Impact of Fermentable Fibres on the Colonic Microbiota Metabolism of Dietary Polyphenols Rutin and Quercetin
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Methods
2.1.1. Faecal Bacterial Donors
2.1.2. Batch Culture Model of Human Colonic Fermentation
2.1.3. Phenolic Acid Quantification by Gas Chromatography Mass Spectrometry
2.1.4. SCFA Quantification by Gas Chromatography—FID (Flame Ionization Detector)
2.2. Statistical Analysis
3. Results
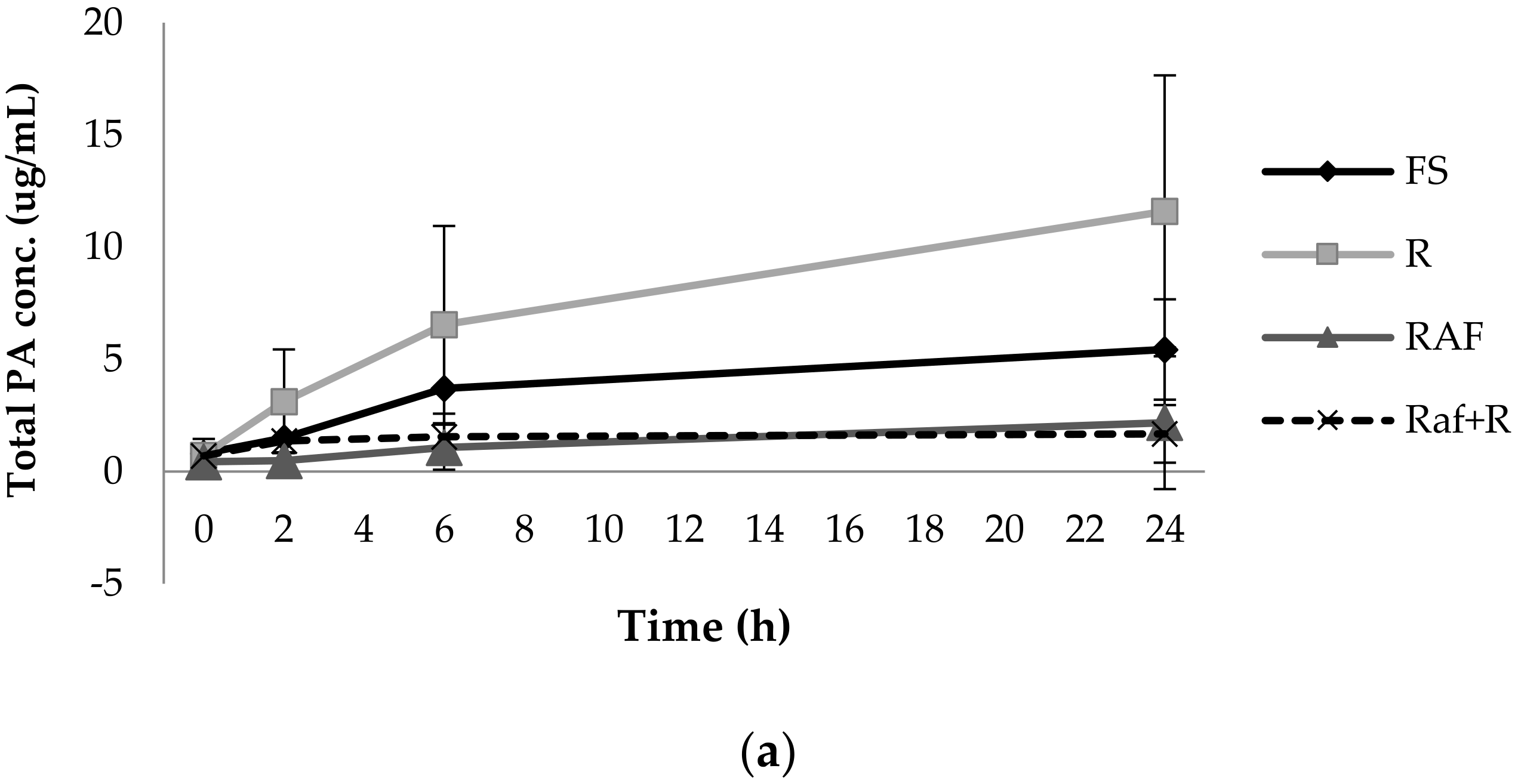
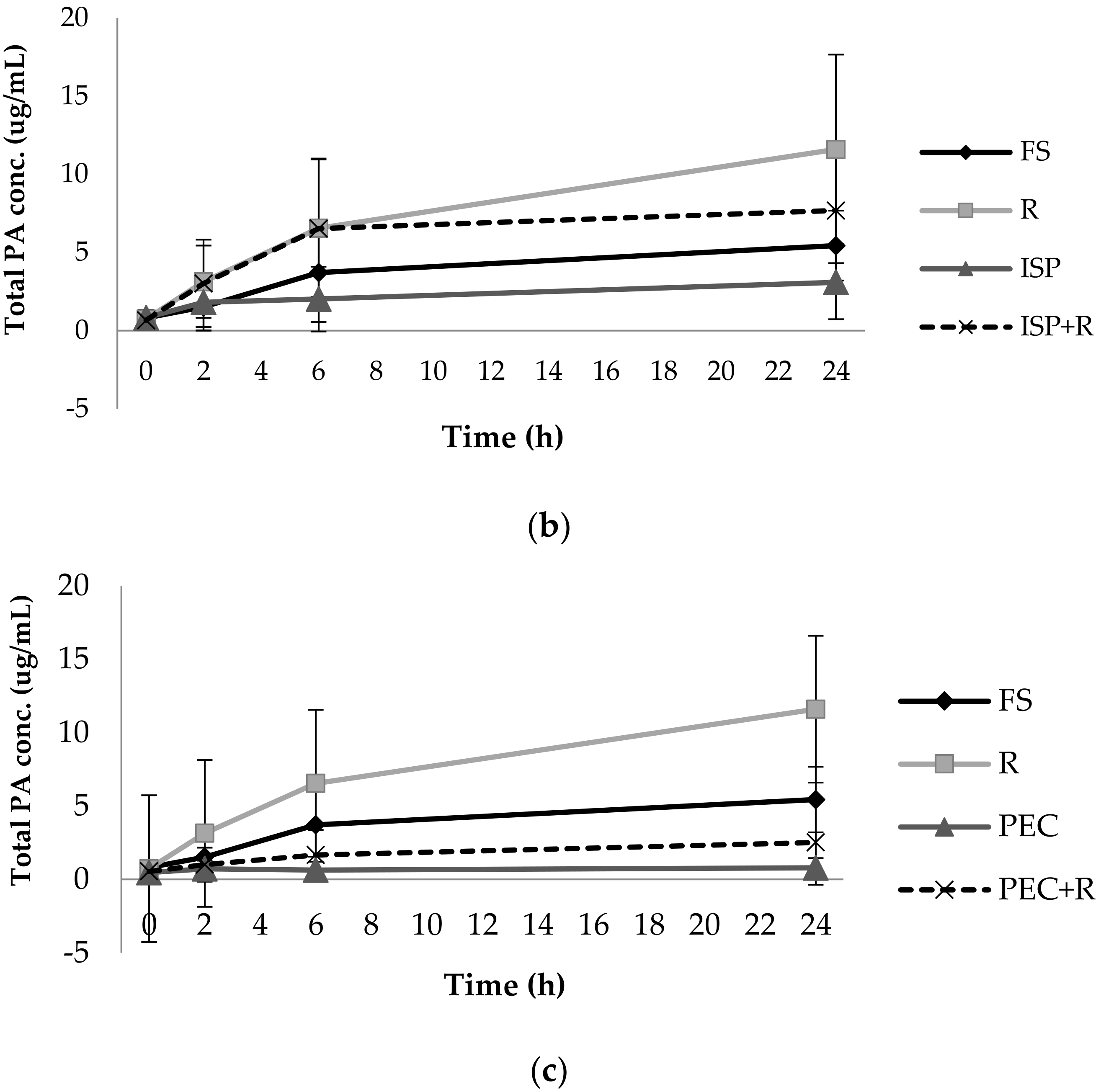
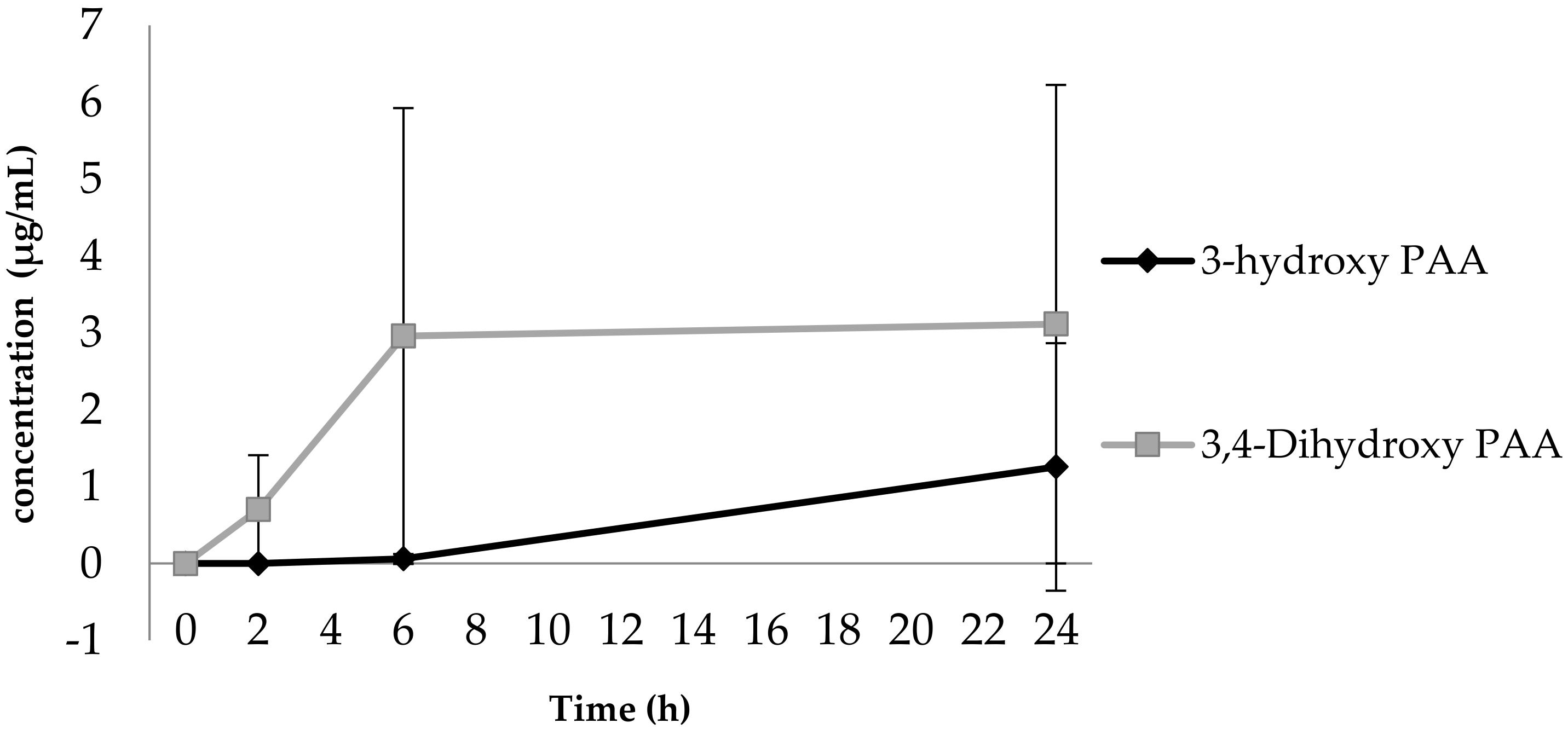
3.1. Phenolic Acid Production from Bacterial Catabolism of Rutin
3.2. Impact of Soluble Fibres on Phenolic Acid Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaganath, I.B.; Mullen, W.; Lean, M.E.; Edwards, C.A.; Crozier, A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic. Biol. Med. 2009, 47, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.; Dumont, J. Impact of flavonols on cardiometabolic biomarkers: A meta-analysis of randomized controlled human trials to explore the role of inter-individual variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Little, C.; Roxburgh, C.; Combet, E. The role of polyphenols in the development of colorectal cancer: A systematic review and meta-analysis of case-controlled studies. Proc. Nutr. Soc. 2014, 73, E13. [Google Scholar] [CrossRef]
- Graf, B.A.; Mullen, W.; Stewart, C.T.; Hartley, R.C.; Duthie, G.G.; Lean, M.E.; Crozier, A.; Edwards, C.A. Disposition and metabolism of [2-14C] quercetin-4′-glucoside in rats. Drug Metab. Dispos. 2005, 33, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jung, E.-A.; Sohng, I.-S.; Han, J.-A.; Kim, T.-H.; Han, M.J. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch. Pharm. Res. 1998, 21, 17–23. [Google Scholar] [CrossRef]
