Weekly Dose-Dense Paclitaxel and Triweekly Low-Dose Cisplatin: A Well-Tolerated and Effective Chemotherapeutic Regimen for First-Line Treatment of Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Treatment
2.3. Assessments
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Pathological Status
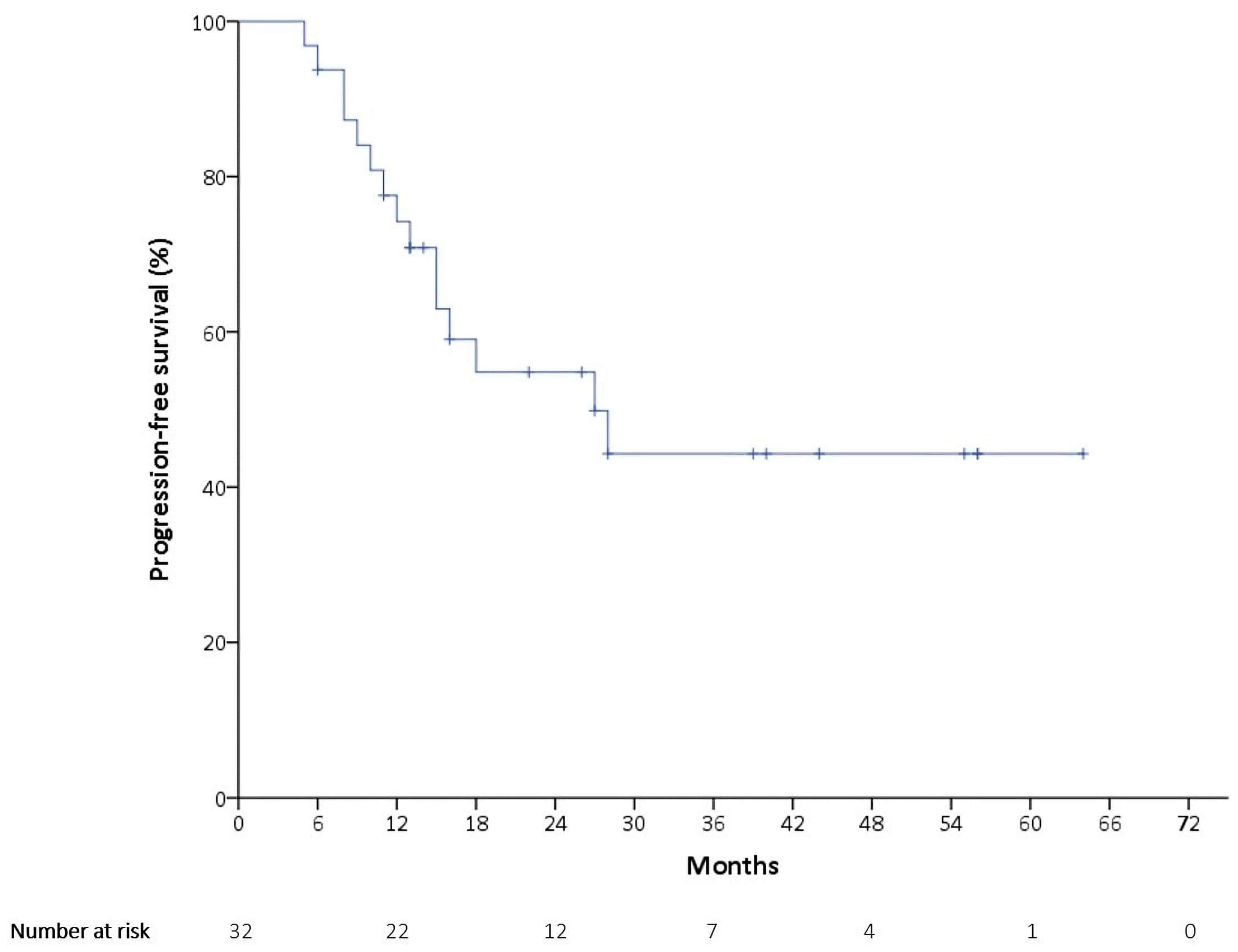
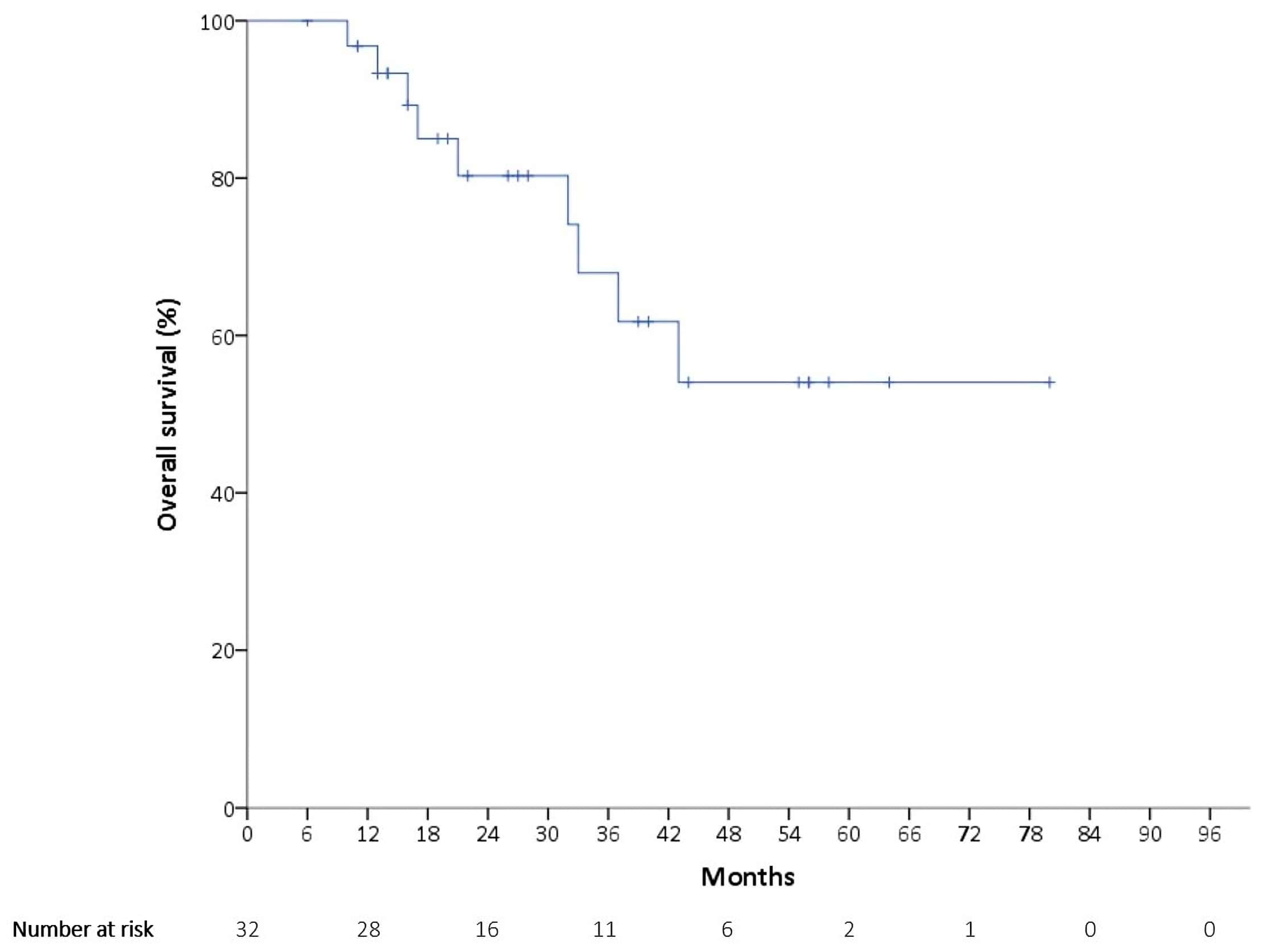
3.2. Outcomes
3.3. Prognostic Factors
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEs | Adverse events |
| AUC | Area under the curve |
| CBR | Clinical benefit rate |
| CT | Computed tomography |
| CI | Confidence interval |
| EOC | Epithelial ovarian cancer |
| eGFR | Estimated glomerular filtration rate |
| FTC | Fallopian tube cancer |
| FIGO | International Federation of Gynecology and Obstetrics |
| ISC | Interval cytoreductive surgery |
| NCI-CTCAE | National Cancer Institute’s Common Terminology Criteria for Adverse Events |
| NACT | Neoadjuvant chemotherapy |
| MRI | Magnetic resonance image |
| ORR | Overall response rate |
| OS | Overall survival |
| PARP inhibitors | Poly(ADP-ribose) polymerase (PARP) inhibitors |
| PPSC | Primary peritoneal serous carcinoma |
| PSC | Primary cytoreductive surgery |
| PFS | Progression-free survival |
| PD | Progressive disease |
| RDI | Relative dose intensity |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RR | Risk ratio |
| VIF | Variance inflation factor |
References
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med. 1996, 334, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.J.; Bertelsen, K.; James, K.; Cassidy, J.; Mangioni, C.; Simonsen, E.; Stuart, G.; Kaye, S.; Vergote, I.; Blom, R.; et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: Three-year results. J. Natl. Cancer Inst. 2000, 92, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.J.; Bertelsen, K.; Stuart, G.; Cassidy, J.; Mangioni, C.; Simonsen, E.; James, K.; Kaye, S.; Vergote, I.; Blom, R.; et al. Long-term follow-up confirms a survival advantage of the paclitaxel-cisplatin regimen over the cyclophosphamide-cisplatin combination in advanced ovarian cancer. Int. J. Gynecol. Cancer 2003, 13, 144–148. [Google Scholar] [PubMed]
- Alberts, D.S. Carboplatin versus cisplatin in ovarian cancer. Semin. Oncol. 1995, 22, 88–90. [Google Scholar]
- du Bois, A.; Neijt, J.P.; Thigpen, J.T. First-line chemotherapy with carboplatin plus paclitaxel in advanced ovarian cancer—a new standard of care? Ann. Oncol. 1999, 10, 51–53. [Google Scholar] [CrossRef]
- Neijt, J.P.; Engelholm, S.A.; Tuxen, M.K.; Sorensen, P.G.; Hansen, M.; Sessa, C.; de Swart, C.A.; Hirsch, F.R.; Lund, B.; van Houwelingen, H.C. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J. Clin. Oncol. 2000, 18, 3084–3092. [Google Scholar] [CrossRef]
