The Impact of Social Disparities on Microbiological Quality of Drinking Water Supply in Ugu District Municipality of Kwazulu-Natal Province, South Africa
Abstract
:1. Introduction
2. Materials and Methods
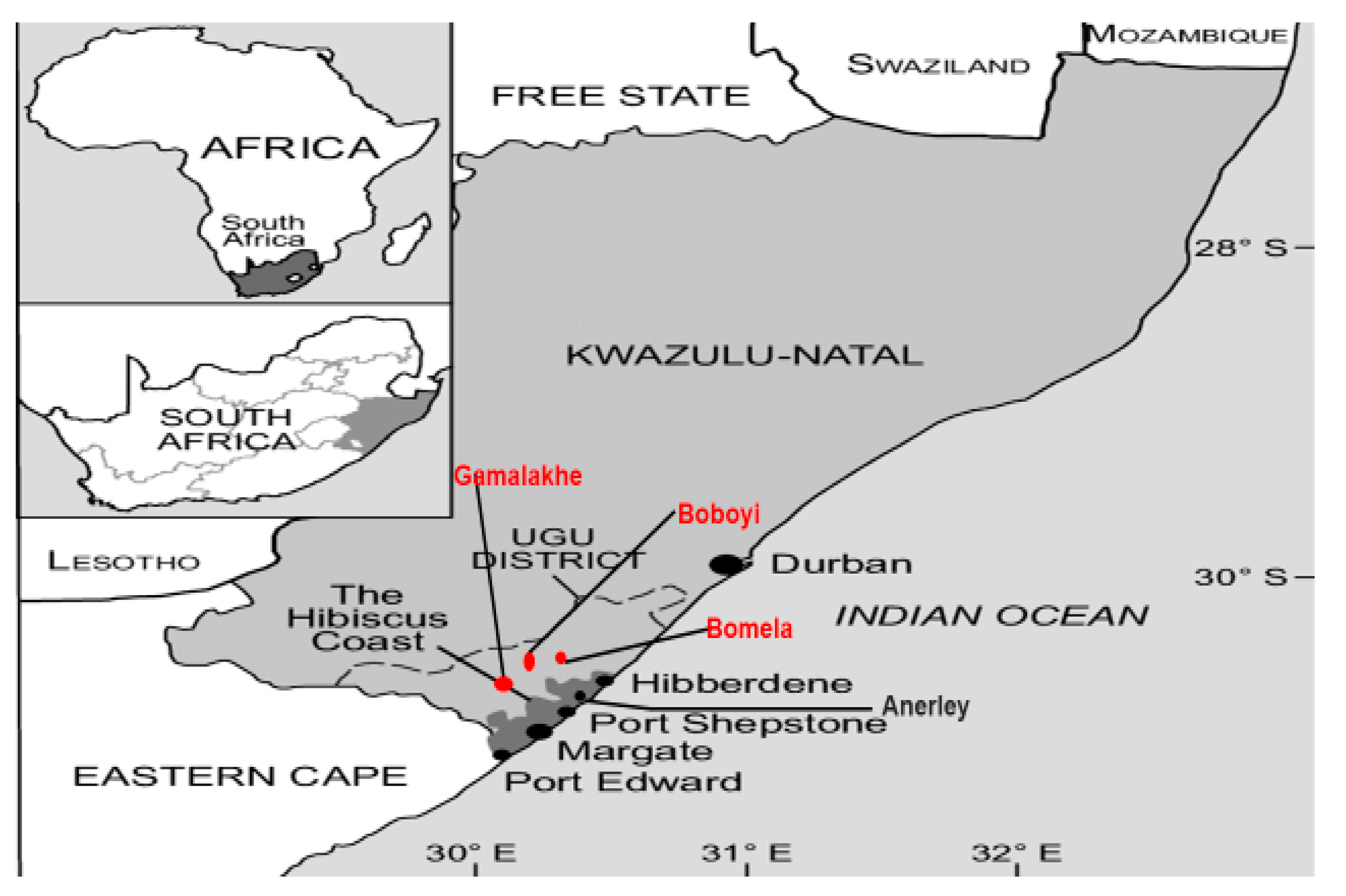
2.1. Description of the Study Site and Population
2.2. Scientific Ethics and Informed Consent
2.3. Study Survey on Social Discrimination
2.4. Collection of Drinking Water Samples
2.5. Analysis of Drinking Water Quality
2.5.1. Physicochemical Characteristics of Drinking Water Samples
2.5.2. Microbiological Characteristics of Drinking Water Samples
Detection and Enumeration of Culturable Thermotolerant Coliforms and E. coli
Molecular Identification of the Isolates
3. Results
3.1. Social Discrimination of the Ugu District during the Study Period
Disparities in Housing Patterns
3.2. Disparities in Water Supply and Sanitation Facilities in the Target Study Area
3.3. Disparities of Employment Rate and Level of Education in the Target Study Area
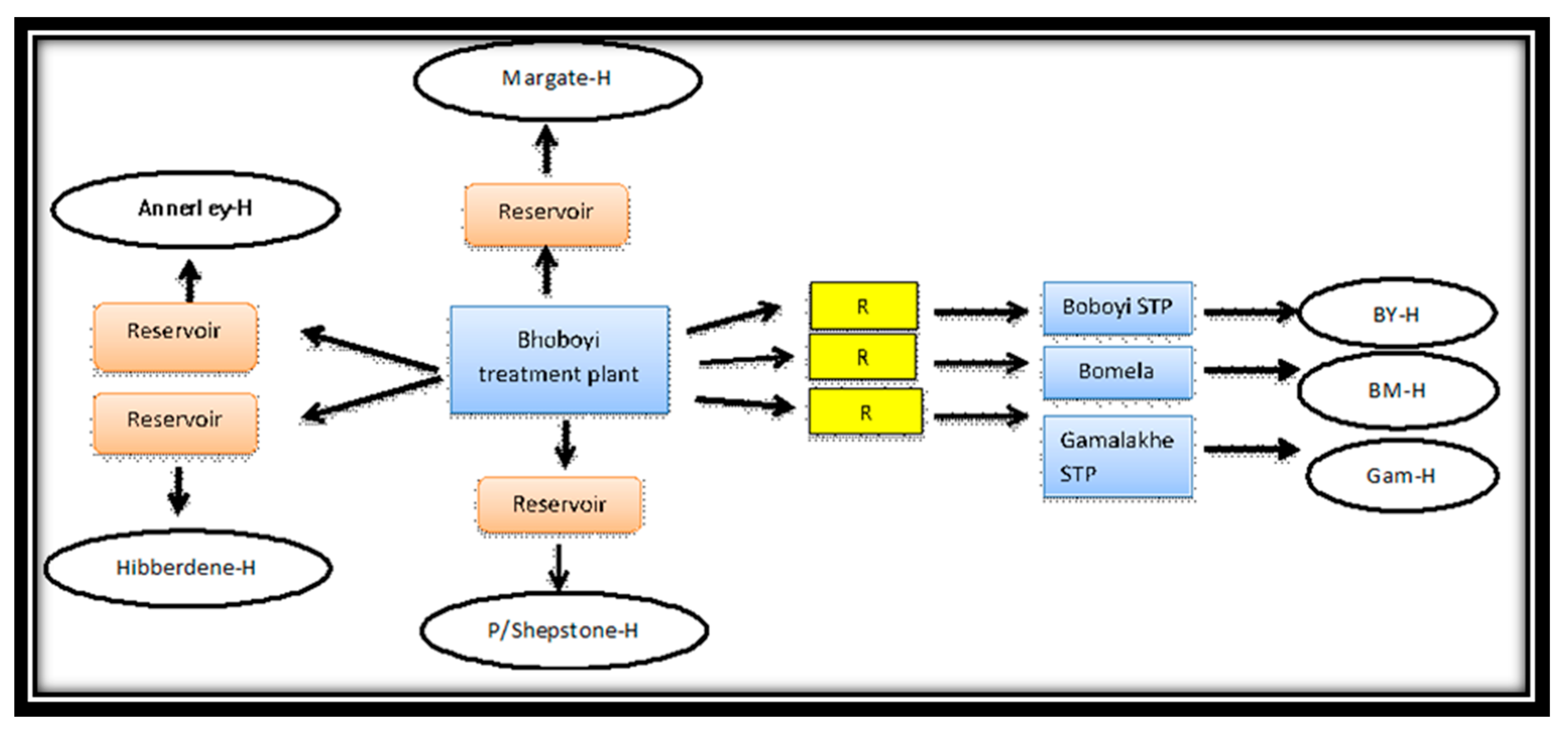
3.4. Disparities in Drinking Water Infrastructure and Mode of Access to Municipal Drinking Water
3.5. Water Quality Analysis
3.5.1. Physicochemical Characteristics of Water Samples from Rural and Urban Areas of Ugu District
3.5.2. General Microbiological Quality of Drinking Water Supply in the Rural and Urban Area of Ugu Municipality
3.5.3. The Prevalence of Pathogenic E. coli Detected in Drinking Water Samples from Rural and Urban Areas of Ugu District after Sequencing of 16S rRNA Genes
4. Discussion
4.1. Social Disparities in Housing, Education Level, Employment Rate, and Health
4.2. Disparities in Sanitation, Water Supply, and Water Quality
5. Conclusions and Recommendation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MacDonald, G.J.; Defelice, N.; Sebastian, D.; Leker, H. Racial disparities in access to community water supply service in Wake County, North Carolina. Front. Public Health Serv. Syst. Res. 2014, 3, 25–35. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Global Water Supply and Sanitation Assessment 2000 Report; UNICEF: Geneva, Switzerland, 2000. [Google Scholar]
- UN/WHO. Clean Water and Sanitation UN-Water Annual Report 2015. Available online: www.unwater.org/publications/un-waterannual-report (accessed on 22 June 2017).
- Bain, R.E.; Gundry, S.W.; Wright, J.A.; Yang, H.; Pedley, S.; Bartram, J.K. Accounting for water quality in monitoring access to safe drinking-water as part of the Millennium Development Goals: Lessons from five countries. Bull. World Health Organ. 2012, 90, 228–235. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization); UNICEF. Progress on Drinking Water and Sanitation: 2014 Update; WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation; WHO Press: Geneva, Switzerland, 2014. [Google Scholar]
