Disinfectant Activity of A Portable Ultraviolet C Equipment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Microbial Species and Materials Tested
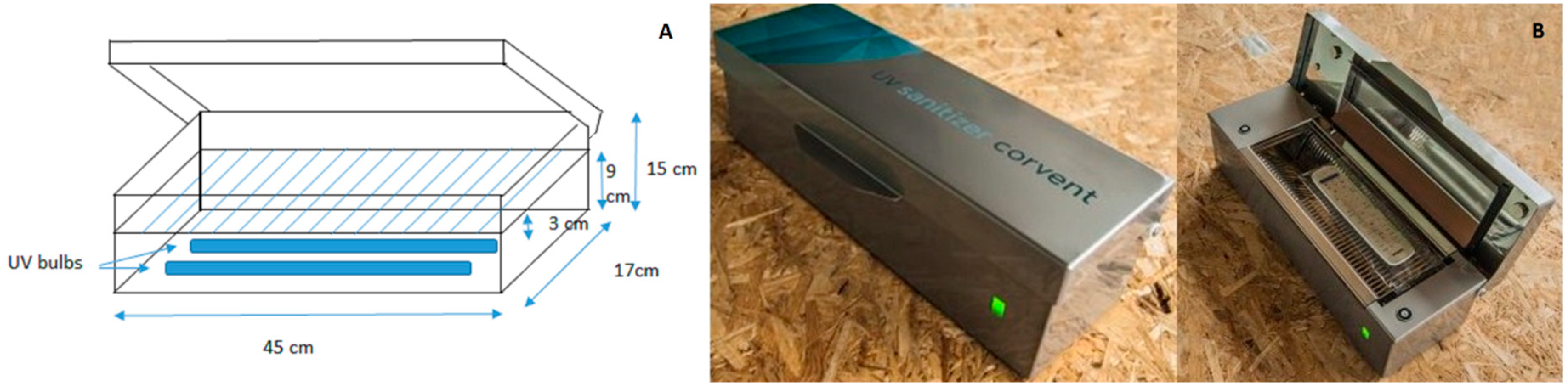
2.3. Disinfection Methods
2.4. Microbial Inoculum Preparation
2.5. Assessment of Disinfectant Capacity on Glassware Slides
2.6. Assessment of the Disinfectant Capacity on Discs of Different Materials
2.7. Colony Counting and Data Analysis
3. Results
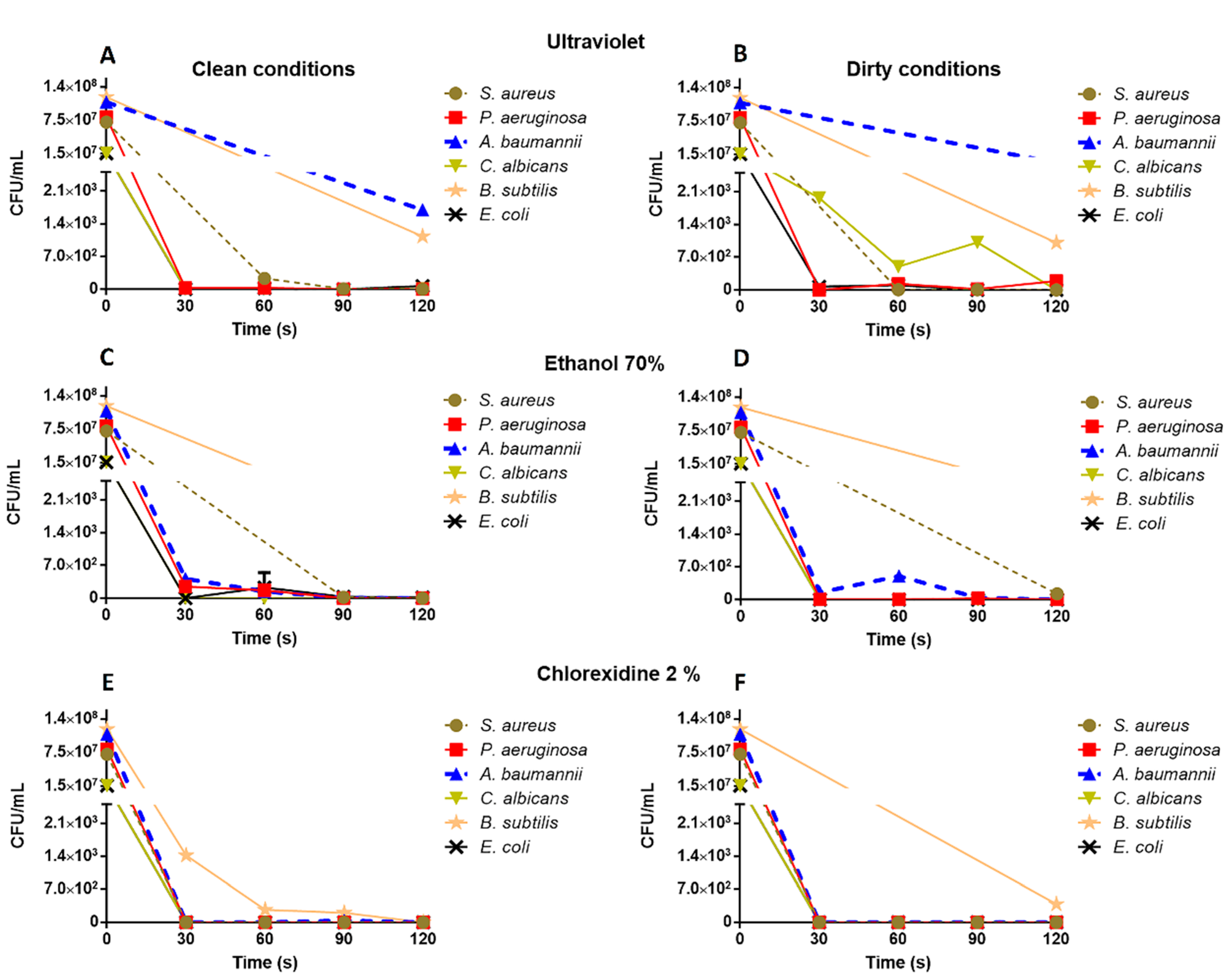
3.1. Assessment of the Disinfectant Capacity on Glassware Slides
3.2. Assessment of the Disinfectant Capacity on Different Materials
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haque, M.; Sartelli, M.; McKimm, J.; Bakar, M.A. Health care-associated infections—An overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Report on the Burden of Endemic Healthcare-Associated Infection Worldwide; WHO: Geneva, Switzerland, 2011; pp. 1–34. [Google Scholar]
- FitzGerald, G.; Moore, G.; Wilson, A.P. Hand hygiene after touching a patient’s surroundings: The opportunities most commonly missed. J. Hosp. Infect. 2013, 84, 27–31. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A. Self-disinfecting surfaces: Review of current methodologies and future prospects. Am. J. Infect. Control. 2013, 41, 31–35. [Google Scholar] [CrossRef]
