Factors Associated with Leptospirosis in Domestic Cattle in Salakphra Wildlife Sanctuary, Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Population
2.2.1. General Information on Domestic Cattle Keeping
2.2.2. Sample Collection and Seroprevalence Examination
2.3. Statistical Analysis
3. Results
3.1. Serological Prevalence to Leptospirosis
3.2. Potential Disease Transmission
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haake, D.A.; Levett, P.N. Leptospirosis in humans. Curr. Top. Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar] [PubMed]
- Suwancharoen, D.; Limlertvatee, S.; Chetiyawan, P.; Tongpan, P.; Sangkaew, N.; Sawaddee, Y.; Inthakan, K.; Wiratsudakul, A. A nationwide survey of pathogenic leptospires in urine of cattle and buffaloes by Loop-mediated isothermal amplification (LAMP) method in Thailand, 2011–2013. J. Vet. Med. Sci. 2016, 78, 1495–1500. [Google Scholar] [CrossRef]
- Van, C.D.; Doungchawee, G.; Suttiprapa, S.; Arimatsu, Y.; Kaewkes, S.; Sripa, B. Association between Opisthorchis viverrini and Leptospira spp. infection in endemic Northeast Thailand. Parasitol. Int. 2017, 66, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Bandara, M.; Ananda, M.; Wickramage, K.; Berger, E.; Agampodi, S. Globalization of leptospirosis through travel and migration. Glob. Health 2014, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.L.; Skelly, C.; Dohnt, M.; Smythe, L.D. The emergence of Leptospira borgpetersenii serovar arborea in Queensland, Australia, 2001 to 2013. BMC Infect. Dis. 2015, 15, 230. [Google Scholar] [CrossRef]
- Ellis, W.A. Animal leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 99–137. [Google Scholar] [PubMed]
- Kurilung, A.; Chanchaithong, P.; Lugsomya, K.; Niyomtham, W.; Wuthiekanun, V.; Prapasarakul, N. Molecular detection and isolation of pathogenic Leptospirafrom asymptomatic humans, domestic animals and water sources in Nan province, arural area of Thailand. Res. Vet. Sci. 2017, 115, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Lilenbaum, W.; Martins, G. Leptospirosis in cattle: A challenging scenario for theunderstanding of the epidemiology. Transbound. Emerg. Dis. 2014, 61 (Suppl. S1), 63–68. [Google Scholar] [CrossRef] [PubMed]