- du Bois, A.; Luck, H.J.; Meier, W.; Adams, H.P.; Mobus, V.; Costa, S.; Bauknecht, T.; Richter, B.; Warm, M.; Schroder, W.; et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer Inst. 2003, 95, 1320–1329. [Google Scholar] [CrossRef]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef]
- Sung, P.L.; Wen, K.C.; Horng, H.C.; Chang, C.M.; Chen, Y.J.; Lee, W.L.; Wang, P.H. The role of α2,3-linked sialylation on clear cell type epithelial ovarian cancer. Taiwan. J. Obstet. Gynecol. 2018, 57, 255–263. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef]
- Vergote, I.; Scambia, G.; O’Malley, D.M.; Van Calster, B.; Park, S.Y.; Del Campo, J.M.; Meier, W.; Bamias, A.; Colombo, N.; Wenham, R.M.; et al. Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 862–876. [Google Scholar] [CrossRef]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N. Engl. J. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- van der Burg, M.E.; van Lent, M.; Buyse, M.; Kobierska, A.; Colombo, N.; Favalli, G.; Lacave, A.J.; Nardi, M.; Renard, J.; Pecorelli, S. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N. Engl. J. Med. 1995, 332, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Trope, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; van der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, S.; Terao, Y.; Hirayama, T.; Fujino, K.; Ujihira, T.; Ota, T.; Takeda, S. Safety and efficacy of neoadjuvant chemotherapy with bevacizumab in advanced-stage peritoneal/ovarian cancer patients. Taiwan. J. Obstet. Gynecol. 2018, 57, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H. Neoadjuvant chemotherapy before definite operative approach for women with advanced-stage epithelial ovarian cancer. Taiwan. J. Obstet. Gynecol. 2018, 57, 623–624. [Google Scholar] [CrossRef]
- Bartels, H.C.; Rogers, A.C.; McSharry, V.; McVey, R.; Walsh, T.; O’Brien, D.; Boyd, W.D.; Brennan, D.J. A meta-analysis of morbidity and mortality in primary cytoreductive surgery compared to neoadjuvant chemotherapy in advanced ovarian malignancy. Gynecol. Oncol. 2019, 154, 622–630. [Google Scholar] [CrossRef]
- Horner, W.; Peng, K.; Pleasant, V.; Brackmann, M.; Ebott, J.; Gutfreund, R.; McLean, K.; Reynolds, R.K.; Uppal, S. Trends in surgical complexity and treatment modalities utilized in the management of ovarian cancer in an era of neoadjuvant chemotherapy. Gynecol. Oncol. 2019, 154, 283–289. [Google Scholar] [CrossRef]