- WHO (World Health Organization). Lack of Sanitation for 2.4 Billion People is Undermining Health Improvements. 2015. Available online: www.who.int (accessed on 19 July 2017).
- Safe Water Alliance; Environmental Justice Coalition for Water. Racial Discrimination and Access to Safe, Affordable Water for Communities of Color in California; A Report Submitted to the Committee on the Elimination of Racial Discrimination in its 85th Session United States’ Compliance with the International Convention on the Elimination of All Forms of Racial Discrimination; Safe Water Alliance: New York, NY, USA, 2014. [Google Scholar]
- Safe Water Alliance; Environmental Justice Coalition for Water. Environmental Justice Coalition for Water, and the International Human Rights Law Clinic; University of California, Berkeley, School of Law: Berkeley, CA, USA, 2014. [Google Scholar]
- Department of Water Affairs & Forestry (DWAF). Water Supply and Sanitation White Paper: Water—An Indivisible National Asset; Department of Water Affairs and Forestry: Pretoria, South Africa, 1994.
- Republic of South Africa. Constitution of the Republic of South Africa, 1996 (Act No. 108 of 1996). Gov. Gaz. 1996, 378, 176–178. [Google Scholar]
- Schwartz, J.; Levin, R.; Goldstein, R. Drinking water turbidity and gastrointestinal illness in the elderly of Philadelphia. J. Epidemiol. Community Health 2000, 54, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momba, M.N.B.; Kaleni, P. Regrowth and survival of indicator microorganisms on the surfaces of household containers used for the storage of drinking water in rural communities of South Africa. Water Res. 2002, 36, 3023–3028. [Google Scholar] [CrossRef]
- Obi, C.L.; Bessong, P.O. Diarrhoegenic bacterial pathogens in HIV-positive patients with diarrhoea in rural communities of Limpopo Province, South Africa. J. Health Popul. Nutr. 2002, 20, 230–234. [Google Scholar]
- Mackintosh, G.; Colvin, C. Failure of rural schemes in South Africa to provide potable water. Environ. Geol. 2003, 44, 101–105. [Google Scholar] [CrossRef]
- Momba, M.N.B.; Tyafa, Z.; Makala, N. Rural water treatment plants fail to provide potable water to their consumers: Alice water treatment plant in the Eastern Cape Province of South Africa. S. Afr. J. Sci. 2004, 100, 307–310. [Google Scholar]
