Inter-Individual Variability in Metabolic Syndrome Severity Score and VO2max Changes Following Personalized, Community-Based Exercise Programming
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Personalized Exercise Training Program
2.3. Statistical Analysis
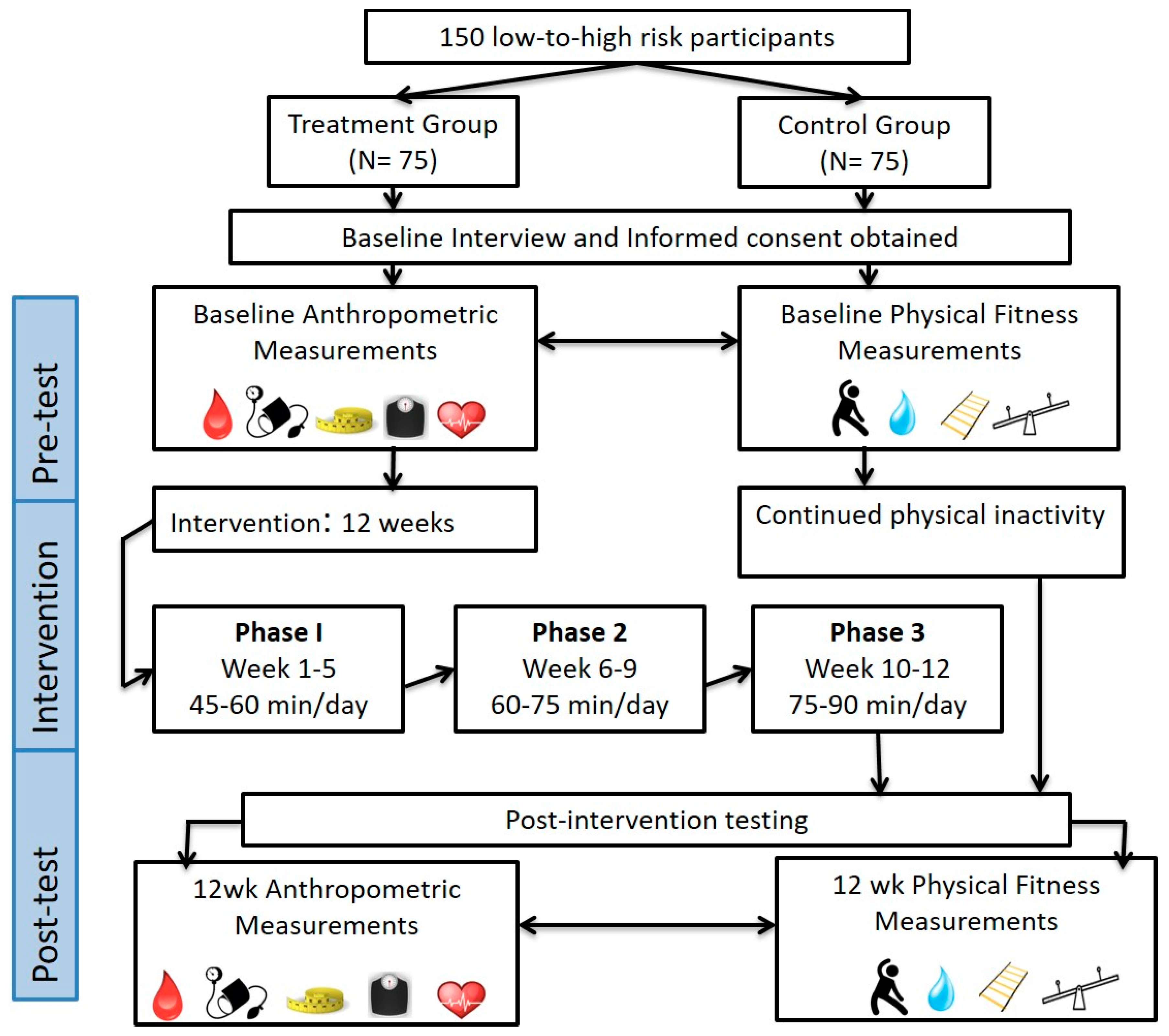
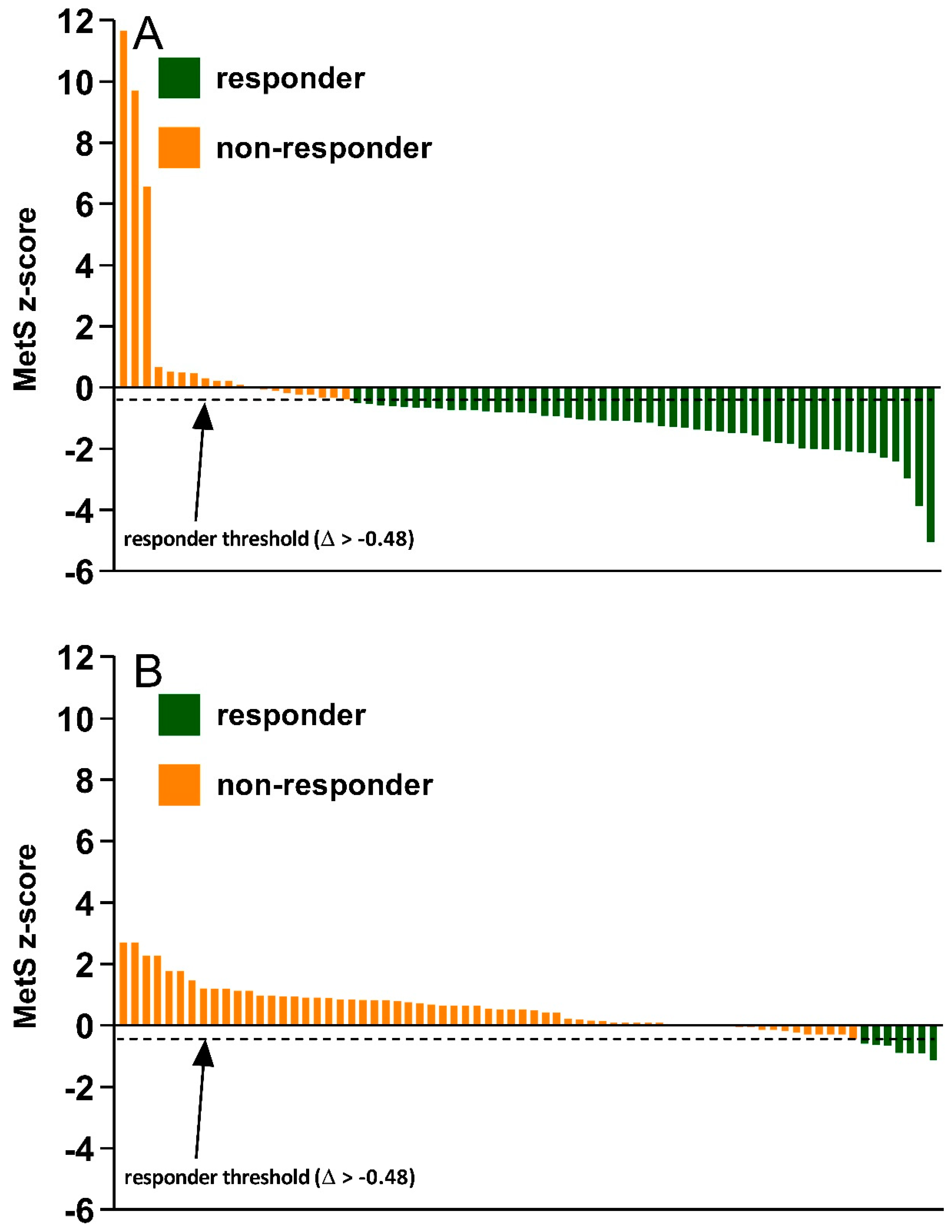
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Larsson, S.C.; Wallin, A.; Håkansson, N.; Stackelberg, O.; Bäck, M.; Wolk, A. Type 1 and type 2 diabetes mellitus and incidence of seven cardiovascular diseases. Int. J. Cardiol. 2018, 262, 66–70. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Report on Diabetes. Available online: https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=C1C77775CDDBC4FF4568A581E746088A?sequence=1 (accessed on 26 November 2019).
