A Cross-Sectional Study of Endogenous Antioxidants and Patterns of Dental Visits of Periodontitis Patients
Abstract
1. Introduction
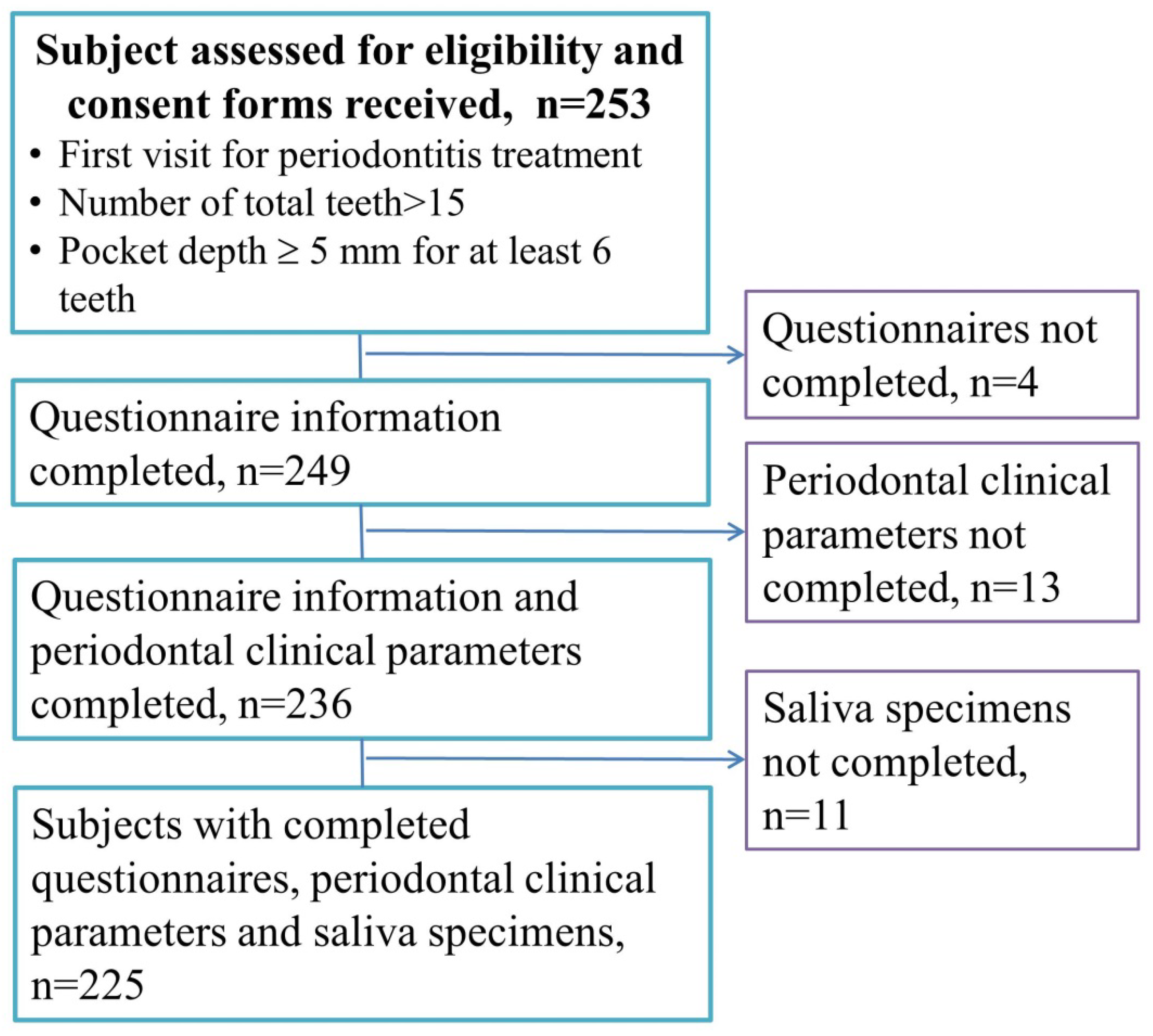
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petersen, P.E.; Ogawa, H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontology 2000 2012, 60, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef]
- Health Promotion Bureau of Taiwan. Bureau of Health Promotion Annual Report, 2009; Health Promotion Bureau of Taiwan: Taipei, Taiwan, 2009; pp. 1–1141.
