Self-Reported Sleep Quality Using the Malay Version of the Pittsburgh Sleep Quality Index (PSQI-M) In Malaysian Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Translation Procedures
2.2. Pilot Study
2.3. Participants
2.4. Pittsburgh Sleep Quality Index Malay Version (PSQI-M)
2.5. Epworth Sleepiness Scale Malay Version (ESS-M)
2.6. Statistical Analyses
3. Results
3.1. Internal Consistency of PSQI-M
3.2. Test Retest Reliability
3.3. Bland–Altman Plot of the Mean PSQI Global Score
3.4. Convergent Validity
3.5. Subject Characteristics, Sleep Quality, and Daytime Sleepiness
3.6. Sleep Quality and Daytime Sleepiness According to Age Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef]
- Krystal, A.D.; Edinger, J.D. Measuring sleep quality. Sleep Med. 2008, 9, S10–S17. [Google Scholar] [CrossRef]
- Ohayon, M.; Wickwire, E.M.; Hirshkowitz, M.; Albert, M.S.; Avida, A.; Daly, F.J.; Dauvilliers, Y.; Ferri, R.; Fung, C.; Gozal, D.; et al. National Sleep Foundation’s sleep quality recommendations: First report. Sleep Health 2017, 3, 6–19. [Google Scholar] [CrossRef]
- Reutrakul, S.; Van Cauter, E. Sleep Influences on Obesity, Insulin Resistance, and Risk of Type 2 Diabetes. Metabolism 2018, 84, 56–66. [Google Scholar] [CrossRef]
- Anothaisintawee, T.; Reutrakul, S.; Van Cauter, E.; Thakkinstian, A. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med. Rev. 2016, 30, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Gadie, A.; Shafto, M.; Leng, Y.; Kievit, R.A. How are age-related differences in sleep quality associated with health outcomes? An epidemiological investigation in a UK cohort of 2406 adults. BMJ Open 2017, 7, e014920. [Google Scholar] [CrossRef]
- Pistollato, F.; Cano, S.S.; Elio, I.; Vergara, M.M.; Giampieri, F.; Battino, M. Associations between sleep, cortisol regulation, and diet: Possible implications for the risk of Alzheimer disease. Adv. Nutr. 2016, 7, 679–689. [Google Scholar] [CrossRef]
- Angarita, G.A.; Emadi, N.; Hodges, S.; Morgan, P.T. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: A comprehensive review. Addict. Sci. Clin. Pract. 2016, 11, 9. [Google Scholar] [CrossRef]
- Tromp, M.D.; Donners, A.A.; Garssen, J.; Verster, J.C. Sleep, eating disorder symptoms, and daytime functioning. Nat. Sci. Sleep 2016, 8, 35–40. [Google Scholar]
