Abstract
Home processing can reduce pesticide residues in agricultural products, and the common forms of treatment include washing, peeling, blanching, and cooking. In this study, the removal effects of tap water, micron calcium solution, alkaline electrolyzed water (AlEW), ozone water, active oxygen, and sodium bicarbonate on 10 typical pesticide residues in kumquat, cucumber, and spinach were investigated. The residue magnitudes were determined by chromatography–tandem mass spectrometry (GC-MS/MS, LC-MS/MS), combined with the QuEChERS pretreatment method. The model tests showed that the results of soaking and greenhouse were close. The removal effects of pesticide residues in kumquat and cucumber washing by alkaline electrolyzed water with a high pH value, micron calcium, and active oxygen solution were better than other washing solutions. The sodium bicarbonate solution, ozone water, and active oxygen solution were more effective in reducing pesticide residues in spinach than others. Active oxygen solution showed a better removal efficiency for the 10 pesticides than other treatments because of its alkalinity and oxidizability. Among the ten pesticides, pyrethroid pesticides had a higher removal rate. Additionally, chlorpyrifos were the most difficult to remove. For the majority of pesticides, the pesticide residue magnitudes showed a gradual reduction when increasing the washing time. The results indicated that alkaline solutions were effective for the reduction of pesticide residues when the washing time was longer than 15 min.
1. Introduction
Pesticides are used to control plant diseases, insect pests, and weeds and regulate plant growth to ensure the quality and quantity of the produce. Pesticides are not made up of one component, but consist of several mixtures and adjuvants. Excessive pesticide residues can do great harm to customers, with effects such as neurotoxicity, carcinogenicity, reproduction abnormality, and cell dysplasia. Nowadays, pesticide remnant is still a major problem affecting the quality and security of fruits and vegetables.
Food processing, such as washing, peeling, blanching, and cooking, plays a common role in the reduction of residues. Washing is the most common and direct form of food processing, is usually the first step before consumption, and is used for removing pesticide residues in fruits and vegetables [1,2]. After harvest, some kinds of produce, such as fresh fruits and vegetables, are often washed with tap water to remove dirty marks on the surface, which are then consumed directly. However, tap water has a limited effect on the removal of pesticide residues, because many pesticides are hydrophobic [3]. Therefore, many detergent solutions are used to degrade pesticides in vegetables and fruits, including sodium chloride solution, acetic acid, sodium carbonate, and sodium bicarbonate. Y. Liang [4] studied the removal of five organophosphorus pesticides in raw cucumber with home preparation, and the research results show that washing by tap water for 20 min only caused a pesticides reduction of 26.7–62.9%. Sodium carbonate and sodium bicarbonate solution caused a pesticides reduction of 66.7–98.9%. Storage in low temperature caused a pesticides reduction of 60.9–90.2% and ultrasonic cleaning for 20 min lowered pesticides by 49.8–84.4% in raw cucumber. Apart from the common detergent solutions, ozone is also used for the removal of the pesticides residue in fruits and vegetables such as carrot, Chinese white cabbage and greenstem bok choy [5], and orange [6], without modifying its physicochemical property and organoleptic characteristics [7]. The highest removal percentages of tetradifon and chlorpyrifos ethyl in lemon and grapefruit matrices that have been achieved with ozonation are 98.6% and 94.2%, respectively. Ozone can also degrade some pesticides in natural waters [8,9]. Meanwhile, electrolytic water is also widely investigated as a disinfectant and detergent in the food industry. There are two types of electrolytic water. Electrolyzed oxidizing (EO) water is extensively used with a low pH and high oxidation–reduction potential. Electrolyzed reduced (ER) water has limited application due to its characteristic of a high pH and low ORP [10,11]. However, researchers found that the ER water could be used as a cleaning solution to reduce pesticide residues in fruits and vegetables; for example, cabbage, leek [2], beans, grapes [3], and cowpea [11]. The removal of six pesticides in cowpea washing by AlEW solution (pH = 12.2) for 45 min was 48–85%. Based on previous studies, alkaline electrolyzed water with two different pH values was selected as a cleaning agent.