- Klein, D.A.; Mann, A.K.; Freeman, A.H.; Liao, C.I.; Kapp, D.S.; Chan, J.K. Chemotherapy alone for patients 75 years and older with epithelial ovarian cancer-is interval cytoreductive surgery still needed? Am. J. Obstet. Gynecol. 2019. [Google Scholar] [CrossRef]
- Markman, M.; Bundy, B.N.; Alberts, D.S.; Fowler, J.M.; Clark-Pearson, D.L.; Carson, L.F.; Wadler, S.; Sickel, J. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol. 2001, 19, 1001–1007. [Google Scholar]
- Markman, M.; Liu, P.Y.; Wilczynski, S.; Monk, B.; Copeland, L.J.; Alvarez, R.D.; Jiang, C.; Alberts, D. Southwest Oncology, Oncology G. Gynecologic, Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: A Southwest Oncology Group and Gynecologic Oncology Group trial. J. Clin. Oncol. 2003, 21, 2460–2465. [Google Scholar] [PubMed]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.; van der Velden, J.; Arts, H.J.; Massuger, L.; et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Marchetti, C.; De Felice, F.; Perniola, G.; Palaia, I.; Musella, A.; Di Donato, V.; Cascialli, G.; Muzii, L.; Tombolini, V.; Benedetti Panici, P. Role of intraperitoneal chemotherapy in ovarian cancer in the platimum-taxane-based era: A meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 136, 64–69. [Google Scholar] [CrossRef]
- Vergote, I.; du Bois, A.; Floquet, A.; Rau, J.; Kim, J.W.; Del Campo, J.M.; Friedlander, M.; Pignata, S.; Fujiwara, K.; Colombo, N.; et al. Overall survival results of AGO-OVAR16: A phase 3 study of maintenance pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced ovarian cancer. Gynecol. Oncol. 2019, 155, 186–191. [Google Scholar] [CrossRef]
- Tsibulak, I.; Zeimet, A.G.; Marth, C. Hopes and failures in front-line ovarian cancer therapy. Crit. Rev. Oncol. Hematol. 2019, 143, 14–19. [Google Scholar] [CrossRef]
- Matanes, E.; Gotlieb, W.H. Immunotherapy of gynecological cancers. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 60, 97–110. [Google Scholar] [CrossRef]
- Wendel Naumann, R.; Coleman, R.L.; Brown, J.; Moore, K.N. Phase III trials in ovarian cancer: The evolving landscape of front line therapy. Gynecol. Oncol. 2019, 153, 436–444. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, H.; Wan, T.; Zhang, C.; Tong, C.; Liu, J. Comparison of PARPis with angiogenesis inhibitors and chemotherapy for maintenance in ovarian cancer: A network meta-analysis. Adv. Ther. 2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Chang, Y.; Wang, P.H. Poly(ADP-ribose) polymerase (PARP) inhibitors and ovarian cancer. Taiwan. J. Obstet. Gynecol. 2017, 56, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Yang, Y.P.; Chuang, J.H.; Chuang, C.M.; Lin, T.W.; Wang, P.H.; Yu, M.H.; Chang, C.C. Discovering the deregulated molecular functions involved in malignant transformation of endometriosis to endometriosis-associated ovarian carcinoma using a data-driven, function-based analysis. Int. J. Mol. Sci. 2017, 18, 2345. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Chiou, S.H.; Yang, M.J.; Yen, M.S.; Wang, P.H. Gene set-based integrative analysis of ovarian clear cell carcinoma. Taiwan. J. Obstet. Gynecol. 2016, 55, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Wang, P.H.; Horng, H.C. Gene se-based analysis of mucinous ovarian carcinoma. Taiwan. J. Obstet. Gynecol. 2017, 56, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Su, K.M.; Lu, K.H.; Lin, C.K.; Wang, P.H.; Li, H.Y.; Wang, M.L.; Lin, C.K.; Yu, M.H.; Chang, C.M. Key immunological functions involved in the progression of epithelial ovarian serous carcinoma discovered by the gene ontology-based immunofunctionome analysis. Int. J. Mol. Sci. 2018, 19, 3311. [Google Scholar] [CrossRef]
- Simon, R.; Norton, L. The Norton-Simon hypothesis: Designing more effective and less toxic chemotherapeutic regimens. Nat. Clin. Pract. Oncol. 2006, 3, 406–407. [Google Scholar] [CrossRef]
- Su, W.H.; Ho, T.Y.; Li, Y.T.; Lu, C.H.; Lee, W.L.; Wang, P.H. Metronomic therapy for gynecologic cancers. Taiwan. J. Obstet. Gynecol. 2012, 51, 167–178. [Google Scholar] [CrossRef]
- Katsumata, N.; Yasuda, M.; Isonishi, S.; Takahashi, F.; Michimae, H.; Kimura, E.; Aoki, D.; Jobo, T.; Kodama, S.; Terauchi, F.; et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 2013, 14, 1020–1026. [Google Scholar] [CrossRef]
- Pignata, S.; Scambia, G.; Katsaros, D.; Gallo, C.; Pujade-Lauraine, E.; De Placido, S.; Bologna, A.; Weber, B.; Raspagliesi, F.; Panici, P.B.; et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 396–405. [Google Scholar] [CrossRef]
- Chan, J.K.; Brady, M.F.; Penson, R.T.; Huang, H.; Birrer, M.J.; Walker, J.L.; DiSilvestro, P.A.; Rubin, S.C.; Martin, L.P.; Davidson, S.A.; et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N. Engl. J. Med. 2016, 374, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Brady, M.F.; Wenzel, L.; Fleming, G.F.; Huang, H.Q.; DiSilvestro, P.A.; Fujiwara, K.; Alberts, D.S.; Zheng, W.; Tewari, K.S.; et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2019, 37, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Kristeleit, R.; McCormack, M.; Raja, F.; Luvero, D.; Widschwendter, M.; MacDonald, N.; Mould, T.; Olatain, A.; Hackshaw, A.; et al. Switching from standard to dose-dense chemotherapy in front-line treatment of advanced ovarian cancer: A retrospective study of feasibility and efficacy. ESMO Open 2017, 1, e000117. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Reimer, D.; Zeimet, A.G. Front-line therapy of advanced epithelial ovarian cancer: Standard treatment. Ann. Oncol. 2017, 28, viii36–viii39. [Google Scholar] [CrossRef]