- Obi, C.L.; Onabolu, B.; Momba, M.N.B.; Igumbor, J.O.; Ramalivahna, J.; Bessong, P.O.; Van Rensburg, E.J.; Lukoto, M.; Green, E.; Mulaudzi, T.B. The interesting cross-paths of HIV/AIDS and water in Southern Africa with special reference to South Africa. Water SA 2006, 32, 56–78. [Google Scholar]
- Momba, M.N.B.; Madoroba, E.; Obi, C.L. Apparent impact of enteric pathogens in drinking water and implications for the relentless saga of HIV/AIDS in South Africa. In Current Research, Technology, and Education Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; Formatex Microbiological Series: Badajoz, Spain, 2010; pp. 615–625. [Google Scholar]
- SANS 241. South African National Standard: Drinking Water Specification; Edition 6.1.; South African National Standard 241: 2006; South African Bureau of Standards: Pretoria, South Africa, 2006. [Google Scholar]
- WHO (World Health Organization); UNICEF. Progress on Sanitation and Drinking Water: 2015 Update and MDG Assessment; UNICEF, WHO: New York, NY, USA, 2015; ISBN 978-92-4-150329-7. [Google Scholar]
- Socio-Economic Profile_Ugu-District 2010. Available online: http://www.ugu.gov.za/pdfs/UGU_DM_INVEST_PROFILE.pdf (accessed on 14 December 2017).
- Pedley, S.; Howard, G. The public health implications of microbiological contamination of groundwater. Q. J. Eng. Geol. 1997, 30, 179–188. [Google Scholar] [CrossRef]
- Stewart, J.R.; Gast, R.J.; Fujioka, R.S.; Solo-Gabriele, H.M.; Meschke, J.S.; Amaral-Zettler, L.A.; Del Castillo, E.; Polz, M.F.; Collier, T.K.; Strom, M.S.; et al. The coastal environment and human health: Microbial indicators, pathogens, sentinels and reservoirs. Environ. Health 2008, 7, S3. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater; The American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF): Washington, DC, USA, 1992; Available online: www.apha.org (accessed on 14 August 2017).
- EPA (United States Environmental Protection Agency). Implementation Guidance for Ambient Water Quality for Bacteria; United States Environmental Protection Agency: Washington, DC, USA, 2004.
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; The American Public Health Association (APHA); Water Environment Federation (WEF); American Water Works Association (AWWA): Washington, DC, USA, 2001; pp. 254–278. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; The American Public Health Association (APHA); Water Environment Federation (WEF); American Water Works Association (AWWA): Washington, DC, USA, 2005; Available online: www.apha.org (accessed on 14 August 2017).
