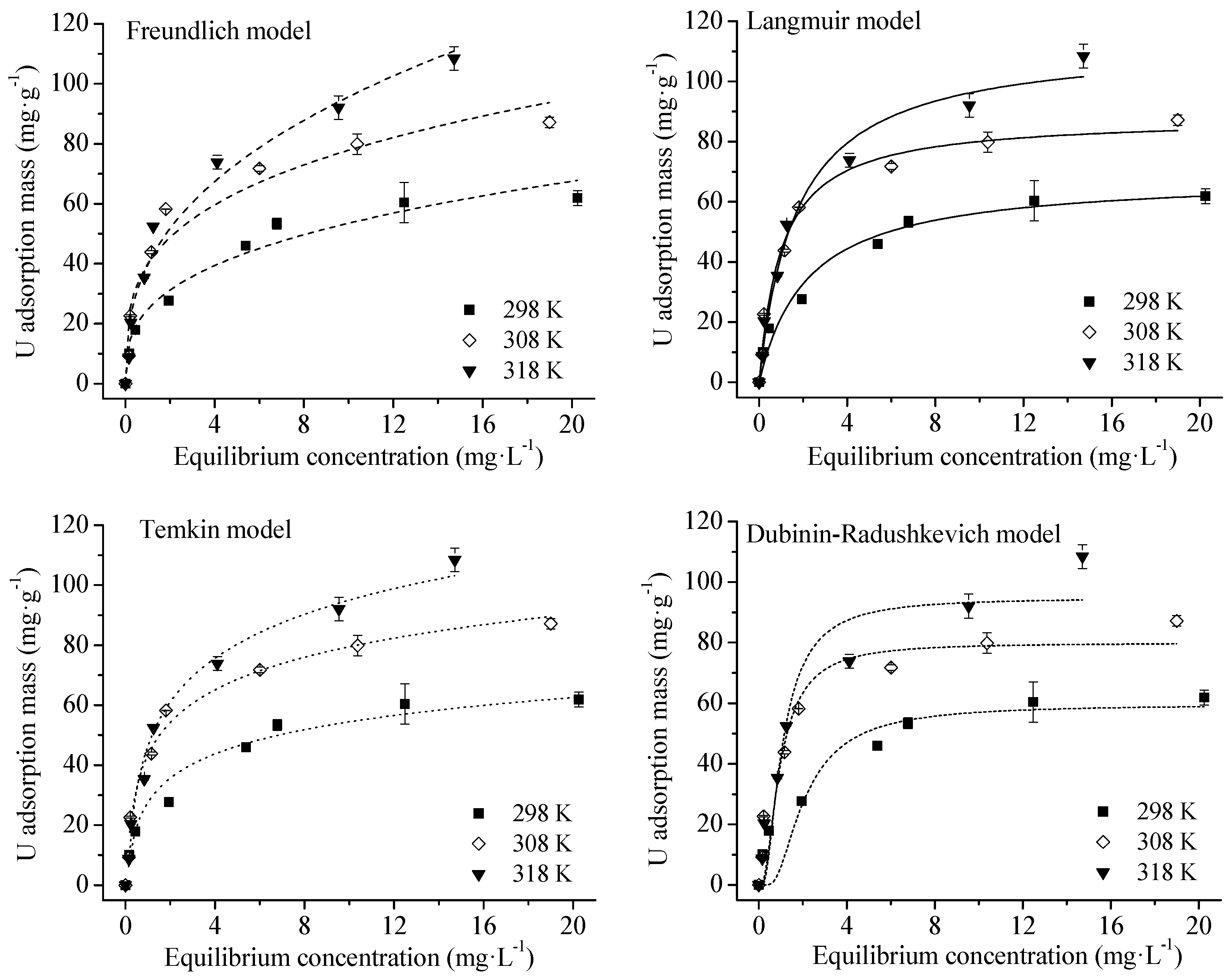
4.3. Adsorption Isotherms
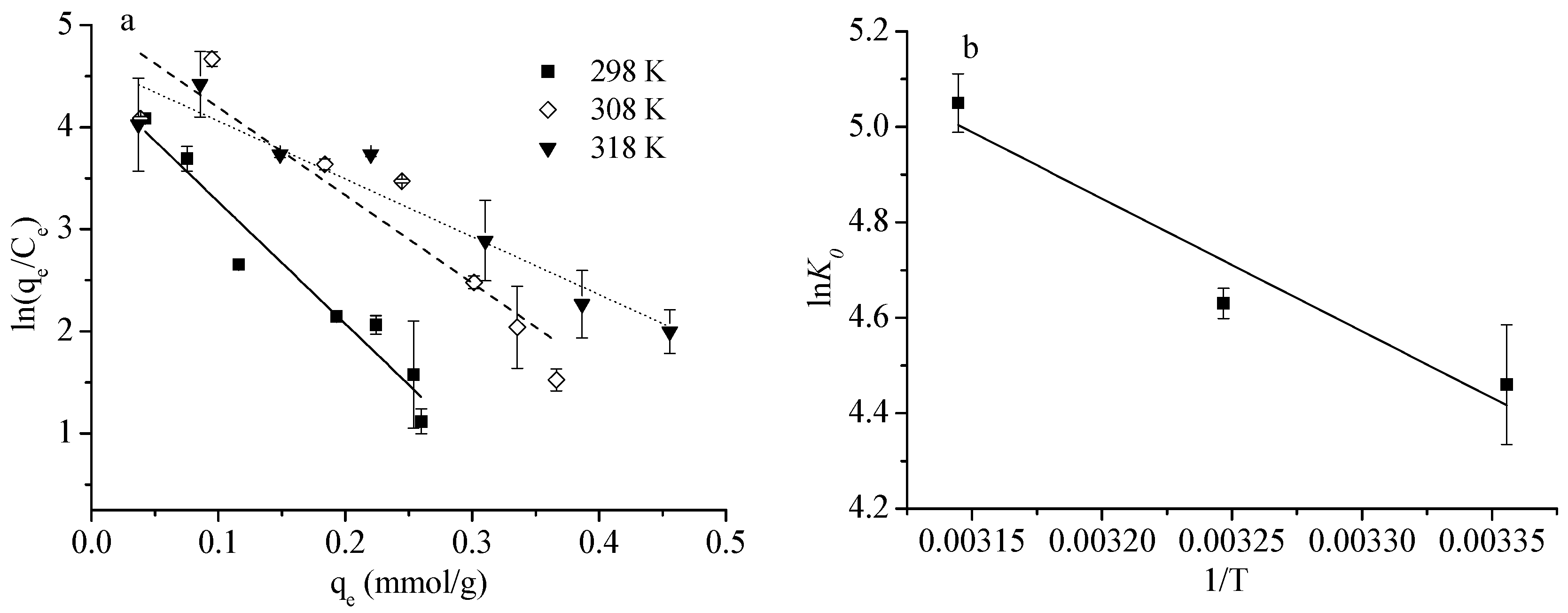
4.4. Adsorption Thermodynamics
4.5. Adsorption Mechanism
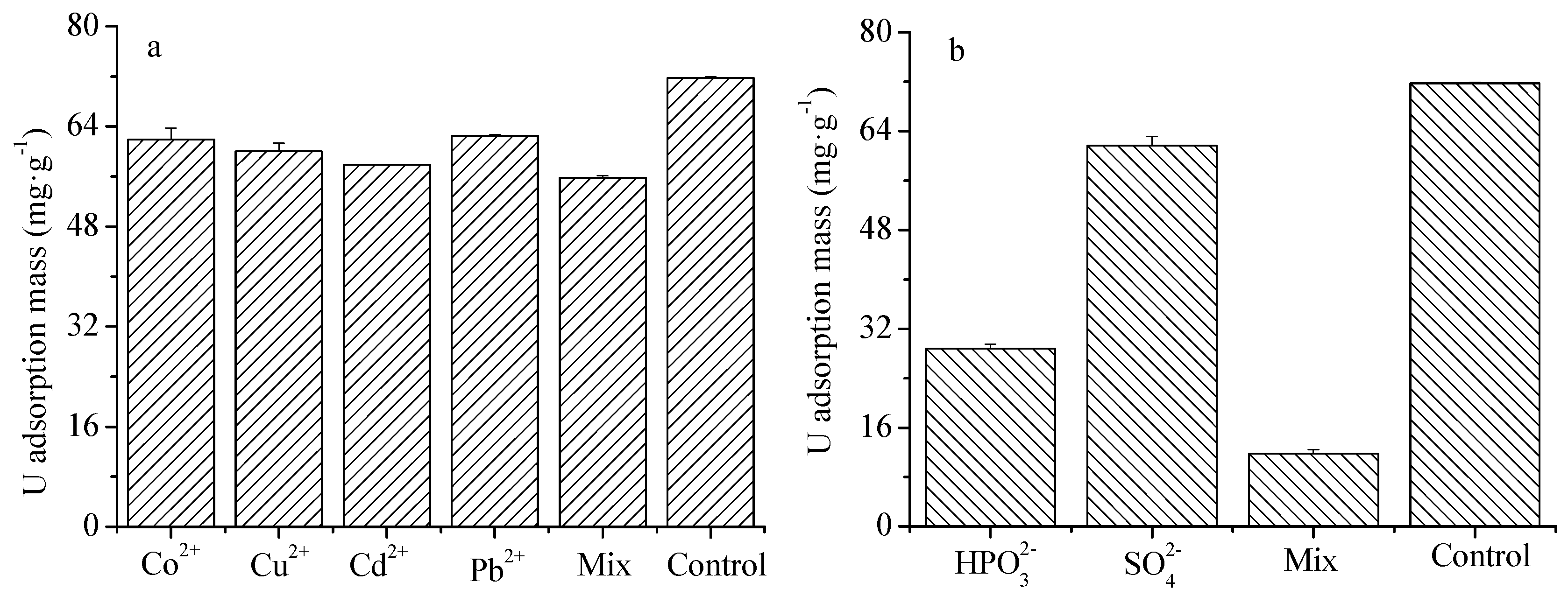
4.6. The Effects of Cations and Anions on U(VI) Adsorption
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rankin, D.W. CRC Handbook of Chemistry and Physics, 88th ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 14–17. [Google Scholar]
- Campos, M.B.; de Azevedo, H.; Nascimento, M.R.L.; Roque, C.V.; Rodgher, S. Environmental assessment of water from a uranium mine (Caldas, Minas Gerais State, Brazil) in a decommissioning operation. Environ. Earth Sci. 2011, 62, 857–863. [Google Scholar] [CrossRef]
- Mudd, G.M.; Patterson, J. Continuing pollution from the Rum Jungle U–Cu project: A critical evaluation of environmental monitoring and rehabilitation. Environ. Pollut. 2010, 158, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yang, J.; Chen, C.; Zhang, X.; Wang, Q.; Zhang, C. Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. J. Environ. Radioactiv. 2008, 99, 126–133. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Drinking Water Standards and Health Advisories; EPA 822-S-12e1001; Office of Water; U.S. Environmental Protection Agency: Washington, DC, USA, 2012.