- Lee, M.X.; Tan, D.S. Weekly versus 3-weekly paclitaxel in combination with carboplatin in advanced ovarian cancer: Which is the optimal adjuvant chemotherapy regimen? J. Gynecol. Oncol. 2018, 29, e96. [Google Scholar] [CrossRef]
- Takaya, H.; Nakai, H.; Murakami, K.; Tobiume, T.; Suzuki, A.; Mandai, M.; Matsumura, N. Efficacy of weekly administration of paclitaxel and carboplatin for advanced ovarian cancer patients with poor performance status. Int. J. Clin. Oncol. 2018, 23, 698–706. [Google Scholar] [CrossRef]
- Marchetti, C.; De Felice, F.; Di Pinto, A.; D’Oria, O.; Aleksa, N.; Musella, A.; Palaia, I.; Muzii, L.; Tombolini, V.; Benedetti Panici, P. Dose-dense weekly chemotherapy in advanced ovarian cancer: An updated meta-analysis of randomized controlled trials. Crit. Rev. Oncol. Hematol. 2018, 125, 30–34. [Google Scholar] [CrossRef]
- Shibutani, T.; Nagao, S.; Suzuki, K.; Kaneda, M.; Yamamoto, K.; Jimi, T.; Yano, H.; Kitai, M.; Shiozaki, T.; Matsuoka, K.; et al. Dose-dense paclitaxel and carboplatin vs. conventional paclitaxel and carboplatin as neoadjuvant chemotherapy for advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer: A retrospective study. Int. J. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Huang, B.S.; Chang, W.H.; Wang, K.C.; Huang, N.; Guo, C.Y.; Chou, Y.J.; Huang, H.Y.; Chen, T.J.; Lee, W.L.; Wang, P.H. Endometriosis might be inversely associated with developing chronic kidney disease: A population-based cohort study in Taiwan. Int. J. Mol. Sci. 2016, 17, 1079. [Google Scholar] [CrossRef]
- Chang, W.H.; Horng, H.C.; Yeh, C.C.; Guo, C.Y.; Chou, Y.J.; Huang, N.; Huang, H.Y.; Chen, Y.J.; Lee, W.L.; Wang, P.H. Risks of female genital tract related cancers (gynecological cancers) or breast cancer in women with and without chronic kidney disease: A population-based cohort study in Taiwan. Medicine 2018, 97, e0157. [Google Scholar] [CrossRef]
- Fiseha, T.; Mengesha, T.; Girma, R.; Kebede, E.; Gebreweld, A. Estimation of renal function in adult outpatients with normal serum creatinine. BMC Res. Notes 2019, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- Schoen, M.W.; Basch, E.; Hudson, L.L.; Chung, A.E.; Mendoza, T.R.; Mitchell, S.A.; St Germain, D.; Baumgartner, P.; Sit, L.; Rogak, L.J.; et al. Software for administering the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events: Usability Study. JMIR Hum. Factors 2018, 5, e10070. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.E.; Shoenbill, K.; Mitchell, S.A.; Dueck, A.C.; Schrag, D.; Bruner, D.W.; Minasian, L.M.; St Germain, D.; O’Mara, A.M.; Baumgartner, P.; et al. Patient free text reporting of symptomatic adverse events in cancer clinical research using the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). J. Am. Med. Inform. Assoc. 2019, 26, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, A.; Randon, G.; Sepe, P.; Claps, M.; Verzoni, E.; de Braud, F.; Procopio, G. The evaluation of response to immunotherapy in metastatic renal cell carcinoma: Open challenges in the clinical practice. Int. J. Mol. Sci. 2019, 20, 4263. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Jiang, R.; Pu, H.; Yang, H.; Tu, D.; Dai, Z.; Cai, Y.; Zhang, Y.; Cheng, X.; Jia, H.; et al. Survival benefits of dose-dense early postoperative intraperitoneal chemotherapy in front-line therapy for advanced ovarian cancer: A randomised controlled study. Br. J. Cancer 2019, 121, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Suidan, R.S.; He, W.; Sun, C.C.; Zhao, H.; Rauh-Hain, J.A.; Fleming, N.D.; Lu, K.H.; Giordano, S.H.; Meyer, L.A. Total and out-of-pocket costs of different primary management strategies in ovarian cancer. Am. J. Obstet. Gynecol. 2019, 221, e1–e136. [Google Scholar] [CrossRef]
- Chelariu-Raicu, A.; Coleman, R.L.; Sood, A.K. Anti-angiogenesis therapy in ovarian cancer: Which patient is it most likely to benefit? Oncology 2019, 33, 629378. [Google Scholar]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Berek, J.S.; Chen, L.M.; Cristea, M.; DeRosa, M.; et al. NCCN guidelines insights: Ovarian cancer, version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 896–909. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. ICON7 trial investigators. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- du Bois, A.; Kristensen, G.; Ray-Coquard, I.; Reuss, A.; Pignata, S.; Colombo, N.; Denison, U.; Vergote, I.; Del Campo, J.M.; Ottevanger, P.; et al. AGO Study Group led Gynecologic Cancer Intergroup/European Network of Gynaecologic Oncology Trials Groups Intergroup Consortium. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016, 17, 78–89. [Google Scholar] [PubMed]
- du Bois, A.; Floquet, A.; Kim, J.W.; Rau, J.; del Campo, J.M.; Friedlander, M.; Pignata, S.; Fujiwara, K.; Vergote, I.; Colombo, N.; et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J. Clin. Oncol. 2014, 32, 3374–3382. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Karihtala, P.; Moschetta, M.; Karathanasi, A.; Sadauskaite, A.; Rassy, E.; Pavlidis, N. Combined strategies with poly (ADP-Ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer: A literature review. Diagnostics 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Beaver, J.A.; Coleman, R.L.; Arend, R.C.; Armstrong, D.K.; Bala, S.; Mills, G.B.; Sood, A.K.; Herzog, T.J. Advancing drug development in gynecologic malignancies. Clin. Cancer Res. 2019, 25, 4874–4880. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 32) |