- Slavish, D.C.; Taylor, D.J.; Lichstein, K.L. Intraindividual variability in sleep and comorbid medical and mental health conditions. Sleep 2019, 42, zsz052. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Firouzi, S.; Poh, B.K.; Ismail, M.N.; Sadeghilar, A. Sleep habits, food intake, and physical activity levels in normal and overweight and obese Malaysian children. Obes. Res. Clin. Pract. 2014, 8, e70–e78. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Gan, J.Y.; Liew, W.L.; Soe, H.H.K.; Nettem, S.; Nettemu, S.K. Association between quality of sleep and chronic periodontitis: A case–control study in Malaysian population. Dent. Res. J. 2019, 16, 29–35. [Google Scholar] [CrossRef]
- Lai, P.-P.; Say, Y.-H. Associated factors of sleep quality and behavior among students of two tertiary institutions in northern malaysia. Med. J. Malays. 2013, 68, 195–203. [Google Scholar]
- Siraj, H.; Salam, A.; Roslan, R.; Hasan, N.A.; Jin, T.H.; Othman, M.N. Sleep pattern and academic performance of undergraduate medical students at university Kebangsaan Malaysia. J. Appl. Pharm. Sci. 2014, 4, 52–55. [Google Scholar]
- Aazami, S.; Mozafari, M.; Shamsuddin, K.; Akmal, S. Work-family conflict and sleep disturbance: The Malaysian working women study. Ind. Health 2016, 54, 50–57. [Google Scholar] [CrossRef]
- Nazatul, S.; Saimy, I.; Moy, F.; Nabila, A. Prevalence of sleep disturbance among nurses in a Malaysian government hospital and its association with work characteristics. J. Univ. Malaya Med. Cent. 2008, 11, 66–71. [Google Scholar]
- Razali, R.; Ariffin, J.; Aziz, A.F.A.; Puteh, S.E.W.; Wahab, S.; Daud, T.I.M. Sleep quality and psychosocial correlates among elderly attendees of an urban primary care centre in Malaysia. Neurol. Asia 2016, 3, 265–273. [Google Scholar]
- Lim, Y.C.; Hoe, V.C.; Darus, A.; Bhoo-Pathy, N. Association between night-shift work, sleep quality and metabolic syndrome. Occup. Environ. Med. 2018, 75, 716–723. [Google Scholar] [CrossRef]
- Yunus, R.M.; Wazid, S.W.; Hairi, N.N.; Choo, W.Y.; Hairi, F.M.; Sooryanarayana, R.; Ahmad, S.N.; Razak, I.A.; Peramalah, D.; Aziz, S.A. Association between elder abuse and poor sleep: A cross-sectional study among rural older Malaysians. PLoS One 2017, 12, e0180222. [Google Scholar]
- Saddichha, S. Diagnosis and treatment of chronic insomnia. Ann. Indian Acad. Neurol. 2010, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D.; Aldrich, M.S.; Pickett, R.; Christian, G. Comparison of the results of the Epworth Sleepiness Scale and the multiple sleep latency test. J. Psychosom. Res. 1997, 42, 145–155. [Google Scholar] [CrossRef]
