Twelve Weeks of Combined Resistance and Aerobic Exercise Improves Cardiometabolic Biomarkers and Enhances Red Blood Cell Hemorheological Function in Obese Older Men: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Anthropometric Characteristics and Body Composition
2.4. Cardiometabolic Biomarkers
2.5. RBC Hemorheological Parameters
2.6. Aerobic Performance
2.7. Statistical Analysis
3. Results
3.1. Body Composition
3.2. Cardiometabolic Biomarkers
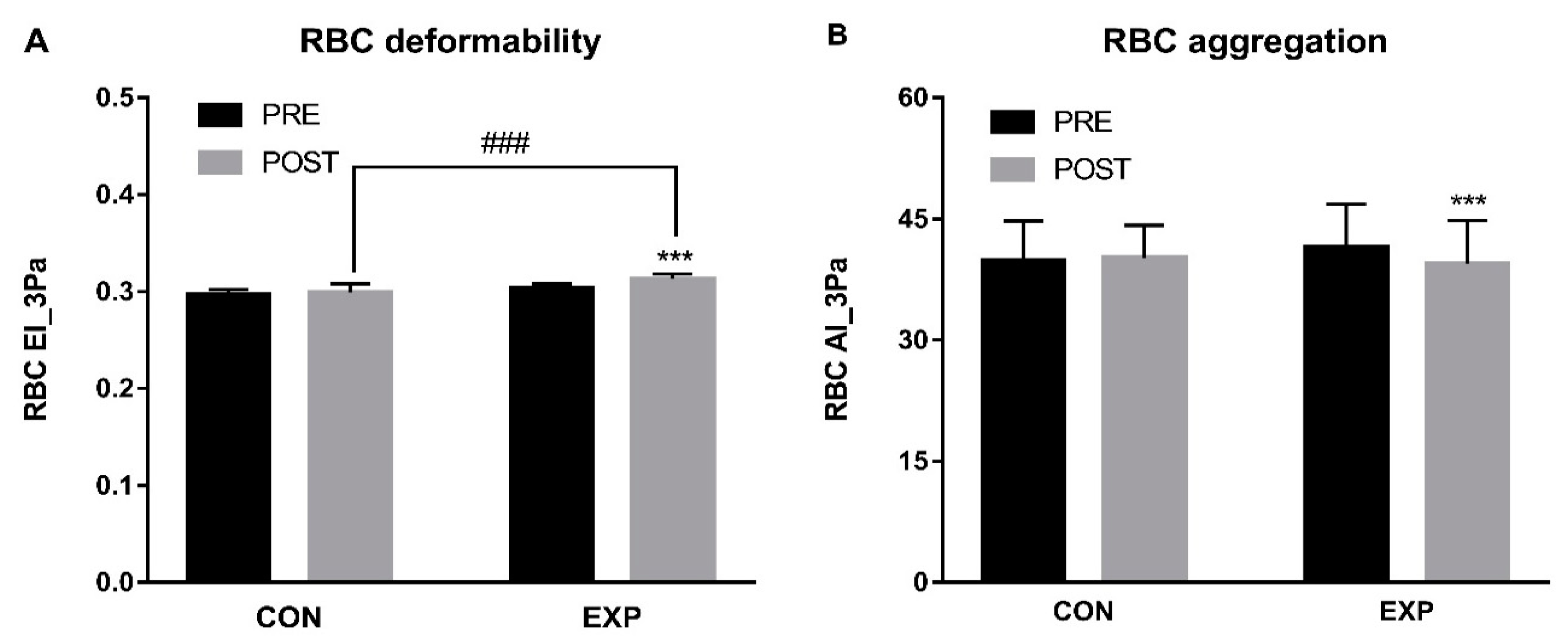
3.3. RBC Hemorheological Parameters
3.4. Aerobic Performance
4. Discussion
5. Limitation of the Study
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.-D. Blood Rheology: Key parameters, impact on blood flow, role in sickle cell disease and effects of exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renoux, C.; Faivre, M.; Bessaa, A.; Da Costa, L.; Joly, P.; Gauthier, A.; Connes, P. Impact of surface-area-to-volume ratio, internal viscosity and membrane viscoelasticity on red blood cell deformability measured in isotonic condition. Sci. Rep. 2019, 9, 6771. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.; Usami, S.; Dellenback, R.J.; Gregersen, M.I. Shear-dependent deformation of erythrocytes in rheology of human blood. Am. J. Physiol. 1970, 219, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilski, J.; Teleglow, A.; Pokorski, J.; Nitecki, J.; Pokorska, J.; Nitecka, E.; Marchewka, A.; Dabrowski, Z.; Marchewka, J. Effects of a meal on the hemorheologic responses to exercise in young males. Biomed. Res. Int. 2014, 2014, 862968. [Google Scholar] [CrossRef] [Green Version]
- Chong-Martinez, B.; Buchanan, T.A.; Wenby, R.B.; Meiselman, H.J. Decreased red blood cell aggregation subsequent to improved glycaemic control in Type 2 diabetes mellitus. Diabet. Med. J. Br. Diabet. Assoc. 2003, 20, 301–306. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Erythrocyte aggregation: Basic aspects and clinical importance. Clin. Hemorheol. Microcirc. 2013, 53, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Toth, A.; Papp, J.; Rabai, M.; Kenyeres, P.; Marton, Z.; Kesmarky, G.; Juricskay, I.; Meiselman, H.J.; Toth, K. The role of hemorheological factors in cardiovascular medicine. Clin. Hemorheol. Microcirc. 2014, 56, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Zeng, N.F.; Mancuso, J.E.; Zivkovic, A.M.; Smilowitz, J.T.; Ristenpart, W.D. Red Blood Cells from Individuals with Abdominal Obesity or Metabolic Abnormalities Exhibit Less Deformability upon Entering a Constriction. PLoS ONE 2016, 11, e0156070. [Google Scholar] [CrossRef] [Green Version]
- Piecuch, J.; Mertas, A.; Nowowiejska-Wiewiora, A.; Zurawel, R.; Gregorczyn, S.; Czuba, Z.; Wiewiora, M. The relationship between the rheological behavior of RBCs and angiogenesis in the morbidly obese. Clin. Hemorheol. Microcirc. 2019, 71, 95–102. [Google Scholar] [CrossRef]
- Capuano, P.; Catalano, G.; Garruti, G.; Trerotoli, P.; Cicco, G.; Martines, G.; Tedeschi, M.; DeTullio, A.; Mallardi, G.; Lucafo, M.A.; et al. The effects of weight loss due to gastric banding and lifestyle modification on red blood cell aggregation and deformability in severe obese subjects. Int. J. Obes. 2012, 36, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, V.; Diederich, L.; Keller, T.C.S.T.; Kramer, C.M.; Luckstadt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef] [PubMed]