- Baldassarre, P.A.; Andersen, A.; Consoli, A.; Knop, F.K.; Vilsboll, T. Cardiovascular biomarkers in clinical studies of type 2 diabetes. Diabetes Obes. Metab. 2008, 20, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D.; Filipp, S.L.; Gurka, M.J. Use of a metabolic syndrome severity Z score to track risk during treatment of prediabetes: An analysis of the diabetes prevention program. Diabetes Care 2018, 41, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Barry, V.W.; Baruth, M.; Beets, M.W.; Durstine, J.L.; Liu, J.; Blair, S.N. Fitness vs. fatness on all-cause mortality: A meta-analysis. Prog. Cardiovasc. Dis. 2014, 56, 382–390. [Google Scholar] [CrossRef]
- Johnson, J.L.; Slentz, C.A.; Houmard, J.A.; Samsa, G.P.; Duscha, B.D.; Aiken, L.B.; McCartney, J.S.; Tanner, C.J.; Kraus, W.E. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise). Am. J. Cardiol. 2007, 100, 1759–1766. [Google Scholar] [CrossRef]
- Ramos, J.S.; Dalleck, L.C.; Borrani, F.; Beetham, K.S.; Wallen, M.P.; Mallard, A.R.; Clark, B.; Gomersall, S.; Keating, S.E.; Fassett, R.G.; et al. Low-volume high intensity interval training is sufficient to ameliorate the severity of metabolic syndrome. Metab. Syndr. Relat. Disord. 2017, 15, 319–328. [Google Scholar] [CrossRef]
- Weatherwax, R.M.; Harris, N.K.; Kilding, A.E.; Dalleck, L.C. Incidence of VO2max responders to personalized versus standardized exercise prescription. Med. Sci. Sports Exerc. 2019, 51, 681–691. [Google Scholar] [CrossRef]
- Byrd, B.R.; Keith, J.; Keeling, S.M.; Weatherwax, R.M.; Nolan, P.B.; Ramos, J.S.; Dalleck, L.C. Personalized Moderate-Intensity Exercise Training Combined with High-Intensity Interval Training Enhances Training Responsiveness. Int. J. Environ. Res. Public Health 2019, 16, 2088. [Google Scholar] [CrossRef]
- Dalleck, L.C.; Van Guilder, G.P.; Quinn, E.M.; Bredle, D.L. Primary prevention of metabolic syndrome in the community using an evidence-based exercise program. Prev. Med. 2013, 57, 392–395. [Google Scholar] [CrossRef]
- Weatherwax, R.M.; Harris, N.K.; Kilding, A.E.; Dalleck, L.C. The incidence of training responsiveness to cardiorespiratory fitness and cardiometabolic measurements following individualized and standardized exercise prescription: Study protocol for a randomized controlled trial. Trials 2016, 17, 601. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2018. [Google Scholar]
- Bryant, C.X.; Green, D.J. ACE Personal Trainer Manual; American Council on Exercise: San Diego, CA, USA, 2010. [Google Scholar]
- Dalleck, L.C.; Haney, D.E.; Buchanan, C.A.; Weatherwax, R.M. Does a personalized exercise prescription enhance training efficacy and limit training unresponsiveness? A randomized controlled trial. J. Fit. Res. 2016, 5, 15–27. [Google Scholar]
- Gurka, M.J.; Guo, Y.; Filipp, S.L.; DeBoer, M.D. Metabolic syndrome severity is significantly associated with future coronary heart disease in type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Unal, B.; Critchley, J.A.; Capewell, S. Modelling the decline in coronary heart disease deaths in England and wales, 1981–2000: Comparing contributions from primary prevention and secondary prevention. BMJ 2005, 331, 614. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Li, C.; Zhao, G.; Pearson, W.S.; Capewell, S. Trends in the prevalence of low risk factor burden for cardiovascular disease among United States adults. Circulation 2009, 120, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Riboli, E. Can noncommunicable diseases be prevented? Lessons from studies of populations and individuals. Science 2012, 337, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.M.; Yanek, L.R.; Johnson, W.R., Jr.; Garrett, D.; Moy, T.F.; Reynolds, S.S.; Blumenthal, R.S.; Vaidya, D.; Becker, L.C. Impact of a community-based multiple risk factor intervention on cardiovascular risk in black families with a history of premature coronary disease. Circulation 2005, 111, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N.; Kampert, J.B.; Kohl, H.W.; Barlow, C.E.; Macera, C.A.; Paffenbarger, R.A., Jr.; Gibbons, L.A. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996, 276, 205–210. [Google Scholar] [CrossRef]
- Meyer, T.; Scharhag, J.; Kindermann, W. Peak oxygen uptake: Myth and truth about an internationally accepted reference value. Z. Kardiol. 2005, 94, 255–264. [Google Scholar] [CrossRef]
- Williams, C.J.; Gurd, B.J.; Bonafiglia, J.T.; Voisin, S.; Li, Z.X.; Harvey, N.; Croci, I.; Taylor, J.L.; Gajanand, T.; Ramos, J.S.; et al. A multi-center comparison of VO2peak trainability between interval training and moderate intensity continuous training. Front. Physiol. 2019, 10, 19. [Google Scholar] [CrossRef]
- Earnest, C.P.; Lupo, M.; Thibodaux, J.; Hollier, C.; Bututta, B.; Lejeune, E.; Johannsen, N.M.; Gibala, M.J.; Church, T.S. Interval training in men at risk for insulin resistance. Int. J. Sports Med. 2013, 34, 355–363. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.F.; Carrington, M.J. A metabolic syndrome severity score: A tool to quantify cardio-metabolic risk factors. Prev. Med. 2016, 88, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.D. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
 ; Submaximal VO2max test (talk test)
; Submaximal VO2max test (talk test)  ; Balance test
; Balance test  ; 8 feet up and go
; 8 feet up and go  ; Shoulder rotation, sit and reach, trunk rotation
; Shoulder rotation, sit and reach, trunk rotation  ; Blood pressure
; Blood pressure  ; Resting heart rate
; Resting heart rate  ; BMI
; BMI  ; Waist circumference
; Waist circumference  .