In this study, the objective was to evaluate the effectiveness of detergent solution in removing the pesticides organophosphates, triazoles, pyrethroids, and neonicotinoids from fresh kumquat, spinach, and cucumber, which are widely used to control pests and diseases in fruits and vegetables. Kumquat and cucumber can be washed and directly eaten without peeling and cooking. Spinach is one of the most common vegetables in daily life and is very nutritional. Therefore, it is of great significance to study the removal of pesticide residues in kumquat, cucumber, and spinach with washing treatments. The main physic-chemical properties and chemical structures of the studied pesticides are presented in Table 1 and Figure 1, respectively. The common home preparation, tap water, and sodium bicarbonate solution [12], were used for comparison with alkaline electrolyzed water and ozone water, which have often been reported in the literature. At the same time, the micron calcium and active oxygen of the pesticide removal products on the market were also compared. Different treatment methods were used to clean the matrix for 5, 15, 20, and 30 min, combined with the QuEChERS pretreatment method [13], as well as chromatography-mass spectrometry technology, in order to find the best cleaning method and the best cleaning time. At the same time, the impact of different treatment methods and pesticides on different substrates was explored.

Table 1.
The main properties of the studied pesticides.
Figure 1.
The chemical structures of the studied pesticides.
2. Materials and Methods
2.1. Standards, Reagents, and Materials
The purities of the ten pesticide standards were from 97% to 99%, which were obtained from the Institute of Control of Agrochemicals, Ministry of Agriculture People’s Republic of China. Standard stock solutions (500 mg/L) for mixture of the ten pesticides were prepared in acetonitrile and stored at −20 °C. The work solution was prepared daily. Acetonitrile was of chromatography grade and obtained from Fisher Scientific (Fair Lawn, NJ, USA). Sodium chloride (NaCl) and anhydrous magnesium sulfate (MgSO4) were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Multi-walled carbon nanotubes (MWCNTs) with average external diameters of 10–20 nm and primary secondary amine (PSA) (40 µm) were acquired from Agilent Technology Co., Ltd. (Beijing, China). Micron calcium was provided by Bai Jia An Bioengineering Co., Ltd. (Liaoning, China). Active oxygen was provided by Guangzhou Zao Gu Biotechnology Co., Ltd. (Guangzhou, China). Alkaline electrolyzed water and ozonated water were prepared by the Specialized preparation machine.
Centrifugation was performed in an Anke TDL–40 B centrifuge equipped with a bucket rotor (8 × 100 mL) (Shanghai, China). An ATARGIN VX–III multitube vortexer was used in sample preparation (Beijing, China).
2.2. GC–MS/MS Analysis
The analysis of the pesticides was carried out with the Thermo Scientific TSQ 8000 EVO triple quadrupole mass spectrometer coupled with a Trace 1300 gas chromatograph and a TriPlus AI 1310 autosampler (Thermo Fisher Scientific, San Jose, CA, USA). An Agilent Technologies capillary column (30 m × 250 μm × 0.25 μm film thickness) was used for chromatographic separation. The column temperature was initially set at 40 °C and held for 0.4 min, and then increased to 180 °C at the rate of 30 °C/min, 280 °C at the rate of 10 °C/min, and finally 290 °C at the rate of 20 °C/min and held for 5 min. The temperature of the injector port was 250 °C and the injection volume was 1 μL. The total running time was 25 min. Helium gas was used as the carrier gas, with a constant flow of 1.0 mL/min, and Argon gas was chosen as the collision gas, with the pressure of 1.5 mTorr. The mass spectrometer was operated in electron ionization (EI) mode at 70 eV. The ion source and transfer line temperatures were set at 280 °C and 280 °C, respectively. Table 2 summarizes the condition of mass spectrum [14,15] and the typical retention time for each analyte.

Table 2.
GC-MS/MS condition for the identification and quantitation of eight pesticides.