- Wiewiora, M.; Piecuch, J.; Gluck, M.; Slowinska-Lozynska, L.; Sosada, K. The effects of weight loss surgery on blood rheology in severely obese patients. Surg. Obes. Relat. Dis. 2015, 11, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- das Gracas Coelho de Souza, M.; Kraemer-Aguiar, L.G.; Bouskela, E. Inflammation-induced microvascular dysfunction in obesity—A translational approach. Clin. Hemorheol. Microcirc. 2016, 64, 645–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connes, P.; Tripette, J.; Mukisi-Mukaza, M.; Baskurt, O.K.; Toth, K.; Meiselman, H.J.; Hue, O.; Antoine-Jonville, S. Relationships between hemodynamic, hemorheological and metabolic responses during exercise. Biorheology 2009, 46, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Houterman, S.; Boshuizen, H.C.; Verschuren, W.M.; Giampaoli, S.; Nissinen, A.; Menotti, A.; Kromhout, D. Predicting cardiovascular risk in the elderly in different European countries. Eur. Heart J. 2002, 23, 294–300. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Sinclair, A.J. Diabetes, Nutrition, and Exercise. Clin. Geriatr. Med. 2015, 31, 439–451. [Google Scholar] [CrossRef]
- Arsenault, B.J.; Boekholdt, S.M.; Kastelein, J.J. Lipid parameters for measuring risk of cardiovascular disease. Nat. Rev. Cardiol. 2011, 8, 197–206. [Google Scholar] [CrossRef]
- Despres, J.P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef] [Green Version]
- Laakso, M.; Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol. 2014, 10, 293–302. [Google Scholar] [CrossRef]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007, 56, 2655–2667. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.C.; Liu, S.; Song, Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015, 4, e002014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghouts, L.B.; Keizer, H.A. Exercise and insulin sensitivity: A review. Int. J. Sports Med. 2000, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Marcell, T.J.; Hawkins, S.A.; Wiswell, R.A. Leg strength declines with advancing age despite habitual endurance exercise in active older adults. J. Strength Cond. Res. 2014, 28, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.L.; Oliveira, R.B.; Fleck, S.J.; Leon, A.C.; Farinatti, P. Influence of strength training variables on strength gains in adults over 55 years-old: A meta-analysis of dose-response relationships. J. Sci. Med. Sport 2014, 17, 337–344. [Google Scholar] [CrossRef]
- Andersen, T.R.; Schmidt, J.F.; Nielsen, J.J.; Randers, M.B.; Sundstrup, E.; Jakobsen, M.D.; Andersen, L.L.; Suetta, C.; Aagaard, P.; Bangsbo, J.; et al. Effect of football or strength training on functional ability and physical performance in untrained old men. Scand. J. Med. Sci. Sports 2014, 24 (Suppl. 1), 76–85. [Google Scholar] [CrossRef] [Green Version]
- Thompson, W.R. Worldwide survey of fitness trends for 2019. ACSMS Health Fit. J. 2018, 22, 10–17. [Google Scholar] [CrossRef]
- Grace, F.; Herbert, P.; Elliott, A.D.; Richards, J.; Beaumont, A.; Sculthorpe, N.F. High intensity interval training (HIIT) improves resting blood pressure, metabolic (MET) capacity and heart rate reserve without compromising cardiac function in sedentary aging men. Exp. Gerontol. 2018, 109, 75–81. [Google Scholar] [CrossRef]
- Hwang, C.L.; Yoo, J.K.; Kim, H.K.; Hwang, M.H.; Handberg, E.M.; Petersen, J.W.; Christou, D.D. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp. Gerontol. 2016, 82, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.T.; Chung, Y.C.; Chen, Y.J.; Ho, S.Y.; Wu, H.J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef]