- Monagas, M.; Khan, N.; Andrés-Lacueva, C.; Urpí-Sardá, M.; Vázquez-Agell, M.; Lamuela-Raventós, R.M.; Estruch, R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br. J. Nutr. 2009, 102, 201–206. [Google Scholar] [CrossRef]
- Edwards, C.; Havlik, J.; Cong, W.; Mullen, W.; Preston, T.; Morrison, D.; Combet, E. Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr. Bull. 2017, 42, 356–360. [Google Scholar] [CrossRef]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef]
- Alkhaldy, A.; Edwards, C.; Combet, E. The effect of ageing on the colonic bacterial metabolism of dietary polyphenols. Proc. Nutr. Soc. 2012, 71, E60. [Google Scholar] [CrossRef]
- Pashikanti, S.; de Alba, D.R.; Boissonneault, G.A.; Cervantes-Laurean, D. Rutin metabolites: Novel inhibitors of nonoxidative advanced glycation end products. Free Radic. Biol. Med. 2010, 48, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, I.B.; Jaganath, I.B.; Mullen, W.; Edwards, C.A.; Crozier, A. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic. Res. 2006, 40, 1035–1046. [Google Scholar] [CrossRef]
- Walle, T.; Otake, Y.; Walle, U.K.; Wilson, F.A. Quercetin glucosides are completely hydrolyzed in ileostomy patients before absorption. J. Nutr. 2000, 130, 2658–2661. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Fuller, R. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J. Nutr. 2000, 130, 391S–395S. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.A.; Gross, G.; Mondot, S.; Possemiers, S.; Marzorati, M.; Van de Wiele, T.; Doré, J.; Vaughan, E.E. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res. Int. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Millhouse, E.; Culshaw, S.; Edwards, C.A.; Ramage, G.; Combet, E. Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2015, 6, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Parrett, M.A. Colonic fermentation-in vitro and in vivo approaches to measurement. Sci. des Aliments 1999, 19, 291–300. [Google Scholar]