- Yu, H.C.; Su, N.Y.; Huang, J.Y.; Lee, S.S.; Chang, Y.C. Trends in the prevalence of periodontitis in Taiwan from 1997 to 2013: A nationwide population-based retrospective study. Medicine (Baltimore) 2017, 96, e8585. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.P.; Mute, B.R.; Pitale, U.M.; Shetty, S.; Hc, S.; Satpute, P.S. Prevalence of periodontal disease and characterization of its extent and severity in an adult population—An observational study. J. Clin. Diagn. Res. 2014, 8, ZC04–ZC07. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Perotto, S.; Castiglione, A.; Mariani, G.M.; Ferrarotti, F.; Romano, F. Prevalence of periodontitis in an adult population from an urban area in North Italy: Findings from a cross-sectional population-based epidemiological survey. J. Clin. Periodontol. 2015, 42, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S. Mapping the pathogenesis of periodontitis: A new look. J. Periodontol. 2008, 79 (Suppl. 8), 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- D’Aiuto, F.; Nibali, L.; Parkar, M.; Patel, K.; Suvan, J.; Donos, N. Oxidative stress, systemic inflammation, and severe periodontitis. J. Dent. Res. 2010, 89, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, F.; El Tantawi, M. Normative prosthodontic care need: Does it impact the daily life of young Saudis with high level of oral diseases? A cross sectional study. BMC Oral Health 2017, 17, 128. [Google Scholar] [CrossRef]
- Lertpimonchai, A.; Rattanasiri, S.; Arj-Ong Vallibhakara, S.; Attia, J.; Thakkinstian, A. The association between oral hygiene and periodontitis: A systematic review and meta-analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontology 2000 2013, 62, 59–94. [Google Scholar] [CrossRef] [PubMed]
- Sculley, D.V.; Langley-Evans, S.C. Salivary antioxidants and periodontal disease status. Proc. Nutr. Soc. 2002, 61, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Netto, L.E.; Antunes, F. The Roles of Peroxiredoxin and Thioredoxin in Hydrogen Peroxide Sensing and in Signal Transduction. Mol. Cells 2016, 39, 65–71. [Google Scholar] [PubMed]
- Giannobile, W.V.; Beikler, T.; Kinney, J.S.; Ramseier, C.A.; Morelli, T.; Wong, D.T. Saliva as a diagnostic tool for periodontal disease: Current state and future directions. Periodontology 2000 2009, 50, 52–64. [Google Scholar] [CrossRef]
- Tothova, L.; Kamodyova, N.; Cervenka, T.; Celec, P. Salivary markers of oxidative stress in oral diseases. Front. Cell. Infect. Microbiol. 2015, 5, 73. [Google Scholar] [CrossRef]