.
 ; Submaximal VO2max test (talk test)
; Submaximal VO2max test (talk test)  ; Balance test
; Balance test  ; 8 feet up and go
; 8 feet up and go  ; Shoulder rotation, sit and reach, trunk rotation
; Shoulder rotation, sit and reach, trunk rotation  ; Blood pressure
; Blood pressure  ; Resting heart rate
; Resting heart rate  ; BMI
; BMI  ; Waist circumference
; Waist circumference  .
.
| Outcome Variable | Control Group (N = 72) | Treatment Group (N = 70) | ||
|---|---|---|---|---|
| Baseline | Post-Program | Baseline | Post-Program | |
| Age (yr) | 45.6 ± 12.5 | ------- | 46.6 ± 16.7 | ------- |
| Body mass (kg) | 75.5 ± 12.3 | 75.7 ± 12.0 * | 77.3 ± 18.7 | 76.7 ± 18.4 *, † |
| Waist circumference (cm) | 82.4 ± 8.8 | 82.7 ± 8.6 | 84.0 ± 14.2 | 83.1 ± 12.9 *, † |
| Systolic BP (mm Hg) | 119.0 ± 11.0 | 121.2 ± 9.6 * | 122.6 ± 14.1 | 117.4 ± 13.1 *, † |
| Diastolic BP (mm Hg) | 79.4 ± 8.4 | 81.4 ± 6.6 * | 79.7 ± 9.7 | 77.3 ± 7.7 *, † |
| Total cholesterol (mg·dL−1) | 201.3 ± 40.0 | 204.4 ± 37.5 | 187.5 ± 39.1 | 185.1 ± 37.7 |
| HDL cholesterol (mg·dL−1) | 50.7 ± 18.2 | 49.4 ± 16.5 * | 54.2 ± 17.9 | 57.8 ± 15.9 *, † |
| LDL cholesterol (mg·dL−1) | 119.9 ± 37.7 | 122.0 ± 36.3 | 107.2 ± 32.9 | 100.6 ± 31.1 |
| Triglycerides (mg·dL−1) | 130.0 ± 64.3 | 136.1 ± 67.2 | 110.8 ± 54.4 | 104.5 ± 45.7 † |
| Blood glucose (mg·dL−1) | 93.1 ± 9.0 | 94.8 ± 9.1 | 92.5 ± 8.6 | 89.7 ± 7.0 *, † |
| VO2max (mL·kg−1·min−1) | 29.0 ± 6.1 | 28.4 ± 5.8 * | 31.4 ± 7.9 | 35.0 ± 8.0 *, † |
| MetS z-score | −4.15 ± 4.01 | −3.68 ± 4.07 * | −3.52 ± 3.82 | −4.12 ± 3.24 *, † |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seward, S.; Ramos, J.; Drummond, C.; Dalleck, A.; Byrd, B.; Kehmeier, M.; Dalleck, L. Inter-Individual Variability in Metabolic Syndrome Severity Score and VO2max Changes Following Personalized, Community-Based Exercise Programming. Int. J. Environ. Res. Public Health 2019, 16, 4855. https://doi.org/10.3390/ijerph16234855
Seward S, Ramos J, Drummond C, Dalleck A, Byrd B, Kehmeier M, Dalleck L. Inter-Individual Variability in Metabolic Syndrome Severity Score and VO2max Changes Following Personalized, Community-Based Exercise Programming. International Journal of Environmental Research and Public Health. 2019; 16(23):4855. https://doi.org/10.3390/ijerph16234855
Chicago/Turabian StyleSeward, Sophie, Joyce Ramos, Claire Drummond, Angela Dalleck, Bryant Byrd, Mackenzie Kehmeier, and Lance Dalleck. 2019. "Inter-Individual Variability in Metabolic Syndrome Severity Score and VO2max Changes Following Personalized, Community-Based Exercise Programming" International Journal of Environmental Research and Public Health 16, no. 23: 4855. https://doi.org/10.3390/ijerph16234855
APA StyleSeward, S., Ramos, J., Drummond, C., Dalleck, A., Byrd, B., Kehmeier, M., & Dalleck, L. (2019). Inter-Individual Variability in Metabolic Syndrome Severity Score and VO2max Changes Following Personalized, Community-Based Exercise Programming. International Journal of Environmental Research and Public Health, 16(23), 4855. https://doi.org/10.3390/ijerph16234855