- Kaur, A.; Rose, D.J.; Rumpagaporn, P.; Patterson, J.A.; Hamaker, B.R. In vitro batch fecal fermentation comparison of gas and short-chain fatty acid production using “slowly fermentable” dietary fibers. J. Food Sci. 2011, 76, H137–H142. [Google Scholar] [CrossRef]
- Combet, E.; Lean, M.E.; Boyle, J.G.; Crozier, A.; Davidson, D.F. Dietary flavonols contribute to false-positive elevation of homovanillic acid, a marker of catecholamine-secreting tumors. Clin. Chim. Acta 2011, 412, 165–169. [Google Scholar] [CrossRef]
- Laurentin, A.; Edwards, C.A. Differential fermentation of glucose-based carbohydrates in vitro by human faecal bacteria. Eur. J. Nutr. 2004, 43, 183–189. [Google Scholar] [CrossRef]
- Justesen, U.; Arrigoni, E.; Larsen, B.R.; Amado, R. Degradation of flavonoid glycosides and aglycones during in vitro fermentation with human faecal flora. LWT-Food Sci. Technol. 2000, 33, 424–430. [Google Scholar] [CrossRef]
- Aura, A.-M.; O’leary, K.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimiä, R.; Nuutila, A.; Oksman-Caldentey, K.-M.; Poutanen, K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J. Agric. Food Chem. 2002, 50, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Labib, S.; Erb, A.; Kraus, M.; Wickert, T.; Richling, E. The pig caecum model: A suitable tool to study the intestinal metabolism of flavonoids. Mol. Nutr. Food Res. 2004, 48, 326–332. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Mena, P.; Ludwig, I.A.; Tomatis, V.B.; Acharjee, A.; Calani, L.; Rosi, A.; Brighenti, F.; Ray, S.; Griffin, J.L.; Bluck, L.J. Inter-individual variability in the production of flavan-3-ol colonic metabolites: Preliminary elucidation of urinary metabotypes. Eur. J. Nutr. 2018, 1–15. [Google Scholar] [CrossRef]