- Farnaud, S.J.; Kosti, O.; Getting, S.J.; Renshaw, D. Saliva: Physiology and diagnostic potential in health and disease. Sci. World J. 2010, 10, 434–456. [Google Scholar] [CrossRef]
- Canakci, V.; Yildirim, A.; Canakci, C.F.; Eltas, A.; Cicek, Y.; Canakci, H. Total antioxidant capacity and antioxidant enzymes in serum, saliva, and gingival crevicular fluid of preeclamptic women with and without periodontal disease. J. Periodontol. 2007, 78, 1602–1611. [Google Scholar] [CrossRef]
- McCord, J.M.; Edeas, M.A. SOD, oxidative stress and human pathologies: A brief history and a future vision. Biomed. Pharmacother. 2005, 59, 139–142. [Google Scholar] [CrossRef]
- Ooshima, T.; Minami, T.; Aono, W.; Izumitani, A.; Sobue, S.; Fujiwara, T.; Kawabata, S.; Hamada, S. Oolong tea polyphenols inhibit experimental dental caries in SPF rats infected with mutans streptococci. Caries Res. 1993, 27, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; You, H.J.; Lian, H.J.; Huang, C.H. Patients receiving comprehensive periodontal treatment have better clinical outcomes than patients receiving conventional periodontal treatment. J. Formos. Med. Assoc. 2016, 115, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Hefti, A.F.; Preshaw, P.M. Examiner alignment and assessment in clinical periodontal research. Periodontology 2000 2012, 59, 41–60. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, N.; Todorovic, T.; Rakic, M.; Milinkovic, I.; Dozic, I.; Jankovic, S.; Aleksic, Z.; Cakic, S. Salivary antioxidants as periodontal biomarkers in evaluation of tissue status and treatment outcome. J. Periodontal Res. 2014, 49, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Glavind, L. Effect of monthly professional mechanical tooth cleaning on periodontal health in adults. J. Clin. Periodontol. 1977, 4, 100–106. [Google Scholar] [CrossRef]
- Lightner, L.M.; O’Leary, J.T.; Drake, R.B.; Crump, P.P.; Allen, M.F. Preventive periodontic treatment procedures: Results over 46 months. J. Periodontol. 1971, 42, 555–561. [Google Scholar] [CrossRef]
- Suomi, J.D.; Smith, L.W.; Chang, J.J.; Barbano, J.P. Study of the effect of different prophylaxis frequencies on the periodontium of young adult males. J. Periodontol. 1973, 44, 406–410. [Google Scholar] [CrossRef]
- Nakamura, H.; Nakamura, K.; Yodoi, J. Redox regulation of cellular activation. Annu. Rev. Immunol. 1997, 15, 351–369. [Google Scholar] [CrossRef]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Day, B.J. Antioxidant therapeutics: Pandora’s box. Free Radic. Biol. Med. 2014, 66, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med. 1991, 91, 14S–22S. [Google Scholar] [CrossRef]