- Stinkens, R.; Brouwers, B. Exercise training-induced effects on the abdominal subcutaneous adipose tissue phenotype in humans with obesity. J. Appl. Physiol. 2018, 125, 1585–1593. [Google Scholar] [CrossRef] [Green Version]
- Blumenthal, J.B.; Gitterman, A.; Ryan, A.S.; Prior, S.J. Effects of Exercise Training and Weight Loss on Plasma Fetuin-A Levels and Insulin Sensitivity in Overweight Older Men. J. Diabetes Res. 2017, 2017, 1492581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberga, A.S.; Prud’homme, D.; Sigal, R.J.; Goldfield, G.S.; Hadjiyannakis, S.; Phillips, P.; Malcolm, J.; Ma, J.; Doucette, S.; Gougeon, R.; et al. Effects of aerobic training, resistance training, or both on cardiorespiratory and musculoskeletal fitness in adolescents with obesity: The HEARTY trial. Appl. Physiol. Nutr. Metab. 2016, 41, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.U.; Lee, J.H.; Kim, J.S.; Hwang, Y.I.; Kim, T.H.; Lim, S.Y.; Yoo, K.H.; Jung, K.S.; Kim, Y.K.; Rhee, C.K. Comparison of World Health Organization and Asia-Pacific body mass index classifications in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2465–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uyuklu, M.; Cengiz, M.; Ulker, P.; Hever, T.; Tripette, J.; Connes, P.; Nemeth, N.; Meiselman, H.J.; Baskurt, O.K. Effects of storage duration and temperature of human blood on red cell deformability and aggregation. Clin. Hemorheol. Microcirc. 2009, 41, 269–278. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Analyzing shear stress-elongation index curves: Comparison of two approaches to simplify data presentation. Clin. Hemorheol. Microcirc. 2004, 31, 23–30. [Google Scholar]
- Bakeman, R. Recommended effect size statistics for repeated measures designs. Behav. Res. Methods 2005, 37, 379–384. [Google Scholar] [CrossRef]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1435–1445. [Google Scholar] [CrossRef] [Green Version]
- Theou, O.; Stathokostas, L.; Roland, K.P.; Jakobi, J.M.; Patterson, C.; Vandervoort, A.A.; Jones, G.R. The effectiveness of exercise interventions for the management of frailty: A systematic review. J. Aging Res. 2011, 2011, 569194. [Google Scholar] [CrossRef] [Green Version]
- Beaufrere, B.; Morio, B. Fat and protein redistribution with aging: Metabolic considerations. Eur. J. Clin. Nutr. 2000, 54 (Suppl. 3), S48–S53. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.S.; Dhaliwal, S.S.; Hills, A.P.; Pal, S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health 2012, 12, 704. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Kim, C.G.; Seo, T.B.; Kim, H.G.; Yoon, S.J. Effects of 8-week combined training on body composition, isokinetic strength, and cardiovascular disease risk factors in older women. Aging Clin. Exp. Res. 2015, 27, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.A.; Arora, P.; Garcia-Bailo, B.; Karmali, M.; El-Sohemy, A.; Badawi, A. The association between obesity, cardiometabolic disease biomarkers, and innate immunity-related inflammation in Canadian adults. Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Badawi, A.; Klip, A.; Haddad, P.; Cole, D.E.; Bailo, B.G.; El-Sohemy, A.; Karmali, M. Type 2 diabetes mellitus and inflammation: Prospects for biomarkers of risk and nutritional intervention. Diabetes Metab. Syndr. Obes. Targets Ther. 2010, 3, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef]
- Bray, G.A.; Clearfield, M.B.; Fintel, D.J.; Nelinson, D.S. Overweight and obesity: The pathogenesis of cardiometabolic risk. Clin. Cornerstone 2009, 9, 30–40. [Google Scholar] [CrossRef]