2.3. LC–MS/MS Analysis
The column of the liquid chromatography was Athena C18-WP (2.1 mm × 50 mm × 3 μm, Agilent, Santa, Clara, CA, USA). The mobile phase was acetonitrile and 0.1% formic acid-water solution and the ratio was 4:6. The flow rate was 0.2 mL/min. The column was kept at 30 °C with the injection volume at 10 μL. A liquid chromatography-tandem mass spectrometer (Agilent 6410B, Agilent, -Santa Clara, CA, USA) coupled with a positive electrospray ionization (ESI+) source using multiple reaction monitoring mode (MRM) was used for analysis. The nitrogen was used as dry gas and atomization gas and the flow rate was 8.0 L/min. The gas temperature was 350 °C and the nebulizer pressure was 35 psi. A summary of the transitions monitored [16], the fragmentor voltage, and the collision energy parameters for acetamiprid and imidacloprid are given in Table 3.

Table 3.
Liquid chromatography-tandem mass spectrometry parameters of two pesticides.
2.4. Sample Preparation and Washing
Fresh vegetables of spinach, cucumber, and kumquat were purchased from a supermarket in Beijing, China. The kumquat and cucumber were steeped in 5 L mixed solution (50 mg/L), which was prepared with the ten pesticide formulations for 15 min. The spinach was immersed in 10 L mixed solution (10 mg/L) for 15 min. The contaminated kumquat, cucumber, and spinach were air-dried in a fume hood for 24 h at room temperature.
After that, 100 g of spinach, cucumber, and kumquat was randomly selected to detect the initial deposits. The polluted sample was washed by six washing methods (Tap water, AlEW solution (pH 12.35, pH 10.5), micron calcium water (10 g micron calcium and 500 mL tap water), 0.4 mg/kg ozone water, 2% active oxygen solution, and 2% NaHCO3) for 5, 15, 20, and 30 min, respectively. The washed samples were rinsed by tap water for 30 s. Following this, the treated samples were air-dried at room temperature and then analyzed.
2.5. Extraction and Purification of Pesticides
The cucumber, spinach, and kumquat were homogenized and processed by the blender, respectively. An amount of (10 ± 0.05 g) homogenized samples was weighed into a 50 mL centrifuge tube, and 10 mL acetonitrile was added. The resulting solution was shaken by the vortex for 5 min. After that, 1 g of sodium chloride and 4 g of anhydrous Magnesium Sulfate were added. The tube was cooled to room temperature and was then shaken for 5 min before centrifugation for 5 min at 3800 rpm. An aliquot of 1 mL supernatant of kumquat was transferred to a 2 mL centrifuge tube containing 5 mg MWCNTs and 30 mg PSA mixed with 150 mg anhydrous MgSO4 (The sorbent of spinach was 7.5 mg MWCNTs mixed with 150 mg anhydrous MgSO4 and the cucumber was 5 mg MWCNTs mixed with 150 mg anhydrous MgSO4). Then, the 2 mL tube was shaken for 1 min and centrifuged for 2 min at 4000 rpm. Finally, the supernatant was filtered through a 0.22 μm membrane into an autosampler vial for analysis.
2.6. Methods Validation
The validation was performed on each matrix, and the method was validated through linearity, the matrix effect, trueness and precision, limit of detection (LOD), and limit of quantification (LOQ). Linearity of the method was studied at five concentrations in the range of 10–1000 μg/kg for 10 pesticides by matrix-matched calibration solutions. Good linearity was found for the pesticides with coefficients of determination (R2) better than 0.990 [14]. LOQs for 10 pesticides were the lowest spike level of the method’s validation and the LOQs were regarded as LODs in this respect [17]. All data are shown in Table 4.

Table 4.
Matrix effects (MEs), calibration curve coefficients (R2), and LOQs (μg/kg) for ten pesticides in kumquat, cucumber, and spinach (n = 5).
The accuracy was evaluated by recovery and the precision was evaluated by the relative standard deviation (RSD). This study was performed at three concentration levels (10, 100, and 500 μg/kg) by spiking standard pesticides for a blank sample. The results are shown in Table 5. The average recoveries of the10 pesticides were in the range of 78 to 118% and the RSDs were <10%.

Table 5.
Average recoveries and relative standard deviations (RSDs) at three spiked levels in kumquat, cucumber, and spinach (n = 5).