- Armitage, G.C.; Robertson, P.B. The biology, prevention, diagnosis and treatment of periodontal diseases: Scientific advances in the United States. J. Am. Dent. Assoc. 2009, 140 (Suppl. 1), 36S–43S. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M.; Brown, L.J.; Brunelle, J.A.; Loe, H. Gingival state and dental calculus in early-onset periodontitis. J. Periodontol. 1996, 67, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lee, C.Y.; Feng, S.W.; Miao, N.F.; Lin, P.H.; Lin, C.T.; Tsai, S.H.; Huang, Y.K. Effects of Salivary Oxidative Markers on Edentulous Patients’ Satisfaction with Prosthetic Denture Treatments: A Pilot Study. PLoS ONE 2016, 11, e0151605. [Google Scholar] [CrossRef]
- Tomas, I.; Diz, P.; Tobias, A.; Scully, C.; Donos, N. Periodontal health status and bacteraemia from daily oral activities: Systematic review/meta-analysis. J. Clin. Periodontol. 2012, 39, 213–228. [Google Scholar] [CrossRef]
- Yang, P.S.; Huang, W.C.; Chen, S.Y.; Chen, C.H.; Lee, C.Y.; Lin, C.T.; Huang, Y.K. Scaling-stimulated salivary antioxidant changes and oral-health behavior in an evaluation of periodontal treatment outcomes. Sci. World J. 2014, 2014, 814671. [Google Scholar] [CrossRef]
- Zschauer, T.C.; Matsushima, S.; Altschmied, J.; Shao, D.; Sadoshima, J.; Haendeler, J. Interacting with thioredoxin-1—Disease or no disease? Antioxid. Redox Signal. 2013, 18, 1053–1062. [Google Scholar] [CrossRef]
- Matthews, J.R.; Wakasugi, N.; Virelizier, J.L.; Yodoi, J.; Hay, R.T. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992, 20, 3821–3830. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kim, O.S.; Kim, O.J.; Kim, Y.J.; Chung, H.J. Antioxidant profile of whole saliva after scaling and root planing in periodontal disease. J. Periodont. Implant Sci. 2010, 40, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Sculley, D.V.; Langley-Evans, S.C. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin. Sci. 2003, 105, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Keskiner, I.; Saygun, I.; Bal, V.; Serdar, M.; Kantarci, A. Dietary supplementation with low-dose omega-3 fatty acids reduces salivary tumor necrosis factor-alpha levels in patients with chronic periodontitis: A randomized controlled clinical study. J. Periodontal Res. 2017, 52, 695–703. [Google Scholar] [CrossRef] [PubMed]
| Periodontal Clinical Parameters | N | PI (%) | BOP (%) | PD (mm) |
|---|---|---|---|---|
| Variables | Mean ± SE | |||
| Gender | 225 | 58.88 ± 1.24 | 43.58 ± 1.4 | 3.44 ± 0.03 |
| Female | 122 | 58.12 ± 1.57 | 43.19 ± 1.77 | 3.41 ± 0.04 |
| Male | 103 | 59.77 ± 1.97 | 44.05 ± 2.25 | 3.48 ± 0.05 |
| p value a | 0.51 | 0.76 | 0.34 | |
| Educational level | ||||