- Balducci, S.; Zanuso, S.; Nicolucci, A.; Fernando, F.; Cavallo, S.; Cardelli, P.; Fallucca, S.; Alessi, E.; Letizia, C.; Jimenez, A.; et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr. Metab. Cardiovasc. Dis. NMCD 2010, 20, 608–617. [Google Scholar] [CrossRef]
- Ha, C.H.; Swearingin, B.; Jeon, Y.K.; Lee, M. Effects of combined exercise on HOMA-IR, HOMA β-cell and atherogenic index in Korean obese female. Sport Sci. Health 2015, 11, 49–55. [Google Scholar] [CrossRef]
- Ha, M.S.; Son, W.M. Combined exercise is a modality for improving insulin resistance and aging-related hormone biomarkers in elderly Korean women. Exp. Gerontol. 2018, 114, 13–18. [Google Scholar] [CrossRef]
- Meier, U.; Gressner, A.M. Endocrine regulation of energy metabolism: Review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin. Chem. 2004, 50, 1511–1525. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L.; Williams, T.D.; Dobbs, W.C. The Effect of Chronic Exercise Training on Leptin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2018, 48, 1437–1450. [Google Scholar] [CrossRef]
- Rostas, I.; Poto, L.; Matrai, P.; Hegyi, P.; Tenk, J.; Garami, A.; Illes, A.; Solymar, M.; Petervari, E.; Szucs, A.; et al. In middle-aged and old obese patients, training intervention reduces leptin level: A meta-analysis. PLoS ONE 2017, 12, e0182801. [Google Scholar] [CrossRef] [PubMed]
- Mairbaurl, H. Red blood cells in sports: Effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 2013, 4, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romain, A.J.; Brun, J.F.; Varlet-Marie, E.; Raynaud de Mauverger, E. Effects of exercise training on blood rheology: A meta-analysis. Clin. Hemorheol. Microcirc. 2011, 49, 199–205. [Google Scholar] [CrossRef]
- Kilic-Toprak, E.; Ardic, F.; Erken, G.; Unver-Kocak, F.; Kucukatay, V.; Bor-Kucukatay, M. Hemorheological responses to progressive resistance exercise training in healthy young males. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, Cr351–Cr360. [Google Scholar] [CrossRef] [Green Version]
- Brun, J.F.; Varlet-Marie, E.; Connes, P.; Aloulou, I. Hemorheological alterations related to training and overtraining. Biorheology 2010, 47, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Influence of regular physical activity on blood rheology. Eur. Heart J. 1987, 8 (Suppl. G), 59–62. [Google Scholar] [CrossRef]
- Sandor, B.; Nagy, A.; Toth, A.; Rabai, M.; Mezey, B.; Csatho, A.; Czuriga, I.; Toth, K.; Szabados, E. Effects of moderate aerobic exercise training on hemorheological and laboratory parameters in ischemic heart disease patients. PLoS ONE 2014, 9, e110751. [Google Scholar] [CrossRef]
- Hurst, C.; Weston, K.L. The effects of same-session combined exercise training on cardiorespiratory and functional fitness in older adults: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2019, 31, 1701–1717. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, H.; Loenneke, J.P.; Thiebaud, R.S.; Abe, T. Resistance training induced increase in VO2 max in young and older subjects. Eur. Rev. Aging Phys. Act. 2013, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Frank, P.; Andersson, E.; Ponten, M.; Ekblom, B.; Ekblom, M.; Sahlin, K. Strength training improves muscle aerobic capacity and glucose tolerance in elderly. Scand. J. Med. Sci. Sports 2016, 26, 764–773. [Google Scholar] [CrossRef]