|---|---|
| Age at diagnosis (years) | 57 (33–79) |
| Age > 60 years | 11 (34.4%) |
| FIGO stage | |
| IIIC | 26 (81.3%) |
| IV | 6 (18.8%) |
| Cancer type | |
| Ovarian | 26 (81.3%) |
| Peritoneum | 4 (12.5%) |
| Fallopian tube | 2 (6.3%) |
| Histology | |
| High-grade serous | 18 (56.3%) |
| Mucinous | 1 (3.1%) |
| Clear cell | 3 (9.4%) |
| Endometrioid | 8 (25%) |
| Others (mixed high-grade serous) | 2 (6.3%) |
| Size of residual tumor | |
| ≤1 cm | 20 (62.5%) |
| >1 cm | 12 (37.5%) |
| Site of residual tumor | |
| Lower abdomen | 12 (37.5%) |
| Upper abdomen | 11 (34.4%) |
| Whole abdomen | 9 (28.1%) |
| Time of treatment | |
| 18 weeks | 18 (56.3%) |
| 18–21 weeks | 11 (34.4%) |
| 21–24 weeks | 0 (0) |
| >24 weeks | 3 (9.4%) |
| ECOG | |
| 0–1 | 30 (93.8%) |
| 2–3 | 2 (6.3%) |
| Characteristic | Number (%) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval (Cl)) | p-Value | Odds Ratio (95% Cl) | p-Value | ||
| Age | |||||
| ≤60 years | 21 (65.6) | Reference | |||
| >60 years | 11 (34.4) | 1.6 (0.37–6.95) | 0.53 | ||
| FIGO stage | |||||
| IIIC | 26 (81.3) | Reference | |||
| IV | 6 (18.8) | 0.5 (0.08–3.22) | 0.466 | ||
| Histology | |||||
| Serous/others | 20 (62.5) | Reference | Reference | ||
| Mucinous/clear cell | 4 (12.5) | 0.67 (0.08–5.75) | 0.712 | 0.41 (0.03–5.25) | 0.494 |
| Endometrioid | 8 (25) | 0.1 (0.01–0.93) | 0.043 | 0.11 (0.01–1.35) | 0.085 |
| Size of residual tumor | |||||
| ≤1 cm | 20 (62.5) | Reference | Reference | ||
| >1 cm | 12 (37.5) | 3.71 (0.82–16.84) | 0.089 | 11.6 (1.07–125.92) | 0.044 |
| Site of residual tumor | |||||
| Lower abdomen | 12 (37.5) | Reference | |||
| Upper abdomen | 11 (34.4) | 1.67 (0.31–9.01) | 0.553 | ||
| Whole abdomen | 9 (28.1) | 4 (0.64–25.02) | 0.138 | ||
| Time of treatment | |||||
| 18 weeks | 18 (56.3) | Reference | Reference | ||
| 18–21 weeks | 11 (34.4) | 2.75 (0.58–12.98) | 0.201 | 5.17 (0.63–42.45) | 0.126 |
| >24 weeks | 3 (9.4) | 0.79 (0.06–10.38) | 0.855 | 0.10 (0.004–2.26) | 0.146 |
| ECOG | |||||
| 0–1 | 30 (93.8) | Reference | |||
| 2–3 | 2 (6.3) | 1.14 (0.07–20.02) | 0.927 | ||
| Events | Any Grade, n (%) | Grade 1/2, n (%) | Grade 3/4, n (%) |
|---|---|---|---|
| Neutropenia | 16 (50) | 9 (28.1) | 7 (21.9) |
| Anemia | 31 (96.9) | 29 (90.6) | 2 (6.3) |
| Thrombocytopenia | 5 (15.6) | 5 (15.6) | 0 |
| Renal toxicity | 3 (9.4%) | 3 (9.4) | 0 |