| High school | 90 | 59.17 ± 1.89 | 46.97 ± 2.16 | 3.52 ± 0.06 |
| University or above | 135 | 58.68 ± 1.64 | 41.36 ± 1.83 | 3.39 ± 0.04 |
| p value a | 0.84 | 0.05 | 0.06 | |
| Smoking status | ||||
| Non-smoker | 159 | 59.74 ± 1.46 | 45.15 ± 1.66 | 3.43 ± 0.04 |
| Smoker | 66 | 56.8 ± 2.33 | 39.7 ± 2.6 | 3.48 ± 0.07 |
| p value a | 0.28 | 0.07 | 0.55 | |
| Alcohol consumption | ||||
| Never or occasional | 200 | 58.55 ± 1.31 | 43.28 ± 1.49 | 3.43 ± 0.03 |
| Regular | 25 | 61.46 ± 3.93 | 46.03 ± 4.22 | 3.55 ± 0.12 |
| p value a | 0.46 | 0.54 | 0.25 | |
| Betel nut chewing | ||||
| Non-chewer | 218 | 58.62 ± 1.27 | 43.54 ± 1.42 | 3.45 ± 0.03 |
| Chewer | 7 | 66.93 ± 4.87 | 44.9 ± 10.33 | 3.3 ± 0.15 |
| p value a | 0.24 | 0.86 | 0.45 | |
| Tooth cleaning frequency | ||||
| Once per day | 23 | 69.29 ± 4.21 | 46.04 ± 5.65 | 3.54 ± 0.11 |
| Twice per day | 132 | 59.93 ± 1.54 | 42.61 ± 1.7 | 3.42 ± 0.04 |
| More than twice per day | 70 | 53.46 ± 2.16 | 44.6 ± 2.61 | 3.46 ± 0.07 |
| p value b | <0.01 | 0.69 | 0.59 | |
| Auxiliary tooth cleaning tools | ||||
| None | 62 | 63.51 ± 2.36 | 47.5 ± 2.98 | 3.52 ± 0.07 |
| Floss or interdental brush | 118 | 57.08 ± 1.72 | 41.16 ± 1.75 | 3.41 ± 0.04 |
| Floss and interdental brush | 45 | 57.19 ± 2.6 | 44.52 ± 3.31 | 3.44 ± 0.08 |
| p value b | 0.07 | 0.15 | 0.34 | |
| Length of time since last dental visit | ||||
| >1 year | 150 | 60.4 ± 1.55 | 45.28 ± 1.77 | 3.45 ± 0.04 |
| <1 year | 75 | 55.82 ± 2.02 | 40.05 ± 2.23 | 3.44 ± 0.07 |
| p value a | 0.08 | 0.08 | 0.97 | |
| Salivary Antioxidant | N | Cu/Zn SOD (µg/mL) | MnSOD (µg/mL) | Prx2 (µg/mL) | Trx1 (µg/mL) | Catalase (mg/mL) |
|---|---|---|---|---|---|---|
| Variable | Mean ± SE | |||||
| All subjects | 225 | 14.87 ± 0.87 | 17.8 ± 2.38 | 4.81 ± 1.36 | 504.88 ± 23.83 | 14.25 ± 3.52 |
| Gender | ||||||
| Female | 122 | 13.79 ± 0.85 | 18.18 ± 3.2 | 2.64 ± 0.53 | 509.23 ± 32.16 | 22.08 ± 6.25 |
| Male | 103 | 16.14 ± 1.61 | 17.34 ± 3.58 | 7.38 ± 2.89 | 499.73 ± 35.65 | 4.98 ± 1.72 |
| p value a | 0.19 | 0.86 | 0.11 | 0.84 | <0.01 | |
| Educational level | ||||||
| High school | 90 | 13.33 ± 1.12 | 11.62 ± 2.7 | 3.13 ± 0.77 | 448.72 ± 33.86 | 23.09 ± 7.41 |
| University or above | 135 | 15.89 ± 1.25 | 21.91 ± 3.51 | 5.93 ± 2.21 | 542.32 ± 32.38 | 8.35 ± 3.09 |
| p value a | 0.12 | 0.02 | 0.23 | 0.05 | 0.07 | |
| Smoking status | ||||||
| Non-smoker | 159 | 15.62 ± 1.07 | 18.19 ± 2.87 | 5.84 ± 1.9 | 540.02 ± 27.78 | 16.93 ± 4.83 |
| Smoker | 66 | 13.05 ± 1.46 | 16.86 ± 4.3 | 2.34 ± 0.6 | 420.23 ± 44.67 | 7.78 ± 2.79 |
| p value a | 0.18 | 0.80 | 0.08 | 0.02 | 0.10 | |