- Van de Maele, I.; Claus, A.; Haesebrouck, F.; Daminet, S. Leptospirosis in dogs: A review with emphasis on clinical aspects. Vet. Rec. 2008, 163, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Callan, R.J. Leptospirosis. In Large Animal Internal Medicine, 4th ed.; Smith, B.P., Ed.; Mosby, Inc.: Maryland Heights, MO, USA, 2009; pp. 967–970. [Google Scholar]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef] [PubMed]
- Piana, R.P.; Marsden, S.J. Impacts of cattle grazing on forest structure and raptor distribution within a neotropical protected area. Biodivers. Conserv. 2014, 23, 559–572. [Google Scholar] [CrossRef]
- Miller, R.S.; Farnsworth, M.L.; Malmberg, J.L. Diseases at the livestock–wildlife interface: Status, challenges, and opportunities in the United States. Prev. Vet. Med. 2013, 110, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.E.; Ojkic, D.; Jardine, C.M. Prevalence of antibodies to Leptospira in wild mammals trapped on livestock farms in Ontario, Canada. J. Wildl. Dis. 2014, 50, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Allan, K.J.; Halliday, J.E.B.; Moseley, M.; Carter, R.W.; Ahmed, A.; Goris, M.G.A.; Hartskeerl, R.A.; Keyyu, J.; Kibona, T.; Maro, V.; et al. Assessment of animal hosts of pathogenic Leptospira in northern Tanzania. PLoS Negl. Trop. Dis. 2018, 12, e0006444. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, T.P.T.; Keuroghlian, A.; Eaton, D.P.; de Freitas, E.B.; Figueiredo, A.; Nakazato, L.; de Oliveira, J.M.; Miranda, F.; Paes, R.C.S.; Monteiro, L.A.R.C.; et al. Prevalence of Leptospira interrogans antibodies in free-ranging Tayassu pecari of the Southern Pantanal, Brazil, an ecosystem where wildlife and cattle interact. Trop. Anim. Health Prod. 2010, 42, 1695–1703. [Google Scholar] [CrossRef]
- Chaiyarat, R.; Srikosamatara, S. Populations of domesticated cattle and buffalo in the Western Forest Complex of Thailand and their possible impacts on the wildlife community. J. Environ. Manag. 2009, 90, 1448–1453. [Google Scholar] [CrossRef]
- Pattanavibool, A.; Nakornchai, T.; Vinitpornsawan, S.; Khewwan, N. Wildlife Rapid Ecological Assessment Technique Manual; Western Forest Complex Ecosystem Management Project Royal Forest Department: Bangkok, Thailand, 2002. (In Thai) [Google Scholar]