3. Results and Discussion
3.1. Establishment of Soaking Model
Taking kumquat as the research object, three models of smearing, soaking, and simulating the field application resulted in eight pesticides being attached to the kumquat. The treatment of the washing matrix with prepared solution (5 g micron calcium and 500 mL tap water) for 15 min was conducted to compare the removal efficiency of eight pesticides, as can be seen in Figure 2. Three models caused a 73–99%, 27–65%, and 23–77% loss of the eight pesticides, respectively. The results of soaking and simulating the field application were close to each other. Thus, the model of soaking was chosen to carry out the next experiment.
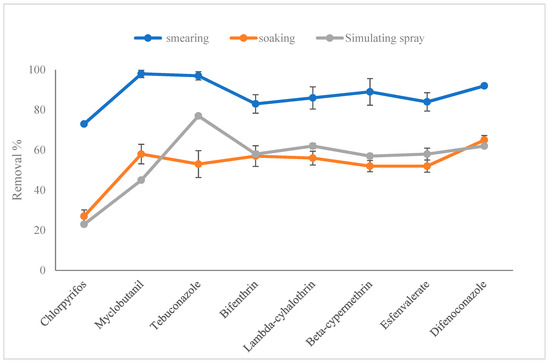
Figure 2.
Removal efficiency of eight pesticides in kumquat treated with smearing, soaking, or simulating the field application by washing with 5 g micron calcium for 15min (n = 3).
Smearing: The pesticide was divided into two groups. The mixture of pesticide formulations (200 mg/L) was mixed with acetone. A syringe was used to remove a mixed solution of 1 mL from the surface of the kumquat. A sample of kumquat (of about 100 g) was determined, and was placed for an hour at room temperature.
Soaking: The kumquat was steeped in 2 L mixed solution (50 mg/L) for 15 min, which was prepared with the eight pesticide formulations. A sample of kumquat (of about 100 g) was determined, and was placed at room temperature for 24 h.
Simulating the field application: The eight pesticides were divided into two groups. According to the highest recommended dosage, two mixed solutions (500 mL) were configured, with four pesticide formulations for each. Then, the mixed solution was sprayed on a group of trees. A group consisted of two kumquat trees. After three days, approximately 200 g kumquat was picked from each group (100 g was cleaned, 100 g was control), and no spray was used in the blank control.
3.2. Effect of Washing Treatments for Pesticide Removal in Kumquat
Tap water and alkaline solution are relatively common washing solutions in our daily lives. The alkaline electrolyzed water (AlEW) is of high pH value and low oxidation reduction potentials, which is gradually being valued [11,18,19,20,21]. Ozone and active oxygen have a strong oxidation capability, which can destroy the unsaturated bonds and oxidize functional groups to decompose most organic compounds, and they do not produce secondary pollutants [22,23]. In this study, the effects of washing by tap water, 2% sodium bicarbonate solution, alkaline electrolyzed water, Micron calcium solution, ozone water (0.4 mg/kg), and 2% active oxygen solution for 5, 15, 20, and 30 min were investigated, and the washing results for kumquat are shown in Table 6. Washing with tap water, as well as detergent solutions, had an effect in reducing pesticide residue in kumquat. The removal of 10 pesticides in kumquat is 20–40% by tap water washing, and the effects of tap water for acetamiprid, imidacloprid, myclobutanil, and tebuconazole were superior to others, which is related to the O/W partition coefficient of pesticides.

Table 6.
Effect of washing treatments for pesticide removal in kumquat (n = 3).
Among these washing processing methods, 2% sodium bicarbonate solution and ozone water caused 20–40% more loss of the 10 pesticides than tap water. The removal effect of the AlEW, whose pH value was 12.35, was better than the one whose pH was 10.50. Micron calcium solution and 2% active oxygen solution were the most effective ways for the elimination of pesticide residues in kumquat. The greatest loss of chlorpyrifos, beta-cypermethrin, and esfenvalerate was 51%, 71%, and 54%, respectively, which was caused by micron calcium solution. The 2% active oxygen solution caused the lowest residual amounts of myclobutanil, tebuconazole, bifenthrin, lambda-cyhalothrin, difenoconazole, acetamiprid, and imidacloprid, which were reduced by 79%, 79%, 65%, 74%, 64%, 59%, and 67%, respectively. The residues of pyrethroid pesticides were the lowest and there was no significant difference on pesticide residue after 20 min washing treatment. Pesticide residues in fruits and vegetables showed a gradual reduction when increasing the treatment time, which is in agreement with Y. Liang [4] and Zhi-Yong Zhang [24].