- World Health Organization. Guidelines for Drinking-Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Bhalara, P.D.; Punetha, D.; Balasubramanian, K. A review of potential remediation techniques for uranium(VI) ion retrieval from contaminated aqueous environment. J. Environ. Chem. Eng. 2014, 2, 1621–1634. [Google Scholar] [CrossRef]
- Meng, F.D.; Yuan, G.D.; Larson, S.L.; Ballard, J.H.; Waggoner, C.A.; Arslan, Z.; Han, F.X. Removing uranium (VI) from aqueous solution with insoluble humic acid derived from leonardite. J. Environ. Radioactiv. 2017, 180, 1–8. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.F.; Sar, P.; Kazy, S.K.; Kubal, B.S. Uranium sorption by Pseudomonas biomass immobilized in radiation polymerized polyacrylamide bio-beads. J. Environ. Sci. Heal. A 2006, 41, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Haddad, D.; Mellah, A.; Nibou, D.; Khemaissia, S. Promising enhancement in the removal of uranium ions by surface-modified activated carbons: Kinetic and equilibrium studies. J. Environ. Chem. Eng. 2018, 144, 04018027. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Chai, X.; Liu, J.; Deng, N. Adsorption of uranium (VI) from aqueous solution on calcined and acid-activated kaolin. Appl. Clay Sci. 2010, 47, 448–451. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, X.; Liang, P.; Liu, Y. Adsorption of uranium from aqueous solution using biochar produced by hydrothermal carbonization. J. Radioanal. Nucl. Ch. 2013, 295, 1201–1208. [Google Scholar] [CrossRef]
- Soler-Rovira, P.; Madejón, E.; Madejón, P.; Plaza, C. In situ remediation of metal contaminated soils with organic amendments: Role of humic acids in copper bioavailability. Chemosphere 2010, 79, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Khalili, F.; Al-Banna, C. Adsorption of uranium (VI) and thorium (IV) by insolubilized humic acid from Ajloun soil–Jordan. J. Environ. Radioactiv. 2015, 146, 16–26. [Google Scholar] [CrossRef]
- Harter, R.D.; Naidu, R. An assessment of environmental and solution parameter impact on trace-metal sorption by soils. Soil Sci. Soc. Am. J. 2001, 65, 597–612. [Google Scholar] [CrossRef]
- International Humic Substances Society. Acidic Functional Groups of IHSS Samples. 2018. Available online: http://humic-substances.org/acidic-functional-groups-of-ihss-samples/#products (accessed on 27 April 2019).