- Saka, K.H.; Akanbi, A.A.; Obasa, T.O.; Raheem, R.A.; Oshodi, A.J.; Kalgo, Z.M. Pathogenic aerobic bacterial contaminants on non-critical hospital surfaces within paediatric ward of a nigerian hospital. J. Med. Microb. Diagn. 2016, 5, 241. [Google Scholar] [CrossRef]
- Russotto, V.; Cortegiani, A.; Raineri, S.M.; Giarratano, A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J. Intensive Care 2015, 3, 54. [Google Scholar] [CrossRef]
- Brady, R.R.; Hunt, A.C.; Visvanathan, A.; Rodrigues, M.A.; Graham, C.; Rae, C.; Gibb, A.P. Mobile phone technology and hospitalized patients: A cross-sectional surveillance study of bacterial colonization, and patient opinions and behaviours. Clin. Microbiol. Infect. 2011, 17, 830–835. [Google Scholar] [CrossRef]
- Tagoe, D.N.A.; Baidoo, S.E.; Dadzie, I.; Tengey, D.; Agede, C. Potential sources of transmission of hospital acquired infections in the volta regional hospital in Ghana. Ghana Med. J. 2011, 45. [Google Scholar] [CrossRef]
- Ustun, C.; Cihangiroglu, M. Health care workers mobile phones: A potential cause of microbial cross-contamination between hospitals and community. J. Occup. Environ. Hyg. 2012, 9, 538–542. [Google Scholar] [CrossRef]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J.; Healthcare Infection Control Practices Advisory Committee. Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html (accessed on 2 October 2019).