- Terwee, C.B.; Bot, S.D.; de Boer, M.R.; van der Windt, D.A.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Kotronoulas, G.C.; Papadopoulou, C.N.; Papapetrou, A.; Patiraki, E. Psychometric evaluation and feasibility of the Greek Pittsburgh Sleep Quality Index (GR-PSQI) in patients with cancer receiving chemotherapy. Support. Care Cancer 2011, 19, 1831–1840. [Google Scholar] [CrossRef]
- Farrahi Moghaddam, J.; Nakhaee, N.; Sheibani, V.; Garrusi, B.; Amirkafi, A. Reliability and validity of the persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep Breath. Schlaf Atm. 2012, 16, 79–82. [Google Scholar] [CrossRef]
- Fontes, F.; Gonçalves, M.; Maia, S.; Pereira, S.; Severo, M.; Lunet, N. Reliability and validity of the Pittsburgh Sleep Quality Index in breast cancer patients. Support. Care Cancer 2017, 25, 3059–3066. [Google Scholar] [CrossRef]
- Suleiman, K.H.; Yates, B.C.; Berger, A.M.; Pozehl, B.; Meza, J. Translating the Pittsburgh Sleep Quality Index into Arabic. West. J. Nurs. Res. 2010, 32, 250–268. [Google Scholar] [CrossRef]
- Sitasuwan, T.; Bussaratid, S.; Ruttanaumpawan, P.; Chotinaiwattarakul, W. Reliability and validity of the Thai version of the Pittsburgh Sleep Quality Index. J. Med. Assoc. Thail. 2014, 97, S57–S67. [Google Scholar]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Beaudreau, S.A.; Spira, A.P.; Stewart, A.; Kezirian, E.; Lui, L.-Y.; Ensrud, K.; Redline, S.; Ancoli-Israel, S.; Stone, K.L. Validation of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older black and white women. Sleep Med. 2012, 13, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: Methodology and discussion. Sleep 2015, 38, 1161–1183. [Google Scholar] [PubMed]
- Jurado-Fasoli, L.; Amaro-Gahete, F.; De-la-O, A.; Dote-Montero, M.; Gutiérrez, Á.; Castillo, M. Association between sleep quality and body composition in sedentary middle-aged adults. Medicina 2018, 54, 91. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yu, Y.; Yuan, J.; Li, C.; Liu, T.; Lin, D.; Lau, A.; Zhong, C.; Xu, T.; Shan, G. Sleep duration and quality among different occupations-China national study. PLoS ONE 2015, 10, e0117700. [Google Scholar] [CrossRef] [PubMed]