- Dikken, H.; Kmety, E. Serological typing methods of leptospires. In Methods in Microbiology; Bergan, T., Norris, J.R., Eds.; Academic Press: London, UK, 1978; Volume 11, pp. 259–307. [Google Scholar]
- Laoluekiat, S.; Jongpipatvanitch, V. The First Report of Leptospirosis Outbreak in Thong Phaphum District, Kanchanaburi Province, May–September 2008; Annual Epidemiological Surveillance Report Thailand; Bureau of Epidemiology, Ministry of Public Health: Nonthaburi, Thailand, 2009; Volume 40, pp. 105–109. (In Thai)
- Bolin, C.A. Diagnosis and control of bovine leptospirosis. In Proceedings of the 6th Western Dairy Management Conference, Reno, ND, USA, 12–14 March 2003. [Google Scholar]
- Sitprija, V.; Pipatanagul, V.; Mertowidjojo, K.; Boonpucknavig, V.; Boonpucknavig, S. Pathogenesis of renal disease in Leptospirosis: Clinical and experimental studies. Kidney Int. 1980, 17, 827–836. [Google Scholar] [CrossRef]
- Cole, J.R.; Sulzer, C.R.; Pursell, A.R. Improved microtechnique for the Leptospiral microscopic agglutination test. Appl. Microbiol. 1973, 25, 976–980. [Google Scholar]
- Nairn, R.C. Fluorescent Protein Tracing, 4th ed.; Churchill & Livingstone: Edinburgh, UK, 1976; pp. 369–371. [Google Scholar]
- McAleece, N.; Gage, J.D.G.; Lambshead, P.J.D.; Paterson, G.L.J. BioDiversity Professional statistics analysis software. Jointly developed by the Scottish Association for Marine Science and the Natural History Museum London. 1997. Available online: http://www.sams.ac.uk/peter-lamont/biodiversity-pro (accessed on 20 January 2014).
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing Ltd.: Malden, MA, USA, 2003. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD: Multivariate Analysis of Ecological Data, Version 5; MjM Software: Gleneden Beach, OR, USA, 1999. [Google Scholar]
- Suwanchareon, D.; Chaisakdanugull, Y.; Thanapongtharm, W.; Yoshida, S. Serological survey of leptospirosis in livestock in Thailand. Epidemiol. Infect. 2013, 141, 2269–2277. [Google Scholar] [CrossRef]
- Tangkanakul, W.; Hinjoy, S.; Smithsuwan, P.; Phulsuksombati, D.; Choomkasien, P. Leptospira serova in humans and animals, Nakhon Ratchasima. Mon. Epidemiol. Surveill. Rep. 2002, 33, 155–162. [Google Scholar]