- Musuuza, J.S.; Guru, P.K.; O’Horo, J.C.; Bongiorno, C.M.; Korobkin, M.A.; Gangnon, R.E.; Safdar, N. The impact of chlorhexidine bathing on hospital-acquired bloodstream infections: A systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 416. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Russell, A.D. Biocide use and antibiotic resistance: The relevance of laboratory findings to clinical and environmental situations. Lancet. Infect. Dis. 2003, 3, 794–803. [Google Scholar] [CrossRef]
- Bolton, J.R.; Cotton, C.A. The Ultraviolet Disinfection Handbook; American Water Works Association (AWWA): Denver, CO, USA, 2008; pp. 25–26. [Google Scholar]
- EN 14561:2006: Chemical Disinfectants and Antiseptics. Quantitative Carrier Test for the Evaluation of Bactericidal Activity for Instruments Used in the Medical Area. Test Method and Requirements (Phase 2, Step 2). Brussels: CEN-European Committee for Standardization. Available online: https://www.en.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0038776 (accessed on 17 October 2019).
- EN 14562:2006: Chemical Disinfectants and Antiseptics. Quantitative Carrier Test for the Evaluation of Fungicidal or Yeasticidal Activity for Instruments Used in the Medical Area. Test Method and Requirements (Phase 2, Step 2). Brussels: CEN–European Committee for Standardization. Available online: https://www.en.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0038777 (accessed on 17 October 2019).
- Gold, N.A.; Avva, U. Alcohol Sanitizer. In StatPearls [Internet]; Treasure Island; StatPearls Publishing: St. Petersburg, FL, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513254/ (accessed on 29 May 2019).
- Graziano, M.U.; Graziano, K.U.; Pinto, F.M.; Bruna, C.Q.; de Souza, R.Q.; Lascala, C.A. Effectiveness of disinfection with alcohol 70% (w/v) of contaminated surfaces not previously cleaned. Rev. Lat. Am. Enfermagem. 2013, 21, 618–623. [Google Scholar] [CrossRef]
- Rutala, W.A.; Gergen, M.F.; Weber, D.J. Room decontamination with UV radiation. Infect. Control Hosp. Epidemiol. 2010, 31, 1025–1029. [Google Scholar] [CrossRef]
- Setlow, P. Resistance of spores of Bacillus species to ultraviolet light. Environ. Mol. Mutagen. 2001, 38, 97–104. [Google Scholar] [CrossRef]
- Thomas, P. Isolation of an ethanol-tolerant endospore-forming Gram-negative Brevibacillus spp. as a covert contaminant in grape tissue cultures. J. Appl. Microbiol. 2006, 101, 764–774. [Google Scholar] [CrossRef]
- Koscova, J.; Hurnikova, Z.; Pistl, J. Degree of bacterial contamination of mobile phone and computer keyboard surfaces and efficacy of disinfection with chlorhexidine digluconate and triclosan to its reduction. J. Environ. Res. Public Health. 2018, 15, 2238. [Google Scholar] [CrossRef]
- Kampf, G. Acquired resistance to chlorhexidine - is it time to establish an ‘antiseptic stewardship’ initiative? J. Hosp. Infect. 2016, 94, 213–227. [Google Scholar] [CrossRef]
- Longtin, J.; Seah, C.; Siebert, K.; McGeer, A.; Simor, A.; Longtin, Y.; Low, D.E.; Melano, R.G. Distribution of antiseptic resistance genes qacA, qacB, and smr in methicillin-resistant Staphylococcus aureus isolated in Toronto, Canada, from 2005 to 2009. Antimicrob. Agents Chemother. 2011, 55, 2999–3001. [Google Scholar] [CrossRef]