- Ho, Y.S. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second-order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Inyang, H.I.; Onwawoma, A.; Bae, S. The Elovich equation as a predictor of lead and cadmium sorption rates on contaminant barrier minerals. Soil Till. Res. 2016, 155, 124–132. [Google Scholar] [CrossRef]
- Juang, R.S.; Chen, M.K. Application of the Elovich equation to the kinetics of metal sorption with solvent-impregnated resins. Ind. Eng. Chem. Res. 1997, 36, 813–820. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Meng, F.D.; Yuan, G.D.; Wei, J.; Bi, D.X.; Wang, H.L. Leonardite-derived humic substances are great adsorbents for cadmium. Environ. Sci. Pollut. R. 2017, 24, 23006–23014. [Google Scholar] [CrossRef] [PubMed]
- Yang, C. Statistical mechanical study on the Freundlich isotherm equation. J. Colloid Interf. Sci. 1998, 208, 379–387. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Sadeek, S.A.; Negm, N.A.; Hefni, H.H.H.; Wahab, M.M.A. Metal adsorption by agricultural biosorbents: Adsorption isotherm, kinetic and biosorbents chemical structures. Int. J. Biol. Macromol. 2015, 81, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, C.; Ungarish, M. Kinetics of activated chemisorption. Part 2—Theoretical models. J. Chem. Soc. Faraday Trans. 1 1977, 73, 456–464. [Google Scholar] [CrossRef]
- Celebi, O.; Kilikli, A.; Erten, H.N. Sorption of radioactive cesium and barium ions onto solid humic acid. J. Hazard. Mater. 2009, 168, 695–703. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Tan, K.H. Humic Matter in Soil and the Environment: Principles and Controversies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Mahmoud, M.A. Kinetics and thermodynamics of U (VI) ions from aqueous solution using oxide nanopowder. Process. Saf. Environ. 2016, 102, 44–53. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Zeng, Z. Comparison of modified montmorillonite adsorbents: Part II: The effects of the type of raw clays and modification conditions on the adsorption performance. Chemosphere 2003, 53, 53–62. [Google Scholar] [CrossRef]
- Biswas, K.; Saha, S.K.; Ghosh, U.C. Adsorption of fluoride from aqueous solution by a synthetic iron (III)−aluminum (III) mixed oxide. Ind. Eng. Chem. Res. 2007, 46, 5346–5356. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Hutson, N.D.; Yang, R.T. Theoretical basis for the Dubinin-Radushkevitch (DR) adsorption isotherm equation. Adsorption 1997, 3, 189–195. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, C.; Zhou, X.; Yang, J.; Zhang, X.; Wang, J. Removal of uranium (VI) from aqueous solution by adsorption of hematite. J. Environ. Radioactiv. 2009, 100, 162–166. [Google Scholar]
- Zareh, M.M.; Aldaher, A.; Hussein, A.E.M.; Mahfouz, M.G.; Soliman, M. Uranium adsorption from a liquid waste using thermally and chemically modified bentonite. J. Radioanal. Nucl. Chem. 2013, 295, 1153–1159. [Google Scholar] [CrossRef]