- Smagula, S.F.; Stone, K.L.; Fabio, A.; Cauley, J.A. Risk factors for sleep disturbances in older adults: Evidence from prospective studies. Sleep Med. Rev. 2016, 25, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Linton, S.J.; Kecklund, G.; Franklin, K.A.; Leissner, L.C.; Sivertsen, B.; Lindberg, E.; Svensson, A.C.; Hansson, S.O.; Sundin, Ö.; Hetta, J.; et al. The effect of the work environment on future sleep disturbances: A systematic review. Sleep Med. Rev. 2015, 23, 10–19. [Google Scholar] [CrossRef]
- Luckhaupt, S.E.; Tak, S.; Calvert, G.M. The prevalence of short sleep duration by industry and occupation in the national health interview survey. Sleep 2010, 33, 149–159. [Google Scholar] [CrossRef]
- Connelly, L.M. Pilot studies. Medsurg Nurs. 2008, 17, 411–412. [Google Scholar]
- Hill, R. What sample size is “enough” in internet survey research? Interpers. Comput. Technol. 1998, 6, 3–4. [Google Scholar]
- Taber, K.S. The use of cronbach’s alpha when developing and reporting research instruments in science education. Res. Sci. Educ. 2018, 48, 1273–1296. [Google Scholar] [CrossRef]
- Yurdugül, H. Minimum sample size for Cronbach’s coefficient alpha: A Monte-Carlo study. Hacet. Univ. J. Educ. 2008, 35, 1–9. [Google Scholar]

| PSQI Subcomponents | Test M ± SD | Retest M ± SD | ICC a | 95% CI | 95% LoA | SEM | |
|---|---|---|---|---|---|---|---|
| PSQI Global Score | 5.30 ± 1.85 | 5.36 ± 2.47 | 0.58 | 0.43–0.69 | −2.81–4.41 | 1.34 | |
| C1 | Sleep Quality | 0.89 ± 0.59 | 0.82 ± 0.60 | 0.59 | 0.45–0.70 | 0.08–0.50 | 0.38 |
| C2 | Sleep Latency | 0.72 ± 0.71 | 0.84 ± 0.76 | 0.54 | 0.40–0.66 | −0.42–0.56 | 0.50 |
| C3 | Sleep Duration | 1.35 ± 0.72 | 1.36 ± 0.78 | 0.62 | 0.50–0.73 | 0.45–1.31 | 0.46 |
| C4 | Sleep Efficiency | 0.49 ± 0.76 | 0.44 ± 0.81 | 0.53 | 0.38–0.65 | −0.05–1.10 | 0.54 |
| C5 | Sleep Disturbance | 1.19 ± 0.50 | 1.13 ± 0.48 | 0.45 | 0.29–0.59 | 0.00–0.52 | 0.36 |
| C7 | Daytime Dysfunction | 0.67 ± 0.53 | 0.66 ± 0.63 | 0.40 | 0.23–0.55 | 0.47–1.29 | 0.45 |
| PSQI Subcomponents | r | p |
|---|---|---|
| PSQI Global score | 0.37 | <0.001 |
| C1 Sleep Quality | 0.18 | 0.345 |
| C2 Sleep Latency | 0.13 | 0.385 |
| C3 Sleep Duration | 0.15 | 0.329 |
| C4 Sleep Efficiency | 0.06 | 0.463 |
| C5 Sleep Disturbance | 0.35 | 0.002 |
| C7 Daytime Dysfunction | 0.48 | 0.007 |
| Characteristics | n (%) | Mean ± SD | Minimum | Maximum |
|---|---|---|---|---|
| Gender | ||||
| Male | 049 (46) | |||
| Female | 057 (54) | |||
| Race | ||||
| Malay | 101 (95) | |||
| Chinese | 003 (3) | |||
| Indian | 002 (2) | |||
| Age (years) | 35.3 ± 7.6 | 21.0 | 57.0 | |
| Young Adult (21–39 years) | 083 (78) | 32.4 ± 4.3 | 21.0 | 39.0 |