- Warren, D.K.; Prager, M.; Munigala, S.; Wallace, M.A.; Kennedy, C.R.; Bommarito, K.M.; Bommarito, K.M.; Mazuski, J.E.; Burnham, C.A. Prevalence of qacA/B genes and mupirocin resistance among methicillin-resistant Staphylococcus aureus (MRSA) isolates in the setting of chlorhexidine bathing without mupirocin. Infect. Control Hosp. Epidemiol. 2016, 37, 590–597. [Google Scholar] [CrossRef]
- Climo, M.W.; Yokoe, D.S.; Warren, D.K.; Perl, T.M.; Bolon, M.; Herwaldt, L.A.; Weinstein, R.A.; Sepkowitz, K.A.; Jernigan, J.A.; Sanogo, K.; et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N. Engl. J. Med. 2013, 368, 533–542. [Google Scholar] [CrossRef]
- Yang, J.H.; Wu, U.I.; Tai, H.M.; Sheng, W.H. Effectiveness of an ultraviolet-C disinfection system for reduction of healthcare-associated pathogens. J. Microbiol. Immunol. Infect. 2019, 52, 487–493. [Google Scholar] [CrossRef]
- Umezawa, K.; Asai, S.; Inokuchi, S.; Miyachi, H. A comparative study of the bactericidal activity and daily disinfection housekeeping surfaces by a new portable pulsed UV radiation device. Curr. Microbiol. 2012, 64, 581–587. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Sousa, C.; Teixeira, P.; Oliveira, R. Influence of surface properties on the adhesion of Staphylococcus epidermidis to acrylic and silicone. Int. J. Biomater. 2009, 718017. [Google Scholar] [CrossRef]
- De-la-Pinta, I.; Cobos, M.; Ibarretxe, J.; Montoya, E.; Eraso, E.; Guraya, T.; Quindós, G. Effect of biomaterials hydrophobicity and roughness on biofilm development. J. Mater. Sci. Mater. Med. 2019, 30, 77. [Google Scholar] [CrossRef]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface topographical factors influencing bacterial attachment. Adv. Colloid Interface Sci. 2012, 179–182, 142–149. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Yoda, I.; Koseki, H.; Tomita, M.; Shida, T.; Horiuchi, H.; Sakoda, H.; Osaki, M. Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiol. 2014, 14, 234. [Google Scholar] [CrossRef]
| Strain | Initial Inoculum (CFU/mL) | Disinfection Conditions | Materials | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Borosilicate | Polycarbonate | Polyurethane | Silicone | Teflon | Titanium | |||||
| P. aeruginosa CECT 108 | 1.7 × 108 | Clean | UVSC | 30″ | 4.6 × 103 ± 41.7 | ≥ | ≥ | ≥ | 4.2 × 103 ± 141.4 | ≥ |
| 120″ | ≤0.5 | 3.3 × 102 ± 128 | 5.5 × 103 ± 50.3 | 1.6 × 103 ± 136.6 | 6.5 × 103 ± 40 | 3.0 × 101 ± 38.3 | ||||
| Ethanol | 30″ | 4.5 × 101 ± 50 | 0.5 × 101 ± 10 | ≥ | 3.0 × 102 ± 31 | 4.5 × 102 ± 137.9 | 1.4 × 102 ± 16.3 | |||
| 120″ | ≤0.5 | 0.5 × 101 ± 10 | 1.5 × 103 ± 244.9 | 3.0 × 101 ± 11.5 | 7.5 × 101 ± 34.1 | ≤0.5 | ||||
| Chlorhexidine | 30″ | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | |||
| 120″ | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ||||
| Dirty | UVSC | 30″ | 5.4 × 102 ± 80 | 3.5 × 103 ± 200.1 | ≥ | ≥ | ≥ | 5.1 × 103 ± 50.4 | ||
| 120″ | ≤0.5 | 6.0 × 101 ± 95.2 | 5.8 × 103 ± 81.6 | ≥ | 3.8 × 103 ± 141.4 | 3.3 × 102 ± 40.8 | ||||
| Ethanol | 30″ | 4.9 × 103 ± 210.2 | 3.6 × 102 ± 91.4 | 4.9 × 103 ± 206.1 | 4.9 × 103 ± 290.6 | ≥ | 1.3 × 103 ± 200.0 | |||
| 120″ | 0.5 × 101 ± 10 | 0.5 × 101 ± 10 | ≥ | 6.5 × 103 ± 150.2 | 3.2 × 102 ± 162.4 | 1.0 × 101 ± 11.5 | ||||