| Alcohol consumption | ||||||
| Never or occasional | 200 | 14.91 ± 0.92 | 18.17 ± 2.62 | 3.56 ± 0.98 | 517.67 ± 25.2 | 14.88 ± 3.93 |
| Regular | 25 | 14.56 ± 2.77 | 14.84 ± 4.82 | 14.82 ± 9.3 | 402.55 ± 71.24 | 9.23 ± 4.14 |
| p value a | 0.90 | 0.54 | 0.23 | 0.12 | 0.32 | |
| Betel nut chewing | ||||||
| Non-chewer | 218 | 15 ± 0.88 | 18.16 ± 2.46 | 4.89 ± 1.4 | 509.24 ± 24.06 | 14.47 ± 3.63 |
| Chewer | 7 | 10.63 ± 5.51 | 6.48 ± 2.18 | 2.48 ± 1.39 | 369.03 ± 161.87 | 7.48 ± 7.22 |
| p value a | 0.38 | <0.01 | 0.23 | 0.30 | 0.40 | |
| Tooth cleaning frequency | ||||||
| Once per day | 23 | 15.61 ± 2.68 | 15.97 ± 6.55 | 12.38 ± 9.86 | 522.5 ± 81.15 | 9.05 ± 4.84 |
| Twice per day | 132 | 13.77 ± 0.86 | 16.91 ± 3.12 | 3.95 ± 1.44 | 502.27 ± 30.57 | 11.77 ± 4.09 |
| More than twice per day | 70 | 16.7 ± 2.11 | 20.08 ± 4.44 | 3.94 ± 1.16 | 504.02 ± 43.45 | 20.62 ± 8.13 |
| p value b | 0.30 | 0.80 | 0.17 | 0.96 | 0.46 | |
| Auxiliary tooth cleaning tools | ||||||
| None | 62 | 16.74 ± 1.48 | 26.83 ± 5.84 | 5.76 ± 3.68 | 445.71 ± 48.68 | 29.01 ± 10.18 |
| Floss or interdental brush | 118 | 14.77 ± 1.38 | 14.38 ± 2.85 | 3.11 ± 0.72 | 553.09 ± 31.48 | 5.98 ± 1.98 |
| Floss and interdental brush | 45 | 12.56 ± 1.26 | 14.31 ± 4.41 | 7.97 ± 4.15 | 460 ± 52.22 | 15.6 ± 8.89 |
| p value b | 0.26 | 0.06 | 0.36 | 0.10 | 0.02 | |
| Length of time since last dental visit | ||||||
| >1 year | 150 | 12.98 ± 0.74 | 15.57 ± 2.81 | 3.52 ± 1.58 | 575.11 ± 28.18 | 21.24 ± 5.19 |
| <1 year | 75 | 18.64 ± 2.11 | 22.26 ± 4.4 | 7.4 ± 2.58 | 364.44 ± 39.48 | 0.26 ± 0.02 |
| p value a | 0.01 | 0.18 | 0.17 | <0.0001 | <0.0001 | |
| Dependent Variable | PI (%) | BOP (%) | PD Mean (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Independent Variable | β | SE | p Value | β | SE | p Value | β | SE | p Value | |
| Gender (male vs. female), N = 225 | 1.65 | 2.48 | 0.50 | 0.86 | 2.82 | 0.76 | 0.06 | 0.06 | 0.33 | |
| Education, N = 225 (university or above vs. high school) | −0.48 | 2.53 | 0.84 | −5.61 | 2.85 | 0.05 | −0.13 | 0.07 | 0.06 | |
| Smoking status, N = 225 (smokers vs. non-smokers) | −2.93 | 2.71 | 0.28 | −5.45 | 3.08 | 0.07 | 0.04 | 0.07 | 0.55 | |
| Alcohol consumption, N = 225 (regular vs. never or occasional) | 2.9 | 3.94 | 0.46 | 2.74 | 4.53 | 0.54 | 0.12 | 0.11 | 0.25 | |
| Betel nut chewing, N = 225 (chewers vs. non-chewers) | 8.31 | 7.12 | 0.24 | 1.36 | 8.05 | 0.85 | −0.14 | 0.2 | 0.45 | |
| Tooth cleaning frequency | ||||||||||
| Twice per day vs. once per day, N = 155 | −9.35 | 4.04 | 0.02 | −3.42 | 4.67 | 0.46 | −0.11 | 0.11 | 0.29 | |