- Chadsuthi, S.; Chalvet-Monfray, K.; Wiratsudakul, A.; Suwancharoen, D.; Cappelle, J. A remotely sensed flooding indicator associated with cattle and buffalo leptospirosis cases in Thailand 2011–2013. BMC Infect. Dis. 2018, 18, 602. [Google Scholar] [CrossRef]
- Ko, A.I.; Goarant, C.; Picardeau, M. Leptospira: The dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat. Rev. Microbiol. 2009, 7, 736–747. [Google Scholar] [CrossRef]
- Doungchawee, G.; Phulsuksombat, D.; Naigowit, P.; Khoaprasert, Y.; Sangjun, N.; Kongtim, S.; Smythe, L. Survey of leptospirosis of small mammals in Thailand. Southeast Asian. J. Trop. Med. Pub. Health 2005, 36, 1516–1522. [Google Scholar]
- Schoonman, L.; Swai, E.S. Herd- and animal-level risk factors for bovine leptospirosis in Tanga region of Tanzania. Trop. Anim. Health Prod. 2010, 42, 1565–1572. [Google Scholar] [CrossRef]
- Oni, O.; Sujit, K.; Kasemsuwan, S.; Sakpuaram, T.; Pfeiffer, D.U. Seroprevalence of leptospirosis in domesticated Asian elephants (Elephas maximus) in north and west Thailand in 2004. Vet. Rec. 2007, 160, 368–371. [Google Scholar] [CrossRef]
- Govindan, B. Detection of leptospira hebdomadis infection in Indian Elephants (Elephas maximas). Indian Vet. J. 2014, 91, 87–88. [Google Scholar]
- Radostits, O.M.; Blood, D.C.; Gay, C.C. Diseases caused by Leptospira spp. In Veterinary Medicine: A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses, 8th ed.; WB Saunders Company Ltd.: Philadelphia, PA, USA, 1994; pp. 884–898. [Google Scholar]
- Meeyam, T.; Tablerk, P.; Petchanok, B.; Pichpol, D.; Padungtod, P. Seroprevalence and risk factors associated with leptospirosis in dogs. Southeast Asian. J. Trop. Med. Public. Health 2006, 37, 148–153. [Google Scholar]
- Adler, B.; de la Pena Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef]
- Delooz, L.; Czaplicki, G.; Gregoire, F.; Dal Pozzo, F.; Pez, F.; Kodjo, A.; Saegerman, C. Serogroups and genotypes of Leptospira spp. strains from bovine aborted fetuses. Transbound. Emerg. Dis. 2018, 65, 158–165. [Google Scholar] [CrossRef]
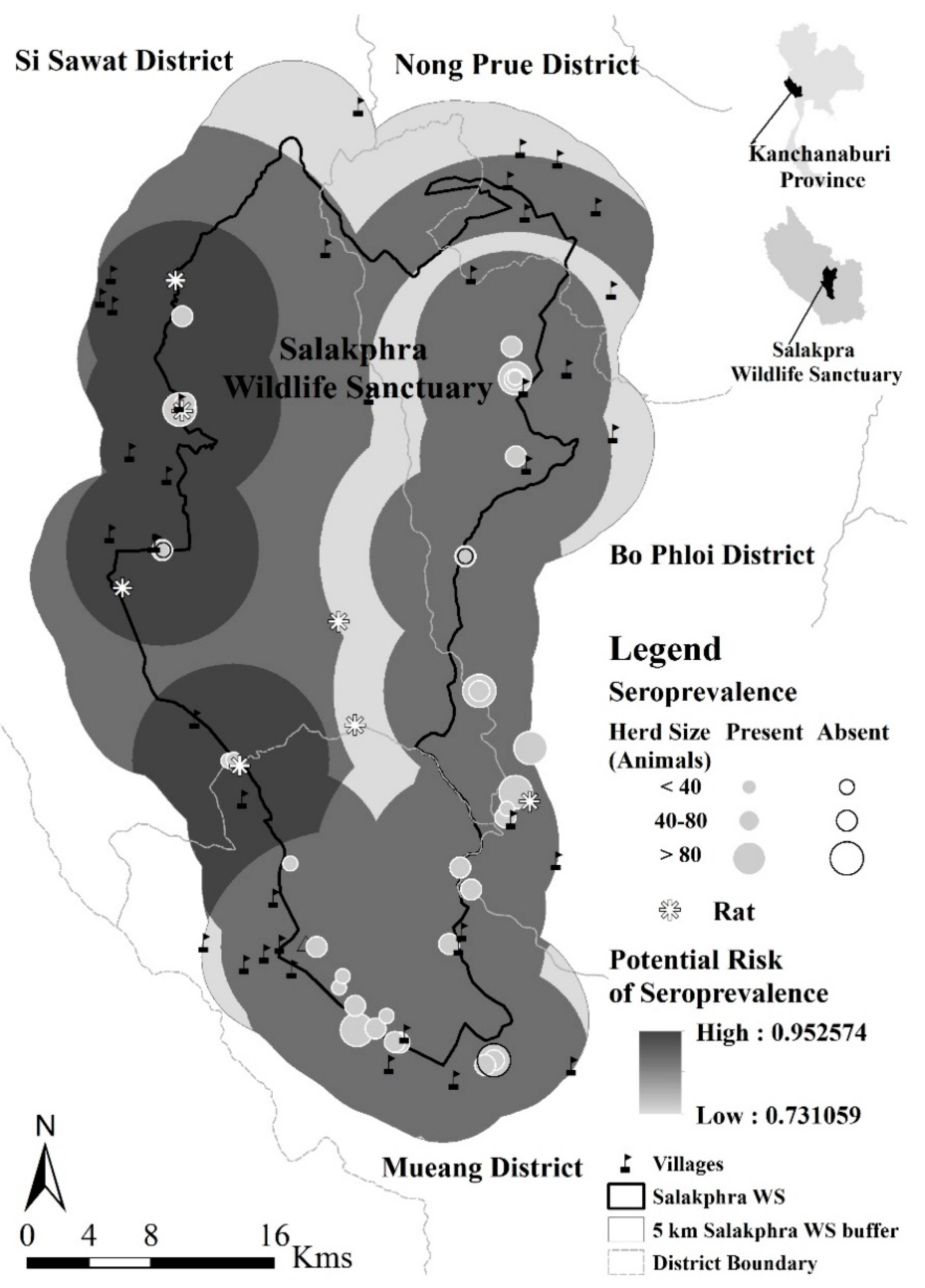
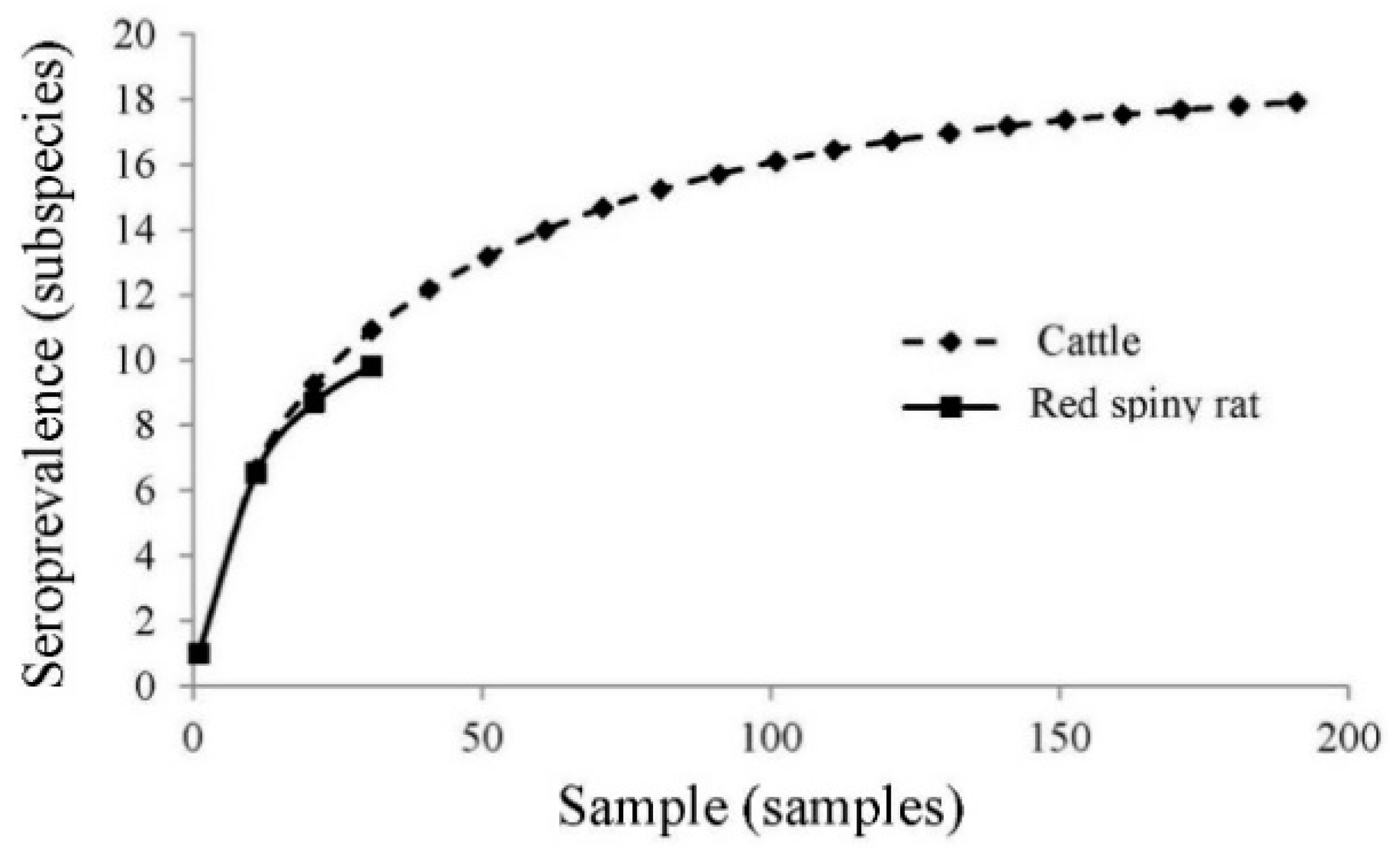
| Herd ID | Herd Size (Individuals) | Sample Size (Individuals (%)) | Location | Seroprevalence of Leptospira interrogans Serovars | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aut | bal | bat | bra | can | cyn | dja | heb | ict | jav | lou | min | pom | pyr | ran | sar | sej | tar | ||||