3.3. Effect of Washing Treatments for Pesticide Removal in Cucumber
The results of the washing solution for removing pesticide residue in cucumber are presented in Table 7. Washing with tap water was a little effective in reducing pesticides in cucumber, and the removal rates of 10 pesticides were less than 35%. The removal effects of AlEW (pH 10.5) and ozone water were not obvious, unlike the pesticides in cucumber, which were effectively removed by washing with AlEW (pH 12.35), micron calcium, and active oxygen, and the removal rate of pyrethroid pesticides was obviously higher than others. Washing with 2% active oxygen solution for 20 min caused a 49%, 41%, 40%, 57%, 58%, 51%, 63%, 53%, 49%, and 50% loss in chlorpyrifos, myclobutanil, tebuconazole, bifenthrin, lambda-cyhalothrin, beta-cypermethrin, esfenvalerate, difenoconazole, acetamiprid, and imidacloprid, respectively. Washing with micron calcium solution for 20 min caused a greater loss of pesticides in cucumber, and the removal efficiency was 50%, 42%, 47%, 67%, 83%, 85% ,86%, 67%, 37%, and 35%, respectively.

Table 7.
Effect of washing treatments for pesticide removal in cucumber (n = 3).
3.4. Effect of Washing Treatments for Pesticide Removal in Spinach
The effect of washing treatments for pesticide removal in spinach are summarized in Table 8. It was difficult to remove pesticides from spinach by tap water, and the order of the removal effects of 10 pesticides in spinach by washing with detergent solution was as follows: ozone water and active oxygen solution > micron calcium solution >AlEW (pH 12.35) and sodium bicarbonate solution > AlEW (pH 10.50) > tap water. These washing methods are two to four times as effective as tap water. The residual amounts of chlorpyrifos, myclobutanil, tebuconazole, bifenthrin, lambda-cyhalothrin, beta-cypermethrin, esfenvalerate, difenoconazole, acetamiprid, and imidacloprid in spinach, which was washed with ozone water for 30 min, were reduced by 53%, 72%, 73%, 62%, 67%, 65%, 78%, 68%, 64%, and 63%, respectively. After being washed with active oxygen solution for 5 min, the removal efficiency of chlorpyrifos, myclobutanil, tebuconazole, bifenthrin, lambda-cyhalothrin, beta-cypermethrin, esfenvalerate, difenoconazole, acetamiprid, and imidacloprid in spinach was 52%, 63%, 65%, 55%, 70%, 71%, 81%, 62%, 50%, and 48%, respectively. According to the experimental result, the pesticides in spinach were easier to remove by oxidizing washing solution.

Table 8.
Effect of washing treatments for pesticide removal in spinach (n = 3).
The optimal treatments of pesticides are shown in Table 9. Active oxygen, micron calcium, and ozone solution are the most effective treatments for kumquat, cucumber, and spinach, respectively. Tap water has a better removal effect on the 10 pesticides in cucumber, and the removal effect of the 10 pesticides in spinach is poor. The effect of 2% active oxygen solution treatment for pesticide removal in kumquat and spinach was superior to cucumber. Micron calcium solution (10 g micron calcium and 500 mL tap water) can effectively remove 10 pesticide residues in kumquat, cucumber, and spinach, and has a pH value of 12.93. The removal efficiency of pesticides from fruits and vegetables by 2% active oxygen solution is better than others because of its alkalinity (pH 10.88) and oxidizability. The pyrethroid pesticides had a higher removal rate as a result of their instability in alkaline solution. These results show that the removal rate of pesticides is associated with the pH of the washing solution, the pesticide properties, and the type of fruits and vegetables.

Table 9.