- Department of Water Affairs and Forestry (DWAF). South African Water Quality Guidelines for Domestic Use, 2nd ed.; Department of Water Affairs and Forestry: Pretoria, South Africa, 1996.
- Prescott, L.M.; Harley, J.P.; Klein, D.A. Microbiology, 2nd ed.; Wm. C. Brown: Dubuque, IA, USA, 1993; pp. 588–591. [Google Scholar]
- Dombek, P.E.; Johnson, L.K.; Zimmerley, S.T.; Sadowsky, M.J. Use of repetitive DNA sequences and the PCR to differentiate Escherichia coli isolates from human and animal sources. Appl. Environ. Microbiol. 2000, 66, 2572–2577. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Ksoll, W.B.; Hicks, R.E.; Sadowsky, M.J. Presence and growth of naturalized Escherichia coli in temperate soils from Lake Superior watersheds. Appl. Environ. Microbiol. 2006, 72, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Oswald, W.E.; Lescano, A.G.; Bern, C.; Calderon, M.M.; Cabrera, L.; Gilman, R.H. Faecal contamination of drinking water within peri-urban households, Lima, Peru. J. Trop. Med. Hyg. 2007, 77, 699–704. [Google Scholar] [CrossRef]
- Yamasaki, S.; Lin, Z.; Shirai, H.; Terai, A.; Oku, Y.; Ito, H.; Ohmura, M.; Karasawa, T.; Tsukamoto, T.; Kurazono, H.; et al. Typing of verotoxin by DNA colony hybridisation with poly- and oligonucleotide probe, a bead enzyme chain reaction. J. Microbiol. Immunol. 1996, 40, 345–352. [Google Scholar] [CrossRef]
- Sethabutr, O.; Venkatesan, M.; Marphy, G.S.; Eampokalap, B.; Hoge, C.W.; Echeverria, P. Detection of Shigella and enteroinvasive E. coli by amplification of the invasion plasmid antigen H DNA sequence in patients with dysentery. J. Infect. Dis. 1993, 167, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Ratchtrachenchai, O.A.; Subpasu, S.; Ito, K. Investigation on enteroaggregative Escherichia coli infection by multiplex PCR. Bull. Dept. Med. Sci. 1997, 39, 211–220. [Google Scholar]
- Mpehle, Z. Socio-economic and spatial inequalities in the provisioning of sustainable housing in South Africa. Politeia 2015, 34, 67–83. [Google Scholar] [CrossRef]
- Ellaway, A.; Macintyre, S.; Bonnefoy, X. Graffiti, Greenery, and Obesity in adults: Secondary analysis of European cross Sectional survey. Br. Med. J. 2005, 331, 611–612. [Google Scholar] [CrossRef]
- Baker, M.; Mcnicholas, A.; Garrett, N.; Jones, N.; Stewart, J.; Koberstein, V.; Lennon, D. Household crowding a major risk factor for epidemic meningococcal disease in Auckland children. Pediatr. Infect. Dis. J. 2000, 19, 983–990. [Google Scholar] [CrossRef]