| A | 70 | 7 (10) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 7 |
| B | 60 | 7 (11.7) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 7 |
| C | 30 | 5 (16.7) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 5 |
| D | 70 | 5 (7.1) | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 5 |
| E | 14 | 9 (64.3) | 1 | 0 | 9 | 0 | 3 | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 5 | 9 |
| F | 7 | 7 (100) | 1 | 0 | 6 | 1 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 6 | 0 | 5 | 6 |
| G | 9 | 2 (22.2) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| H | 42 | 2 (4.8) | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| I | 44 | 9 (20.5) | 2 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 9 |
| J | 70 | 4 (5.7) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| K | 37 | 3 (8.1) | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 |
| L | 80 | 1 (1.3) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| M | 120 | 2 (1.7) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| N | 60 | 4 (6.7) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 4 |
| O | 24 | 3 (12.5) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 2 |
| P | 27 | 3 (11.1) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 |
| Q | 42 | 4 (9.5) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 4 |
| R | 59 | 6 (10.2) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 4 |
| S | 60 | 6 (10) | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 6 |
| T | 60 | 2 (3.3) | 3 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | - | - | 3 |
| U | 31 | 7 (22.6) | 3 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 1 | 2 | 7 |
| W | 70 | 7 (10) | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| X | 110 | 4 (3.6) | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Y | 50 | 6 (12) | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 |
| Z | 143 | 17 (11.9) | 3 | 0 | 14 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 2 | 13 |
| AA | 34 | 5 (14.7) | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 4 |
| BB | 65 | 8 (12.3) | 3 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 6 |