- Deng, L.; Su, Y.; Su, H.; Wang, X.; Zhu, X. Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J. Hazard. Mater. 2007, 143, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Qu, R.; Sun, C.; Wang, C.; Ji, C.; Zhang, Y.; Yin, P. Adsorption behaviors of Hg (II) on chitosan functionalized by amino-terminated hyperbranched polyamidoamine polymers. J. Hazard. Mater. 2009, 172, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, Z. Hydrogel-supported nanosized hydrous manganese dioxide: Synthesis, characterization, and adsorption behavior study for Pb2+, Cu2+, Cd2+ and Ni2+ removal from water. Chem. Eng. J. 2015, 281, 69–80. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Pandey, P.; Chakraborty, T. Blue-and red-shifting CH··O hydrogen bonded complexes between haloforms and ethers: Correlation of donor νC−H spectral shifts with C−O–C angular strain of the acceptors. J. Phys. Chem. A 2010, 114, 5026–5033. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresource Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.; Wang, J.; Xu, C.; Liang, Y.; Yao, W.; Wang, X.; Yu, S.; Chen, C.; Sun, Y.; et al. Superior immobilization of U (VI) and 243Am (III) on polyethyleneimine modified lamellar carbon nitride composite from water environment. Chem. Eng. J. 2017, 326, 863–874. [Google Scholar] [CrossRef]
- Futalan, C.M.; Kan, C.C.; Dalida, M.L.; Hsien, K.J.; Pascua, C.; Wan, M.W. Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite. Carbohyd. Polym. 2011, 83, 528–536. [Google Scholar] [CrossRef]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Gong, J.L.; Zeng, G.M.; Niu, Q.Y.; Zhang, H.Y.; Niu, C.G.; Deng, J.H.; Yan, M. Adsorption of Cd(II) and Zn(II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem. Eng. J. 2010, 162, 487–494. [Google Scholar] [CrossRef]
- Jiang, M.; Jin, X.; Lu, X.Q.; Chen, Z. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- Bachmaf, S.; Planer-Friedrich, B.; Merkel, B.J. Effect of sulfate, carbonate, and phosphate on the uranium (VI) sorption behavior onto bentonite. Radiochim. Acta 2008, 96, 359–366. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cha, W.S.; Kim, J.G.; Baik, M.H.; Jung, E.C.; Jeong, J.T.; Kim, K.; Chung, S.Y.; Lee, Y.J. Uranium (IV) remobilization under sulfate reducing conditions. Chem. Geol. 2014, 370, 40–48. [Google Scholar] [CrossRef]
- Sanding, A.; Bruno, J. The solubility of (UO2)3(PO4)2•4H2O (s) and the formation of U(VI) phosphate complexes: Their influence in uranium speciation in natural waters. Geochim. Cosmochim. Acta 1992, 56, 4135–4145. [Google Scholar] [CrossRef]
- Mehta, V.S.; Maillot, F.; Wang, Z.; Catalano, J.G.; Giammar, D.E. Effect of co-solutes on the products and solubility of uranium (VI) precipitated with phosphate. Chem. Geol. 2014, 364, 66–75. [Google Scholar] [CrossRef]
- Perassi, I.; Borgnino, L. Adsorption and surface precipitation of phosphate onto CaCO3–montmorillonite: Effect of pH, ionic strength and competition with humic acid. Geoderma 2014, 232, 600–608. [Google Scholar] [CrossRef]

| Adsorbents | pH (H2O) | Ash (%) | C (%) | H (%) | N (%) | S (%) | O (%) | -COOH (mol/kg) | Phenolic-OH (mol/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Leonardite | 3.99 | 17.77 | 49.21 | 3.48 | 1.01 | 0.47 | 28.06 | 3.04 | 1.10 |
| HA | 2.78 | 7.48 | 56.71 | 3.78 | 1.16 | 0.36 | 30.51 | 3.64 | 1.03 |