- Sandler, M.; Ruthven, C.; Goodwin, B.; Lees, A.; Stern, G. Phenylacetic acid in human body fluids: High correlation between plasma and cerebrospinal fluid concentration values. J. Neurol. Neurosurg. Psychiatry 1982, 45, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Brusilow, S.W. Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion. Pediatr. Res. 1991, 29, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Hommes, F.A. The assay of phenylacetic acid and 4-phenylbutyric acid in physiological fluids. Clin. Chim. Acta Int. J. Clin. Chem. 1999, 284, 109–111. [Google Scholar] [CrossRef]
- Yu, X.; Thompson, M.M.; Shi, D.; Tuchman, M. Quantification of benzoic, phenylacetic, and phenylbutyric acids from filter-paper blood spots by gas chromatography–mass spectrometry with stable isotope dilution. Clin. Chem. 2001, 47, 351–354. [Google Scholar]
- Arima, H.; Ashida, H.; Danno, G.-i. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef]
- Vaquero, M.R.; Alberto, M.; de Nadra, M.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar] [PubMed]
- Massot-Cladera, M.; Pérez-Berezo, T.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch. Biochem. Biophys. 2012, 527, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Product Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; Du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Serrano, J.; Tabernero, M.; Arranz, S.; Díaz-Rubio, M.E.; García-Diz, L.; Goñi, I.; Saura-Calixto, F. Bioavailability of phenolic antioxidants associated with dietary fiber: Plasma antioxidant capacity after acute and long-term intake in humans. Plant Foods Hum. Nutr. 2009, 64, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804. [Google Scholar] [CrossRef]
- Greenhill, C. Gut microbiota: Anti-cancer therapies affected by gut microbiota. Nature Rev. Gastroenterol. Hepatol. 2013, 11, 1. [Google Scholar] [CrossRef] [PubMed]
| Peak | Retention Time (tR) in Minutes | Identifying Ion (m/z) | Qualifying Ions (m/z) | Phenolic Acid |