| Middle-Aged (40–60 years) | 023 (22) | 48.5 ± 4.0 | 40.0 | 57.0 |
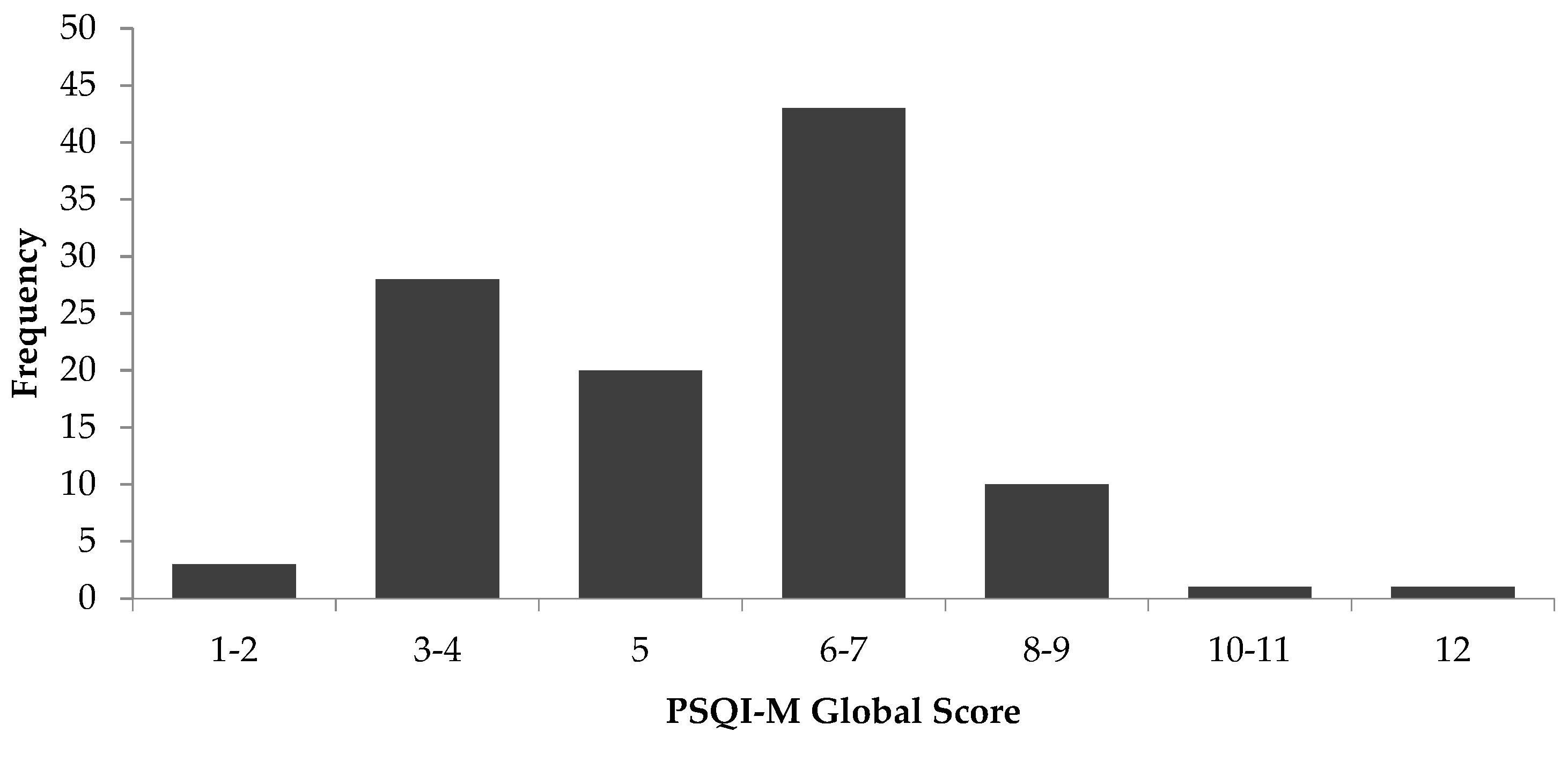
| PSQI Global Score | 5.25 ± 1.85 | 1 | 12 | |
| (C1) Sleep Quality | 0.89 ± 0.59 | 0 | 3 | |
| (C2) Sleep Latency | 0.72 ± 0.71 | 0 | 3 | |
| (C3) Sleep Duration | 1.87 ± 0.61 | 0 | 3 | |
| (C4) Sleep Efficiency | 0.49 ± 0.75 | 0 | 3 | |
| (C5) Sleep Disturbance | 1.19 ± 0.50 | 0 | 3 | |
| (C6) Sleep Medication | 0.00 ± 0.00 | 0 | 3 | |
| (C7) Daytime Dysfunction | 0.67 ± 0.53 | 0 | 2 | |
| Total Sleep Duration (hour) | 5.95 ± 1.05 | 4 | 9 | |
| ESS Total Score | 7.26 ± 3.73 | 0 | 19 |
| Domains | Mean ± SD | p Value | Cohen’s d | |
|---|---|---|---|---|
| Young Adult (21–39 years) | Middle-Aged (40–60 years) | |||
| Global PSQI Score | 4.4 ± 2.2 | 5.5 ± 2.2 | 0.06 | −0.18 |
| (C1) Sleep Quality | 0.7 ± 0.6 | 0.9 ± 0.6 | 0.13 | −0.15 |
| (C2) Sleep Latency | 1.0 ± 1.0 | 0.8 ± 0.7 | 0.36 | −0.09 |
| (C3) Sleep Duration | 0.9 ± 0.5 | 1.5 ± 0.8 | <0.001 | −0.33 |
| (C4) Sleep Efficiency | 0.4 ± 0.8 | 0.5 ± 0.8 | 0.52 | −0.06 |
| (C5) Sleep Disturbance | 1.0 ± 0.4 | 1.2 ± 0.5 | 0.04 | −0.20 |
| (C7) Daytime Dysfunction | 0.5± 0.5 | 0.7 ± 0.7 | 0.31 | −0.10 |
| ESS Overall Score | 5.6 ± 3.0 | 7.7 ± 3.8 | 0.02 | −0.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farah, N.M.; Saw Yee, T.; Mohd Rasdi, H.F. Self-Reported Sleep Quality Using the Malay Version of the Pittsburgh Sleep Quality Index (PSQI-M) In Malaysian Adults. Int. J. Environ. Res. Public Health 2019, 16, 4750. https://doi.org/10.3390/ijerph16234750
Farah NM, Saw Yee T, Mohd Rasdi HF. Self-Reported Sleep Quality Using the Malay Version of the Pittsburgh Sleep Quality Index (PSQI-M) In Malaysian Adults. International Journal of Environmental Research and Public Health. 2019; 16(23):4750. https://doi.org/10.3390/ijerph16234750
Chicago/Turabian StyleFarah, Nor MF, Teh Saw Yee, and Hanif Farhan Mohd Rasdi. 2019. "Self-Reported Sleep Quality Using the Malay Version of the Pittsburgh Sleep Quality Index (PSQI-M) In Malaysian Adults" International Journal of Environmental Research and Public Health 16, no. 23: 4750. https://doi.org/10.3390/ijerph16234750
APA StyleFarah, N. M., Saw Yee, T., & Mohd Rasdi, H. F. (2019). Self-Reported Sleep Quality Using the Malay Version of the Pittsburgh Sleep Quality Index (PSQI-M) In Malaysian Adults. International Journal of Environmental Research and Public Health, 16(23), 4750. https://doi.org/10.3390/ijerph16234750