| Temperature (K) | qe (mg·g−1) | Isotherm Model | ||||
|---|---|---|---|---|---|---|
| Pseudo First-Order | Pseudo Second-Order | Elovich | ||||
| q1 (mg·g−1) | R2 | q2 (mg·g−1) | R2 | R2 | ||
| 298 | 28.60 | 27.58 | 0.986 ** | 28.86 | 0.997 ** | 0.986 ** |
| 308 | 29.96 | 28.36 | 0.971 ** | 29.99 | 0.994 ** | 0.991 ** |
| 318 | 34.79 | 33.56 | 0.989 ** | 34.89 | 0.999 ** | 0.992 ** |
| Temperature (K) | ki (mg·g−1·h0.5) | C (mg·g−1) | R2 |
|---|---|---|---|
| 298 | 3.99 | 22.50 | 0.553 ** |
| 308 | 5.17 | 21.89 | 0.684 ** |
| 318 | 4.24 | 28.21 | 0.599 ** |
| Isotherm | Parameters | R2 | ||
|---|---|---|---|---|
| Freundlich model | Temperature (K) | n | kF (mg(1−n)·Ln·g−1) | |
| 298 | 2.99 | 24.76 | 0.967 ** | |
| 308 | 3.44 | 39.88 | 0.940 ** | |
| 318 | 2.62 | 39.66 | 0.973 ** | |
| Langmuir model | Temperature (K) | qL (mg·g−1) | kL (L·mg−1) | R2 |
| 298 | 68.60 | 0.46 | 0.970 ** | |
| 308 | 88.12 | 0.99 | 0.985 ** | |
| 318 | 113.34 | 0.59 | 0.982 ** | |
| Temkin model | Temperature (K) | kT (L·mg−1) | b (J·mol−1) | R2 |
| 298 | 10.62 | 212.28 | 0.972 ** | |
| 308 | 15.93 | 163.39 | 0.988 ** | |
| 318 | 9.00 | 125.22 | 0.988 ** | |
| Dubinin–Radushkevich model | Temperature (K) | qD (mg·g−1) | kD (mol2·J−2) | R2 |
| 298 | 59.55 | 2.29E−8 | 0.785 ** | |
| 308 | 79.93 | 6.32E−9 | 0.851 ** | |
| 318 | 94.74 | 6.89E−9 | 0.878 ** | |
| Materials | C (mg U6+/L) | pH | qm (mg/g) | References |
|---|---|---|---|---|
| HA | 0−100 | 3.0 | 68.60 | This study |
| Kaolin | 20−80 | 5.0 | 4.52 | [10] |
| Biochar | 0−100 | 6.0 | 62.70 | [11] |
| Activated Carbon | 100−200 | 6.0 | 24.94 | [9] |
| Hematite | 0−100 | - | 3.36 | [36] |
| Modified bentonite | 100−600 | 6.0 | 29.6 | [37] |
| Temperature (K) | lnK0 | ΔG0 (kJ·mol−1) |
|---|---|---|
| 298 | 4.46 * | −11.1 |
| 308 | 4.63 ** | −12.2 |
| 318 | 5.05 ** | −12.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, F.; Yuan, G.; Larson, S.L.; Ballard, J.H.; White, J.R.; Arslan, Z.; Han, F.X. Kinetics and Thermodynamics of Uranium (VI) Adsorption onto Humic Acid Derived from Leonardite. Int. J. Environ. Res. Public Health 2019, 16, 1552. https://doi.org/10.3390/ijerph16091552
Meng F, Yuan G, Larson SL, Ballard JH, White JR, Arslan Z, Han FX. Kinetics and Thermodynamics of Uranium (VI) Adsorption onto Humic Acid Derived from Leonardite. International Journal of Environmental Research and Public Health. 2019; 16(9):1552. https://doi.org/10.3390/ijerph16091552
Chicago/Turabian StyleMeng, Fande, Guodong Yuan, Steven L. Larson, John H. Ballard, Jeremy R. White, Zikri Arslan, and Fengxiang X. Han. 2019. "Kinetics and Thermodynamics of Uranium (VI) Adsorption onto Humic Acid Derived from Leonardite" International Journal of Environmental Research and Public Health 16, no. 9: 1552. https://doi.org/10.3390/ijerph16091552
APA StyleMeng, F., Yuan, G., Larson, S. L., Ballard, J. H., White, J. R., Arslan, Z., & Han, F. X. (2019). Kinetics and Thermodynamics of Uranium (VI) Adsorption onto Humic Acid Derived from Leonardite. International Journal of Environmental Research and Public Health, 16(9), 1552. https://doi.org/10.3390/ijerph16091552