|---|---|---|---|---|
| Peak 1 | 7.45 | 164 | 73, 91, 164 | 4-OHBA |
| Peak 2 | 13.22 | 164 | 73, 147, 75 | PAA |
| Peak 3 | 13.82 | 267 | 73, 267, 193 | 3-OHPAA |
| Peak 4 | 14.15 | 179 | 73, 164, 281, 252 | 4-OHPAA |
| Peak 5 | 17.17 | 205 | 192, 177, 73, 310 | 3,4-diOHPAA |
| Peak 6 | 18.44 | 179 | 192, 73, 75, 177 | 3-OHPPA |
| Peak 7 | 21.1 | 179 | 73, 267, 384 | 4-OHPPA |
| 4-OHBA (µg/mL) | PAA (µg/mL) | 3-OHPAA (µg/mL) | 4-OHPAA (µg/mL) | 3,4-diOHPAA (µg/mL) | 3-OHPPA (µg/mL) | 4-OHPPA (µg/mL) | |
|---|---|---|---|---|---|---|---|
| Detection frequency | 2/10 | 10/10 | 9/10 | 10/10 | 10/10 | 10/10 | 10/10 |
| FS | - | 4.33 ± 1.67 | 0.006 ± 0.02 | 0.50 ± 0.43 | - | 0.21 ± 0.14 | 0.75 ± 1.23 |
| R | 0.04 ± 0.02 | 5.59 ± 3.35 | 1.25 ± 1.60 | 0.79 ± 0.66 | 3.11 ± 4.27 | 0.43 ± 0.28 | 0.39 ± 0.68 |
| RAF | - | 0.72 ± 1.29 | 0.002 ± 0.007 | 0.18 ± 0.24 | - | 0.24 ± 0.18 | 0.15 ± 0.16 |
| RR | 0.05 ± 0.0 | 0.41 ± 0.90 | 0.02 ± 0.06 | 0.19 ± 0.25 | 0.56 ± 0.92 | 0.34 ± 0.15 | 0.12 ± 0.10 |
| % Change | ↑40% | ↓92.5% ** | ↓98.4% ** | ↓76% ** | ↓81.6% ** | ↓20.9% | ↓39.1% |
| ISP | - | 2.19 ± 1.86 | 0.002 ± 0.007 | 0.25 ± 0.46 | - | 0.39 ± 0.43 | 0.56 ± 0.71 |
| ISP-R | - | 2.94 ± 2.61 | 0.96 ± 1.24 | 0.33 ± 0.62 | 2.29 ± 2.86 | 0.69 ± 0.80 | 0.47 ± 0.69 |
| % Change | ↓100% ** | ↓47.2% ** | ↓23.2% | ↓58.2% | ↓27.6% * | ↑37.6% | ↑10.8% |
| PEC | - | 0.28 ± 0.57 | 0.001 ± 0.005 | 0.16 ± 0.18 | - | 0.25 ± 0.13 | 0.08 ± 0.09 |
| PR | - | 0.29 ± 0.54 | 0.12 ± 0.26 | 0.2 ± 0.21 | 1.03 ± 1.60 | 0.39 ± 0.18 | 0.1 ± 0.1 |
| % Change | ↓100% ** | ↓95% ** | ↓89.6% ** | ↓74.6% ** | ↓66.8% ** | ↓9.30% | ↓74.3% ** |
| Substrate | Total SCFA Concentration at 6 h (mmol/L) | Total SCFA Concentration at 24 h (mmol/L) | pH at 24 h | Gas Production at 24 h (mL) |
|---|---|---|---|---|
| Raftiline | 49.6 ± 8.4 | 80.3 ± 8.2 | 4.27 ± 0.4 | 21.0 ± 10.4 |