| Chlorhexidine | 30″ | ≤0.5 | ≤0.5 | 2.5 × 101 ± 50 | ≤0.5 | ≤0.5 | ≤0.5 | |||
| 120″ | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ||||
| S. aureus CECT 435 | 1.2 × 108 | Clean | UVSC | 30″ | ≥ | ≥ | ≥ | ≥ | ≥ | ≥ |
| 120″ | 2.8 × 102 ± 78.3 | ≥ | ≥ | 6.0 × 102 ± 66.5 | ≤0.5 | 5.1 × 102 ± 91.4 | ||||
| Ethanol | 30″ | 6.3 × 103 ± 76.7 | ≥ | 3.8 × 103 ± 50.3 | ≥ | ≥ | 3.9 × 103 ± 180.3 | |||
| 120″ | ≤0.5 | ≥ | 1.5 × 101 ± 19.1 | 5.5 × 102 ± 92.9 | 2.6 × 103 ± 91.0 | ≤0.5 | ||||
| Chlorhexidine | 30″ | ≤0.5 | 1 × 101 ± 20 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | |||
| 120″ | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ||||
| Dirty | UVSC | 30″ | ≥ | ≥ | ≥ | ≥ | ≥ | ≥ | ||
| 120″ | 9.5 × 102 ± 17 | ≥ | ≥ | ≥ | 3.7 × 102 ± 26.7 | 3.7 × 102 ± 26.7 | ||||
| Ethanol | 30″ | ≤0.5 | ≥ | ≥ | ≥ | ≥ | ≥ | |||
| 120″ | ≤0.5 | ≥ | 9.8 × 102 ± 42.4 | ≥ | ≥ | ≤0.5 | ||||
| Chlorhexidine | 30″ | ≤0.5 | 0.5 × 101 ± 10 | 4.5 × 101 ± 10 | ≤0.5 | ≤0.5 | ≤0.5 | |||
| 120″ | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ||||
| C. albicans MYA-2876 | 1.4 × 107 | Clean | UVSC | 30″ | 3.5 × 102 ± 131 | 9.9 × 102 ± 158.7 | ≥ | 4.5 × 103 ± 120.7 | 5.0 × 103 ± 170.0 | 3.1 × 103 ± 140.4 |
| 120″ | ≤0.5 | 1.3 × 102 ± 44.3 | 2.8 × 103 ± 150.5 | 5.0 × 102 ± 48.9 | 2.5 × 101 ± 5.7 | 0.5 × 101 ± 10 | ||||
| Ethanol | 30″ | ≥ | 3.5 × 101 ± 30 | ≤0.5 | 2.8 × 102 ± 50 | 2.8 × 103 ± 228.2 | 7.5 × 101 ± 19.1 | |||
| 120″ | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | 0.5 × 101 ± 10 | ≤0.5 | ||||
| Chlorhexidine | 30″ | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | |||
| 120″ | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ||||
| Dirty | UVSC | 30″ | 2.0 × 101 ± 9.1 | 2.0 × 102 ± 82.2 | ≥ | ≥ | ≥ | 1.3 × 103 ± 98.9 | ||
| 120″ | ≤0.5 | 1.1 × 102 ± 10 | 3.8 × 103 ± 280.5 | 5.7 × 103 ± 90.8 | 1.3 × 102 ± 5 | 7.0 × 101 ± 25.8 | ||||
| Ethanol | 30″ | 4.4 × 103 ± 140.0 | 6.4 × 103 ± 136.6 | ≥ | ≥ | ≥ | 2.6 × 103 ± 160.7 | |||
| 120″ | ≤0.5 | ≤0.5 | ≤0.5 | 3.5 × 101 ± 10 | 4.1 × 102 ± 131.2 | ≤0.5 | ||||
| Chlorhexidine | 30″ | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | |||
| 120″ | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guridi, A.; Sevillano, E.; de la Fuente, I.; Mateo, E.; Eraso, E.; Quindós, G. Disinfectant Activity of A Portable Ultraviolet C Equipment. Int. J. Environ. Res. Public Health 2019, 16, 4747. https://doi.org/10.3390/ijerph16234747
Guridi A, Sevillano E, de la Fuente I, Mateo E, Eraso E, Quindós G. Disinfectant Activity of A Portable Ultraviolet C Equipment. International Journal of Environmental Research and Public Health. 2019; 16(23):4747. https://doi.org/10.3390/ijerph16234747
Chicago/Turabian StyleGuridi, Andrea, Elena Sevillano, Iñigo de la Fuente, Estibaliz Mateo, Elena Eraso, and Guillermo Quindós. 2019. "Disinfectant Activity of A Portable Ultraviolet C Equipment" International Journal of Environmental Research and Public Health 16, no. 23: 4747. https://doi.org/10.3390/ijerph16234747
APA StyleGuridi, A., Sevillano, E., de la Fuente, I., Mateo, E., Eraso, E., & Quindós, G. (2019). Disinfectant Activity of A Portable Ultraviolet C Equipment. International Journal of Environmental Research and Public Health, 16(23), 4747. https://doi.org/10.3390/ijerph16234747