The optimal treatments of pesticides in kumquat, cucumber, and spinach.
4. Conclusions
The removal effects of ten pesticide residues in kumquat, cucumber, and spinach when using different detergent solutions were investigated. After soaking, the deposition of pesticides in fruits and vegetables were different, which made the experimental data generate an inevitable error. However, the overall trend is obvious. Pesticide residues in fruits and vegetables showed a gradual reduction when increasing the treatment time for the majority of pesticides. It was obvious that the removal effect of washing for 15 min was vastly different from 5 min, and there was no significant difference in pesticide residue after 15 min washing treatment. Pesticides in cucumber were more easily removed by alkaline solutions, such as AlEW, micron calcium, and sodium bicarbonate solution, compared with oxidizing solutions. On the contrary, the pesticides in spinach were easily removed by oxidizing solutions. The removal efficiency of other washing solutions outperformed the tap water; tap water washing only caused a 10–40% loss of the 10 pesticides, and the AlEW, micron calcium, and active oxygen solution caused a 40–90% loss of the 10 pesticides. The data indicated that the lower Kow the pesticides had, the easier they were removed by washing with tap water, but it was inadequate when washing with other solutions. Pyrethroid pesticides adhering to plant superficies were removed more easily by washing, which is instable in the presence of alkaline solution and sunlight. The removal percentage of pesticides depended on the different washing solutions and the time of treatment, as well as the characteristics of pesticides, such as the lower octanol–water partition coefficient (Kow), mode of action, and the stability of hydrolysis and photolysis. These results clearly indicate that washing samples with detergent solution could effectively reduce pesticide residues in fruits and vegetables and ensure that humans have a healthy diet. Though the removal effects of different washing treatments have been studied in the existing literature, there has been no further study on the effects of detergents on the quality of fruit and vegetable and human health. The effect of cleaning agent residues will be studied in future work.
Author Contributions
Data curation, Y.W.; Formal analysis, Y.W. and Q.A.; Investigation, Y.W.; Methodology, Y.W. and Q.A.; Resources, Y.W., Q.A., D.L., J.W., and C.P.; Supervision, Q.A. and D.L.; Validation, Y.W.; Writing—original draft, Y.W.; Writing—review & editing, Y.W. and C.P.
Funding
The authors are grateful for the support from National Key R&D Program of China (2017YFD0800700) and Guangxi Science and Technology Major Projects [grant number AA17204043].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaushik, G.; Satya, S.; Naik, S.N. Food processing a tool to pesticide residue dissipation—A review. Food Res. Int. 2009, 42, 26–40. [Google Scholar] [CrossRef]
- Hao, J.; Wuyundalai; Liu, H.; Chen, T.; Zhou, Y.; Su, Y.C.; Li, L. Reduction of pesticide residues on fresh vegetables with electrolyzed water treatment. J. Food Sci. 2011, 76, C520–C524. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Huang, Q.; Hung, Y.C. Effectiveness of electrolyzed oxidizing water treatment in removing pesticide residues and its effect on produce quality. Food Chem. 2018, 239, 561–568. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, W.; Shen, Y.; Liu, Y.; Liu, X.J. Effects of home preparation on organophosphorus pesticide residues in raw cucumber. Food Chem. 2012, 133, 636–640. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lin, Y.J.; Kuo, W.C. Pesticide residue removal from vegetables by ozonation. J. Food Eng. 2013, 114, 404–411. [Google Scholar] [CrossRef]
- Kusvuran, E.; Yildirim, D.; Mavruk, F.; Ceyhan, M. Removal of chloropyrifos ethyl, tetradifon and chlorothalonil pesticide residues from citrus by using ozone. J. Hazard. Mater. 2012, 241–242, 287–300. [Google Scholar]