| CC | 100 | 15 (15) | 3 | 0 | 9 | 0 | 2 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 1 | 14 | 0 | 0 | 15 |
| DD | 70 | 7 (10) | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 6 |
| EE | 130 | 1 (0.8) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FF | 100 | 10 (10) | 1 | 0 | 4 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 1 |
| GG | 100 | 4 (4) | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 3 |
| HH | 40 | 5 (12.5) | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 2 | 5 |
| II | 57 | 8 (14) | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 4 |
| JJ | 107 | 10 (9.3) | 1 | 0 | 4 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 5 | 9 |
| KK | 33 | 10 (30.3) | 1 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 10 | 0 | 9 | 9 |
| MM | 60 | 20 (33.3) | 1 | 2 | 12 | 0 | 0 | 9 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 13 | 13 | 2 | 3 | 13 |
| TT | 40 | 13 (32.5) | 3 | 5 | 5 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 7 |
| UU | 47 | 10 (21.3) | 3 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| VV | 60 | 12 (20) | 3 | 12 | 12 | 0 | 3 | 1 | 1 | 2 | 3 | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 0 | 4 | 12 |
| WW | 105 | 18 (17.1) | 3 | 13 | 13 | 0 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 4 | 18 |
| XX | 2,537 | 225 (8.9) | 1 | 36 | 129 | 2 | 29 | 11 | 2 | 4 | 56 | 2 | 1 | 1 | 2 | 3 | 19 | 179 | 7 | 44 | 225 |
| Seroprevalence (individuals (%)) | 72 (14) | 258 (50.3) | 4 (0.8) | 58 (11.3) | 22 (4.3) | 4 (0.8) | 8 (1.6) | 112 (21.8) | 4 (0.8) | 2 (0.4) | 2 (0.4) | 4 (0.8) | 6 (1.2) | 38 (7.4) | 358 (69.8) | 14 (2.7) | 88 (17.2) | 450 (87.7) | |||
| Serovars | Cattle Herd (% Prevalence) | Red Spiny Rat (% Prevalence) | Titer Range | χ2 | df | p-value |
|---|---|---|---|---|---|---|
| Leptospira interrogans | ||||||
| Tarassovi | 39 (92.9) | 4 (30.8) | 50–800 | 28.5 | 1 | <0.001 |
| Ranarum | 37 (88.1) | 4 (30.8) | 50–400 | 26.6 | 1 | <0.001 |
| Hebdomadis | 30 (71.4) | - | 5–800 | 30 | 1 | N/A |
| Ballum | 22 (52.4) | - | 50–400 | 22 | 1 | N/A |
| Bratislava | 17 (40.5) | - | 50–400 | 17 | 1 | N/A |
| Sejroe | 12 (28.6) | 1 (7.7) | 50–400 | 9.3 | 1 | 0.002 |
| Autumnalis | 6 (14.3) | - | 50–100 | 6 | 1 | N/A |