| Proteinuria | 6 (18.8) | 6 (18.8) | 0 |
| Peripheral neuropathy | 9 (28.1) | 9 (28.1) | 0 |
| Nausea | 11 (34.4) | 11 (34.4) | 0 |
| Authors | Population | n | Regimen (Intravenous) | Median PFS | Median OS | Wbc | Plt | Rbc | SN | V |
|---|---|---|---|---|---|---|---|---|---|---|
| Katsumata et al. [39] | EOC FIGO II–IV | 312 | P 80mg/m2 (D1,8,15), C AUC 6 (D1) | 28.2 months | 100.5 months | 92% | 44% | 69% | 7% | 3% |
| Chan et al. [41] | EOC FIGO II–IV | 340 | P 80mg/m2 (D1,8,15), C AUC 6 (D1), optional bevacizumab 15mg/kg (D1) | 14.7 months | - | 72% | 20% | 36% | 3% | 6% |
| 55 | P 80mg/m2 (D1,8,15), C AUC 6 (D1), without bevacizumab | 14.2 months | - | - | - | - | - | - | ||
| Walker et al. [42] | EOC FIGO II–IV | 521 | P 80mg/m2 (D1,8,15), C AUC 6 (D1), bevacizumab 15mg/kg (D1) | 24.9 months | 75.5 months | 72% | 18% | 27% | 6% | 5% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.; Lee, H.H.; Chang, W.-H.; Lee, N.-R.; Huang, H.-Y.; Chen, Y.-J.; Horng, H.-C.; Lee, W.-L.; Wang, P.-H. Weekly Dose-Dense Paclitaxel and Triweekly Low-Dose Cisplatin: A Well-Tolerated and Effective Chemotherapeutic Regimen for First-Line Treatment of Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer. Int. J. Environ. Res. Public Health 2019, 16, 4794. https://doi.org/10.3390/ijerph16234794
Cheng M, Lee HH, Chang W-H, Lee N-R, Huang H-Y, Chen Y-J, Horng H-C, Lee W-L, Wang P-H. Weekly Dose-Dense Paclitaxel and Triweekly Low-Dose Cisplatin: A Well-Tolerated and Effective Chemotherapeutic Regimen for First-Line Treatment of Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer. International Journal of Environmental Research and Public Health. 2019; 16(23):4794. https://doi.org/10.3390/ijerph16234794
Chicago/Turabian StyleCheng, Min, Howard Hao Lee, Wen-Hsun Chang, Na-Rong Lee, Hsin-Yi Huang, Yi-Jen Chen, Huann-Cheng Horng, Wen-Ling Lee, and Peng-Hui Wang. 2019. "Weekly Dose-Dense Paclitaxel and Triweekly Low-Dose Cisplatin: A Well-Tolerated and Effective Chemotherapeutic Regimen for First-Line Treatment of Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer" International Journal of Environmental Research and Public Health 16, no. 23: 4794. https://doi.org/10.3390/ijerph16234794
APA StyleCheng, M., Lee, H. H., Chang, W.-H., Lee, N.-R., Huang, H.-Y., Chen, Y.-J., Horng, H.-C., Lee, W.-L., & Wang, P.-H. (2019). Weekly Dose-Dense Paclitaxel and Triweekly Low-Dose Cisplatin: A Well-Tolerated and Effective Chemotherapeutic Regimen for First-Line Treatment of Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer. International Journal of Environmental Research and Public Health, 16(23), 4794. https://doi.org/10.3390/ijerph16234794