| More than twice per day vs. once per day, N = 93 | −15.83 | 4.46 | <0.001 | −1.43 | 5.56 | 0.79 | −0.08 | 0.13 | 0.54 | |
| More than twice per day vs. twice per day, N = 202 | −6.47 | 2.63 | 0.01 | 1.99 | 3.00 | 0.50 | 0.03 | 0.08 | 0.65 | |
| Floss or interdental brush vs. none, N = 180 | −6.42 | 2.93 | 0.02 | −6.34 | 3.24 | 0.05 | −0.12 | 0.0.8 | 0.13 | |
| Floss and interdental brush vs. none, N = 107 | −6.32 | 3.54 | 0.07 | −2.98 | 4.48 | 0.50 | −0.07 | 0.11 | 0.45 | |
| Floss and interdental brush vs. floss or interdental brush, N = 163 | 0.11 | 3.22 | 0.97 | 3.36 | 3.48 | 0.33 | 0.04 | 0.09 | 0.66 | |
| Length of time since last dental visit (<1 year vs. >1 year), N = 225 | −4.58 | 2.61 | 0.08 | −5.23 | 2.98 | 0.08 | −2.52 × 10−3 | 0.07 | 0.97 | |
| Dependent Variable: PI (%) | Model I § | Model II ¶ | |||||
|---|---|---|---|---|---|---|---|
| Independent Variable | β | SE | p Value | β | SE | p Value | |
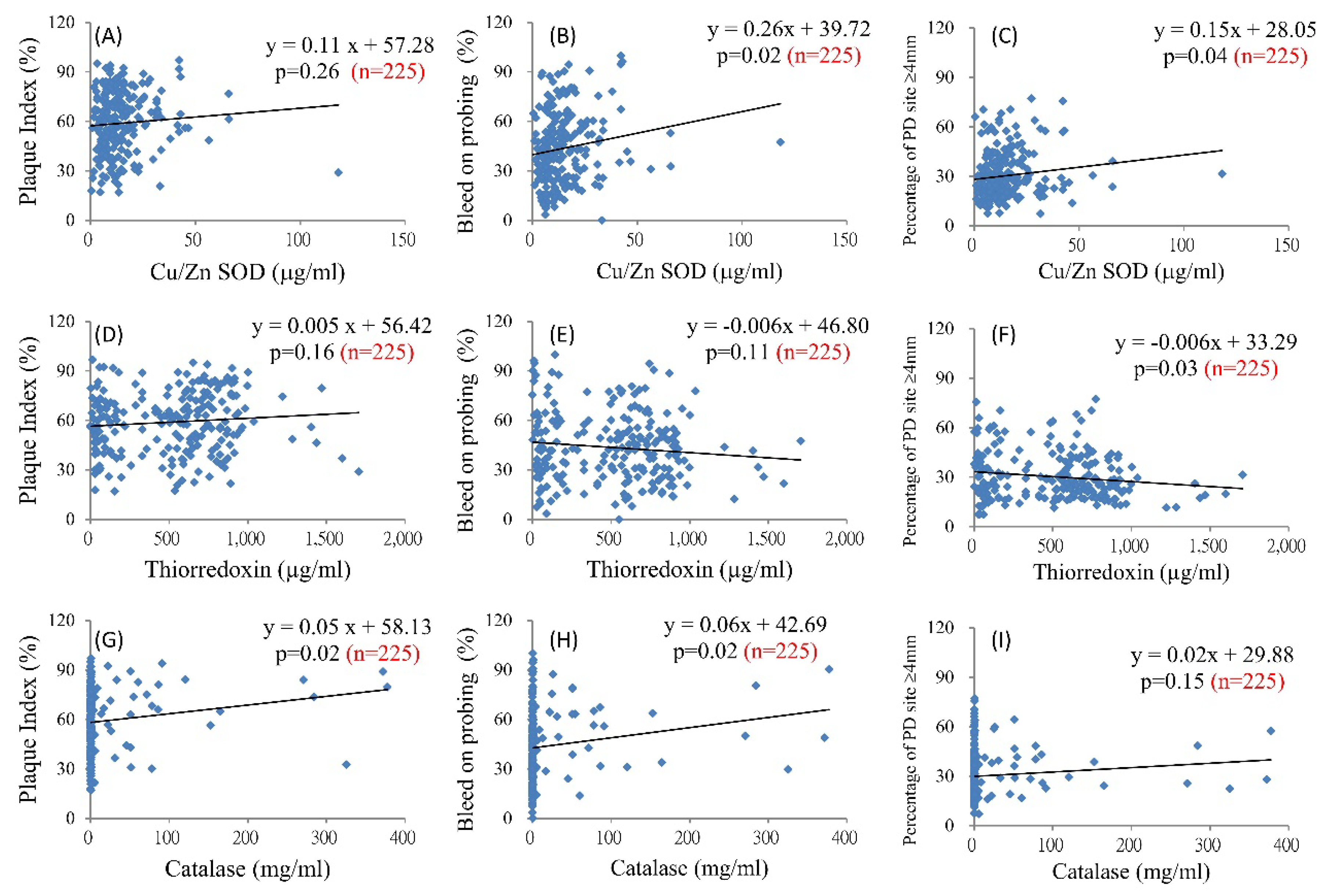
| Cu-Zn SOD (µg/mL) | 0.47 | 0.18 | <0.01 | 0.38 | 0.18 | 0.04 | |
| MnSOD (µg/mL) | −0.03 | 0.05 | 0.05 | −0.02 | 0.05 | 0.64 | |
| Prx2 (µg/mL) | 0.05 | 0.08 | 0.56 | −0.01 | 0.08 | 0.92 | |
| Trx1 (µg/mL) | 5.14 × 10−3 | 4.46 × 10−3 | 0.25 | 3.89 × 10−3 | 4.45 × 10−3 | 0.38 | |
| Catalase (mg/mL) | 0.05 | 0.03 | 0.07 | 0.05 | 0.02 | 0.04 | |
| Dependent variable: BOP (%) | Model III § | Model IV ¶ | |||||
| Independent variable | β | SE | p value | β | SE | p value | |
| Cu-Zn SOD (µg/mL) | 0.64 | 0.19 | <0.001 | 0.56 | 0.19 | <0.01 | |
| MnSOD (µg/mL) | 0.03 | 0.05 | 0.51 | 0.05 | 0.05 | 0.35 | |
| Prx2 (µg/mL) | 0.10 | 0.08 | 0.23 | 0.05 | 0.09 | 0.54 | |
| Trx1 (µg/mL) | −0.02 | 4.75 × 10−3 | <0.001 | −0.02 | 4.82 × 10−3 | <.0001 | |
| Catalase (mg/mL) | 0.04 | 0.02 | 0.09 | 0.04 | 0.03 | 0.15 | |
| Dependent variable: PD mean (mm) | Model V § | Model VI ¶ | |||||
| Independent variable | β | SE | p value | β | SE | p value | |
| Cu-Zn SOD (µg/mL) | 8.37 × 10−3 | 4.07 × 10−3 | 0.04 | 7.42 × 10−3 | 4.25 × 10−3 | 0.08 | |
| MnSOD (µg/mL) | 5.12 × 10−4 | 1.08 × 10−3 | 0.64 | 7.96 × 10−4 | 1.11 × 10−3 | 0.47 | |
| Prx2 (µg/mL) | 3.08 × 10−3 | 1.82 × 10−3 | 0.09 | 1.47 × 10−3 | 1.93 × 10−3 | 0.45 | |
| Trx1 (µg/mL) | −4.91 × 10−4 | 1.03 × 10−4 | <0.0001 | −4.89 × 10−4 | 1.05 × 10−4 | <0.0001 | |
| Catalase (mg/mL) | 6.51 × 10−4 | 5.54 × 10−4 | 0.24 | 6.54 × 10−4 | 5.82 × 10−4 | 0.26 | |
| Dependent Variable: PI (%) | Model I § | Model II ¶ | |||||
|---|---|---|---|---|---|---|---|
| Independent Variable | β | SE | p Value | β | SE | p Value | |
| Cu-Zn SOD (µg/mL) | −0.03 | 0.20 | 0.87 | 3.54 × 10−3 | 0.21 | 0.99 | |
| MnSOD (µg/mL) | −0.14 | 0.09 | 0.11 | −0.14 | 0.09 | 0.12 | |