| Pyrogenes | 6 (14.3) | - | 50–400 | 2 | 1 | 0.153 |
| Bataviae | 5 (11.9) | 2 (15.4) | 50–100 | 5 | 1 | N/A |
| Sarmin | 5 (11.9) | 1 (7.7) | 50–100 | 2.7 | 1 | 0.098 |
| Canicola | 4 (9.5) | 4 (30.8) | 50–100 | 0 | 1 | N/A |
| Djasiman | 4 (9.5) | - | 50 | 4 | 1 | N/A |
| Icterohaemorrhagica | 3 (7.1) | - | 50–100 | 3 | 1 | N/A |
| Pomona | 3 (7.1) | 3 (23.1) | 50 | 0 | 1 | N/A |
| Cynopteri | 2 (4.8) | 1 (7.7) | 5–100 | 0.3 | 1 | 0.571 |
| Mini | 2 (4.8) | - | 50 | 2 | 1 | N/A |
| Javanica | 1 (2.4) | - | 100 | 1 | 1 | N/A |
| Louisiana | 1 (2.4) | - | 50 | 1 | 1 | N/A |
| Shermani | - | 9 (69.2) | 50 | 9 | 1 | N/A |
| Patoc I | - | 4 (30.8) | 50 | 4 | 1 | N/A |
| H′ | 1.027 | 0.905 | 173.4 | 40 | <0.001 |
| Environmental Factor | Level | Seroprevalence n (%) | χ2 | df | p-Value a |
|---|---|---|---|---|---|
| Herd size (individuals) | 1 = small (<40) 2 = medium (40–80) 3 = large (>80) | 11 (26.2) 22 (52.4) 9 (21.4) | 7 | 2 | 0.03 * |
| Raising patterns - Dry season - Wet season | 1 = free grazing 2 = stationed 1 = free grazing 2 = stationed | 40 (95.2) 2 (4.8) 34 (80.9) 8 (19.1) | 42.9 67 | 1 1 | <0.001 *** <0.001 *** |
| Raising distance (km/day) | 1 = <1 2 = 2–3 3 = 4–5 4 = >5 | 3 (7.1) 9 (21.4) 8 (19.1) 22 (52.4) | 18.8 | 3 | <0.001 *** |
| Water resource | 1 = natural source 2 = artificial ponds | 23 (54.8) 19 (45.2) | 1.5 | 1 | 0.22 ns |
| Number of years that livestock were kept (years) | 1 = <1 2 = 1–3 3 = 4–5 4 = 6–10 5 = >10 | 1 (2.4) 4 (9.5) 5 (11.9) 10 (23.1) 22 (52.4) | 32.5 | 4 | <0.001 *** |
| Raising in the same route with other herds | 0 = no 1 = yes | 26 (61.9) 16 (38.1) | 2.4 | 1 | 0.12 ns |
| Sharing feeding ground with other herds | 0 = no 1 = yes | 16 (38.1) 26 (61.9) | 2.4 | 1 | 0.12 ns |
| Introduced of new animal into herd | 0 = no 1 = yes | 31 (73.8) 11 (26.2) | 9.5 | 1 | 0.002 ** |
| Pets in the farm | 0 = no 1 = yes | 9 (21.42) 33 (78.6) | 18.7 | 1 | <0.001 *** |
| Abortion history | 0 = not aborted 1 = aborted | 27 (64.3) 15 (35.7) | 3.4 | 1 | 0.06 ns |
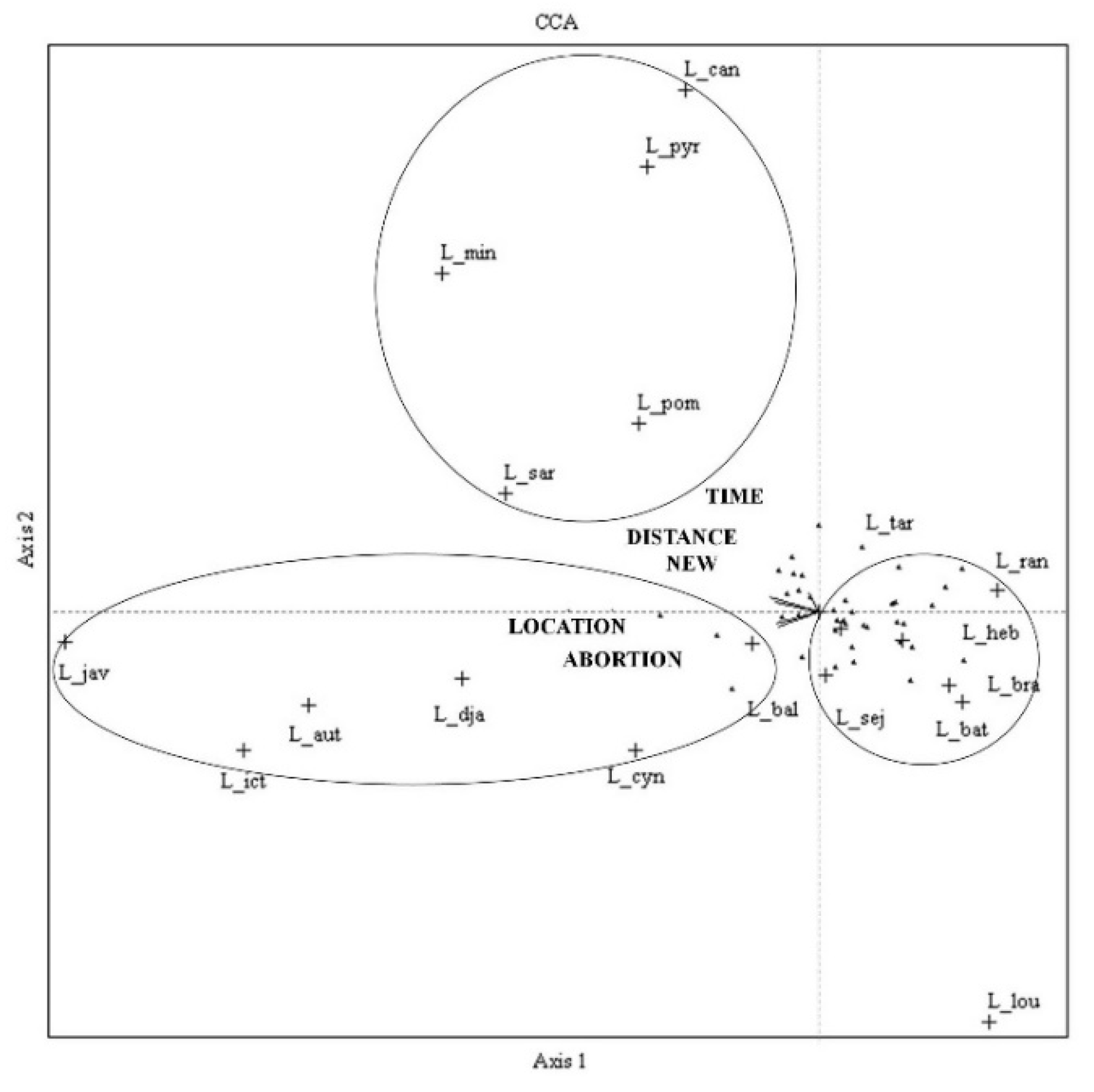
| Parameter | PC1 | PC2 | PC3 |
|---|---|---|---|
| Risk factor | |||
| Location of herd * | −0.265 | 0.062 | −0.165 |
| Herd size | −0.017 | −0.146 | −0.054 |