- Souza, L.P.; Faroni, L.R.D.; Heleno, F.F.; Pinto, F.G.; Queiroz, M.; Prates, L.H.F. Ozone treatment for pesticide removal from carrots: Optimization by response surface methodology. Food Chem. 2018, 243, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Chelme-Ayala, P.; El-Din, M.G.; Smith, D.W. Kinetics and mechanism of the degradation of two pesticides in aqueous solutions by ozonation. Chemosphere 2010, 78, 557–562. [Google Scholar] [CrossRef]
- Chelme-Ayala, P.; El-Din, M.G.; Smith, D.W.; Adams, C.D. Oxidation kinetics of two pesticides in natural waters by ozonation and ozone combined with hydrogen peroxide. Water Res. 2011, 45, 2517–2526. [Google Scholar] [CrossRef]
- Huang, Y.-R.; Hung, Y.-C.; Hsu, S.-Y.; Huang, Y.-W.; Hwang, D.-F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Han, Y.; Song, L.; An, Q.; Pan, C. Removal of six pesticide residues in cowpea with alkaline electrolysed water. J. Sci. Food Agric. 2017, 97, 2333–2338. [Google Scholar] [CrossRef]
- Rodrigues, A.A.Z.; De Queiroz, M.; De Oliveira, A.F.; Neves, A.A.; Heleno, F.F.; Zambolim, L.; Freitas, J.F.; Morais, E.H.C. Pesticide residue removal in classic domestic processing of tomato and its effects on product quality. J. Environ. Sci. Health B 2017, 52, 850–857. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, L.; Zhou, L.; Zhang, F.; Kang, S.; Pan, C. Multi-walled carbon nanotubes as alternative reversed-dispersive solid phase extraction materials in pesticide multi-residue analysis with QuEChERS method. J. Chromatogr. A 2012, 1225, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, J.; He, Y.; Han, Y.; Zou, N.; Li, Y.; Chen, R.; Li, X.; Pan, C. Automated Multiplug Filtration Cleanup for Pesticide Residue Analyses in Kiwi Fruit (Actinidia chinensis) and Kiwi Juice by Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 6082–6090. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Song, L.; Zou, N.; Qin, Y.; Li, X.; Pan, C. Rapid multiplug filtration cleanup method for the determination of 124 pesticide residues in rice, wheat, and corn. J. Sep. Sci. 2017, 40, 878–884. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Yu, C.; Pan, C. Determination of six neonicotinoid insecticides residues in spinach, cucumber, apple and pomelo by QuEChERS method and LC-MS/MS. Bull. Environ. Contam. Toxicol. 2012, 88, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Guidance Document on Analytical Quality Control And Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed. SANTE/11813/2017. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf (accessed on 15 September 2018).
- Lyu, F.; Gao, F.; Zhou, X.; Zhang, J.; Ding, Y. Using acid and alkaline electrolyzed water to reduce deoxynivalenol and mycological contaminations in wheat grains. Food Control 2018, 88, 98–104. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, F.; Liu, S.; Yu, C.; Guan, D.; Pan, C. Removal of aflatoxin B(1) in edible plant oils by oscillating treatment with alkaline electrolysed water. Food Chem. 2013, 141, 3118–3123. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.; Jin, Y.G.; Oh, D.H. Combination treatment of alkaline electrolyzed water and citric acid with mild heat to ensure microbial safety, shelf-life and sensory quality of shredded carrots. Food Microbiol. 2011, 28, 484–491. [Google Scholar] [CrossRef]
- Shimamura, Y.; Shinke, M.; Hiraishi, M.; Tsuchiya, Y.; Masuda, S. The application of alkaline and acidic electrolyzed water in the sterilization of chicken breasts and beef liver. Food Sci. Nutr. 2016, 4, 431–440. [Google Scholar] [CrossRef]
- Lozowicka, B.; Jankowska, M.; Hrynko, I.; Kaczynski, P. Removal of 16 pesticide residues from strawberries by washing with tap and ozone water, ultrasonic cleaning and boiling. Environ. Monit. Assess. 2016, 188, 51. [Google Scholar] [CrossRef]
- Tamaki, M.; Ikeur, H. Removal of residual pesticides in vegetables using ozone microbubbles. J. Hazard. Mater. 2011, 186, 956–959. [Google Scholar]
- Zhang, Z.-Y.; Liu, X.-J.; Hong, X.-Y. Effects of home preparation on pesticide residues in cabbage. Food Control 2007, 18, 1484–1487. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).