| Prx2 (µg/mL) | −0.14 | 0.10 | 0.16 | −0.17 | 0.09 | 0.09 | |
| Trx1 (µg/mL) | 1.14 × 10−3 | 0.01 | 0.91 | −1.67 × 10−3 | 0.01 | 0.87 | |
| Catalase (mg/mL) | 0.03 | 0.02 | 0.07 | 0.03 | 0.01 | 0.08 | |
| Dependent variable: BOP (%) | Model III § | Model IV ¶ | |||||
| Independent variable | β | SE | p value | β | SE | p value | |
| Cu-Zn SOD (µg/mL) | −0.05 | 0.22 | 0.82 | −0.07 | 0.24 | 0.79 | |
| MnSOD (µg/mL) | −0.16 | 0.09 | 0.09 | −0.14 | 0.11 | 0.19 | |
| Prx2 (µg/mL) | −0.10 | 0.10 | 0.31 | −0.11 | 0.11 | 0.30 | |
| Trx1 (µg/mL) | 0.01 | 0.01 | 0.30 | 0.01 | 0.01 | 0.41 | |
| Catalase (mg/mL) | 0.04 | 0.02 | 0.04 | 0.04 | 0.02 | 0.07 | |
| Dependent variable: PD mean (mm) | Model V § | Model VI ¶ | |||||
| Independent variable | β | SE | p value | β | SE | p value | |
| Cu-Zn SOD (µg/mL) | 2.08 × 10−3 | 6.65 × 10−3 | 0.76 | 3.71 × 10−3 | 6.79 × 10−3 | 0.59 | |
| MnSOD (µg/mL) | −6.61 × 10−3 | 2.80 × 10−3 | 0.02 | −7.55 × 10−3 | 2.92 × 10−3 | 0.01 | |
| Prx2 (µg/mL) | −1.18 × 10−3 | 3.08 × 10−3 | 0.70 | 3.51 × 10−4 | 3.15 × 10−3 | 0.91 | |
| Trx1 (µg/mL) | 1.36 × 10−4 | 3.26 × 10−4 | 0.68 | 4.93 × 10−5 | 3.32 × 10−4 | 0.88 | |
| Catalase (mg/mL) | 1.39 | 0.60 | 0.02 | 1.63 | 0.61 | 0.01 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-Y.; Choy, C.-S.; Lai, Y.-C.; Chang, C.-C.; Teng, N.-C.; Huang, W.-T.; Lin, C.-T.; Huang, Y.-K. A Cross-Sectional Study of Endogenous Antioxidants and Patterns of Dental Visits of Periodontitis Patients. Int. J. Environ. Res. Public Health 2019, 16, 180. https://doi.org/10.3390/ijerph16020180
Lee C-Y, Choy C-S, Lai Y-C, Chang C-C, Teng N-C, Huang W-T, Lin C-T, Huang Y-K. A Cross-Sectional Study of Endogenous Antioxidants and Patterns of Dental Visits of Periodontitis Patients. International Journal of Environmental Research and Public Health. 2019; 16(2):180. https://doi.org/10.3390/ijerph16020180
Chicago/Turabian StyleLee, Chang-Yu, Cheuk-Sing Choy, Yu-Cheng Lai, Chao-Chien Chang, Nai-Chia Teng, Wan-Ting Huang, Che-Tong Lin, and Yung-Kai Huang. 2019. "A Cross-Sectional Study of Endogenous Antioxidants and Patterns of Dental Visits of Periodontitis Patients" International Journal of Environmental Research and Public Health 16, no. 2: 180. https://doi.org/10.3390/ijerph16020180
APA StyleLee, C.-Y., Choy, C.-S., Lai, Y.-C., Chang, C.-C., Teng, N.-C., Huang, W.-T., Lin, C.-T., & Huang, Y.-K. (2019). A Cross-Sectional Study of Endogenous Antioxidants and Patterns of Dental Visits of Periodontitis Patients. International Journal of Environmental Research and Public Health, 16(2), 180. https://doi.org/10.3390/ijerph16020180