| Place | −0.100 | −0.031 | 0.098 |
| Raising distance (km/day) * | −0.076 | 0.111 | 0.081 |
| Together | −0.080 | −0.251 | 0.029 |
| Togeth_a | −0.061 | 0.069 | 0.088 |
| Number of years that domestic cattle were kept (years) * | 0.032 | 0.250 | −0.241 |
| Introduced of new animal into herd * | −0.257 | 0.052 | 0.015 |
| Abortion history * | −0.094 | −0.223 | −0.014 |
| Domestic cattle | 0.099 | 0.200 | 0.103 |
| Leptospira interrogans in domestic cattle | |||
| Autumnalis | −2.948 | −0.884 | −0.673 |
| Ballum | −0.380 | −0.293 | 0.617 |
| Bataviae | 0.831 | −0.853 | 0.345 |
| Bratislava | 0.758 | −0.695 | −2.133 |
| Canicola | −0.770 | 4.907 | 0.283 |
| Cynopteri | −1.054 | −1.306 | 0.478 |
| Djasiman | −2.056 | −0.632 | −0.288 |
| Hebdomadis | 0.483 | −0.274 | −1.459 |
| Icterohaemorrhagica | −3.320 | −1.305 | −0.360 |
| Javanica | −4.350 | −0.279 | −2.689 |
| Louisiana | 0.982 | −3.859 | 7.542 |
| Mini | −2.176 | 3.175 | −0.534 |
| Pomona | −1.037 | 1.766 | −1.483 |
| Pyrogenes | −0.994 | 4.179 | 0.566 |
| Ranarum | 1.033 | 0.209 | −0.066 |
| Sarmin | −1.804 | 1.108 | −1.141 |
| Sejroe | 0.041 | −0.602 | 2.720 |
| Tarassovi | 0.129 | −0.160 | −0.142 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yatbantoong, N.; Chaiyarat, R. Factors Associated with Leptospirosis in Domestic Cattle in Salakphra Wildlife Sanctuary, Thailand. Int. J. Environ. Res. Public Health 2019, 16, 1042. https://doi.org/10.3390/ijerph16061042
Yatbantoong N, Chaiyarat R. Factors Associated with Leptospirosis in Domestic Cattle in Salakphra Wildlife Sanctuary, Thailand. International Journal of Environmental Research and Public Health. 2019; 16(6):1042. https://doi.org/10.3390/ijerph16061042
Chicago/Turabian StyleYatbantoong, Nantawan, and Rattanawat Chaiyarat. 2019. "Factors Associated with Leptospirosis in Domestic Cattle in Salakphra Wildlife Sanctuary, Thailand" International Journal of Environmental Research and Public Health 16, no. 6: 1042. https://doi.org/10.3390/ijerph16061042
APA StyleYatbantoong, N., & Chaiyarat, R. (2019). Factors Associated with Leptospirosis in Domestic Cattle in Salakphra Wildlife Sanctuary, Thailand. International Journal of Environmental Research and Public Health, 16(6), 1042. https://doi.org/10.3390/ijerph16061042