| Raftiline + Rutin | 42.6 ± 11.3 | 76.6 ± 7 | 4.68 ± 0.88 | 23.2 ± 11.9 |
| Raftiline + Quercetin | 41.9 ± 17.8 | 80.6 ± 7.4 | 4.30 ± 0.50 | 26.9 ± 8.0 |
| Ispaghula | 28.5 ± 11.8 | 76.8 ± 7.8 | 5.33 ± 0.53 | 20.7 ± 4.1 |
| Ispaghula + Rutin | 29.1 ± 9.5 | 66.2 ± 4.5 | 5.46 ± 0.64 | 17.5± 4.2 |
| Ispaghula + Quercetin | 28.6 ± 9.7 | 63 ± 16.5 | 5.42 ± 0.63 | 15.7 ± 6.4 |
| Pectin | 39.9 ± 15.3 | 68.7 ± 47.6 | 3.92 ± 0.64 | 22.7 ± 12.4 |
| Pectin + Rutin | 38.8 ± 9.3 | 67.5 ± 21.8 | 4.11 ± 0.60 | 21.6 ± 10.4 |
| Pectin + Quercetin | 38 ± 13.6 | 59.8 ± 29.2 | 4.01 ± 0.55 | 17.7 ± 8.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansoorian, B.; Combet, E.; Alkhaldy, A.; Garcia, A.L.; Edwards, C.A. Impact of Fermentable Fibres on the Colonic Microbiota Metabolism of Dietary Polyphenols Rutin and Quercetin. Int. J. Environ. Res. Public Health 2019, 16, 292. https://doi.org/10.3390/ijerph16020292
Mansoorian B, Combet E, Alkhaldy A, Garcia AL, Edwards CA. Impact of Fermentable Fibres on the Colonic Microbiota Metabolism of Dietary Polyphenols Rutin and Quercetin. International Journal of Environmental Research and Public Health. 2019; 16(2):292. https://doi.org/10.3390/ijerph16020292
Chicago/Turabian StyleMansoorian, Bahareh, Emilie Combet, Areej Alkhaldy, Ada L. Garcia, and Christine Ann Edwards. 2019. "Impact of Fermentable Fibres on the Colonic Microbiota Metabolism of Dietary Polyphenols Rutin and Quercetin" International Journal of Environmental Research and Public Health 16, no. 2: 292. https://doi.org/10.3390/ijerph16020292
APA StyleMansoorian, B., Combet, E., Alkhaldy, A., Garcia, A. L., & Edwards, C. A. (2019). Impact of Fermentable Fibres on the Colonic Microbiota Metabolism of Dietary Polyphenols Rutin and Quercetin. International Journal of Environmental Research and Public Health, 16(2), 292. https://doi.org/10.3390/ijerph16020292