- Karavirta, L.; Hakkinen, K.; Kauhanen, A.; Arija-Blazquez, A.; Sillanpaa, E.; Rinkinen, N.; Hakkinen, A. Individual responses to combined endurance and strength training in older adults. Med. Sci. Sports Exerc. 2011, 43, 484–490. [Google Scholar] [CrossRef] [PubMed]

| Variables | CON (n = 10) | EXP (n = 10) | t-value |
|---|---|---|---|
| Age (years) | 68.5 ± 0.85 | 69.1 ± 0.88 | –1.555 |
| Body height (cm) | 165.8 ± 4.82 | 164.1 ± 3.79 | 0.846 |
| Body weight (kg) | 71.6 ± 5.00 | 70.7 ± 3.84 | 0.434 |
| BMI (kg/m2) | 26.0 ± 0.43 | 26.2 ± 0.48 | 0.301 |
| Fat free mass (kg) | 45.4 ± 3.17 | 44.8 ± 2.43 | 0.434 |
| Percent body fat (%) | 32.7 ± 1.78 | 32.4 ± 1.37 | 0.434 |
| Variables | CON | EXP | F-value (ηp2) | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Time | Group | Interaction | |
| Body weight (kg) | 71.6 ± 5.00 | 72.3 ± 5.05 | 70.7 ± 3.84 | 69.2 ± 4.09 *** | 1.921 (0.096) | 0.970 (0.051) | 14.229 (0.442) ††† |
| Fat free mass (kg) | 45.4 ± 3.17 | 44.4 ± 3.10 * | 44.8 ± 2.43 | 45.2 ± 2.67 | 2.379 (0.117) | 0.014 (0.001) | 13.994 (0.437) ††† |
| Fat mass (kg) | 23.5 ± 2.97 | 24.9 ± 3.14 ***,### | 22.9 ± 2.23 | 21.1 ± 2.22 *** | 1.063 (0.056) | 3.337 (0.156) | 87.840 (0.830) ††† |
| Percent body fat (%) | 32.7 ± 1.78 | 34.3 ± 1.90 ***,### | 32.4 ± 1.37 | 30.4 ± 1.38 *** | 1.151 (0.060) | 8.577 (0.323) † | 282.897 (0.940) ††† |
| Variables | CON | EXP | F-value (ηp2) | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Time | Group | Interaction | |
| Glucose (mg/dL) | 114.38 ± 12.02 | 116.53 ± 6.63 | 120.04 ± 7.76 | 114.32 ± 7.04 | 0.488 (0.026) | 0.354 (0.019) | 2.383 (0.117) |
| Insulin (µU/mL) | 3.39 ± 0.36 | 3.68 ± 0.19 *,### | 3.43 ± 0.22 | 3.27 ± 0.20 | 0.732 (0.039) | 4.885 (0.213) † | 9.337 (0.342) †† |
| HOMA-IR | 0.97 ± 0.20 | 1.06 ± 0.12 # | 1.02 ± 0.13 | 0.93 ± 0.11 | 0.000 (0.000) | 0.736 (0.039) | 5.179 (0.223) † |
| HOMA-ꞵ | 24.53 ± 3.79 | 24.93 ± 1.71 | 21.85 ± 1.69 | 23.16 ± 2.05 | 1.306 (0.068) | 7.533 (0.295) † | 0.370 (0.020) |
| Leptin (μg/L) | 22.08 ± 8.82 | 19.75 ± 6.53 | 22.56 ± 8.01 | 17.03 ± 7.00 ** | 7.459 (0.293) † | 0.132 (0.007) | 1.232 (0.064) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-W.; Jung, W.-S.; Park, W.; Park, H.-Y. Twelve Weeks of Combined Resistance and Aerobic Exercise Improves Cardiometabolic Biomarkers and Enhances Red Blood Cell Hemorheological Function in Obese Older Men: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2019, 16, 5020. https://doi.org/10.3390/ijerph16245020
Kim S-W, Jung W-S, Park W, Park H-Y. Twelve Weeks of Combined Resistance and Aerobic Exercise Improves Cardiometabolic Biomarkers and Enhances Red Blood Cell Hemorheological Function in Obese Older Men: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2019; 16(24):5020. https://doi.org/10.3390/ijerph16245020
Chicago/Turabian StyleKim, Sung-Woo, Won-Sang Jung, Wonil Park, and Hun-Young Park. 2019. "Twelve Weeks of Combined Resistance and Aerobic Exercise Improves Cardiometabolic Biomarkers and Enhances Red Blood Cell Hemorheological Function in Obese Older Men: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 16, no. 24: 5020. https://doi.org/10.3390/ijerph16245020
APA StyleKim, S.-W., Jung, W.-S., Park, W., & Park, H.-Y. (2019). Twelve Weeks of Combined Resistance and Aerobic Exercise Improves Cardiometabolic Biomarkers and Enhances Red Blood Cell Hemorheological Function in Obese Older Men: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 16(24), 5020. https://doi.org/10.3390/ijerph16245020