- Jazairy, I.; Alamgir, M.; Panuccio, T. The State of World Rural Poverty: An Inquiry into Its Causes and Consequences; New York University Press (NYU Press): New York, NY, USA, 1992; ISBN 9789290720034. [Google Scholar]
- Sahn, D.E.; Stifel, D.C. Urban-rural inequality in living standards in Africa. J. Afr. Econ. 2003, 12, 564–597. [Google Scholar] [CrossRef]
- Wilson, S.M.; Heaney, C.D.; Cooper, J.; Wilson, O. Built environment issues in unserved and underserved African-American neighborhoods in North Carolina. Environ. Justice 2008, 1, 63–72. [Google Scholar] [CrossRef]
- Wilson, S.M.; Heaney, C.D.; Wilson, O. Governance structures and the lack of basic amenities: Can community engagement be effectively used to address environmental injustice in underserved black communities? Environ. Justice 2010, 3, 125–133. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Women and Health: Today’s Evidence Tomorrow’s Agenda; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- WHO/UNICEF 2016. Joint Monitoring Programme for Water Supply, Sanitation and Hygiene; 2016 Annual Report; World Health Organisation: Geneva, Switzerland, 2016. [Google Scholar]
- PieTrucha-urbaniK, K.; Studziński, A. Case study of failure simulation of pipelines conducted in chosen water supply system. Eksploatacja i Niezawodność 2017, 19. [Google Scholar] [CrossRef]
- Sorenson, S.B.; Morssink, C.; Campos, P.A. Safe access to safe water in low-income countries: Water fetching in current times. Soc. Sci. Med. 2011, 72, 1522–1526. [Google Scholar] [CrossRef]
- House, S.; Ferron, S.; Sommer, M.; Cavill, S. Violence, Gender and WASH: A Practitioner’s Toolkit—Making Water, Sanitation and Hygiene Safer Through Improved Programming and Services; Water Aid and Sanitation and Hygiene Applied Research for Equity (SHARE): London, UK, 2014. [Google Scholar]
- Firestone, L.; Kaswan, A.; Meraz, S. Environmental justice: Access to clean drinking water. Hastings Law J. 2006, 57, 1367–1386. [Google Scholar]
- Heaney, C.D.; Wing, S.; Wilson, S.; Campbell, R.; Cadwell, D.; Hopkins, B.; O’shea, S.; Yeatts, K. Public infrastructure disparities and the microbiological and chemical safety of drinking and surface water supplies in a community bordering a landfill. J. Environ. Health 2013, 75, 24–36. [Google Scholar]
- Doyle, J.T.; Kindness, L.; Bear, D.W.; Realbird, J.; Eggers, M.J. Addressing disparities in safe drinking water access on the Crow Reservation, Montana. In Proceedings of the Environmental Health Disparities and Environmental Justice Meeting, Raleigh, NC, USA, 29–31 July 2013; p. 28. [Google Scholar]
- Islam, M.S.; Hossain, M.A.; Khan, S.I.; Khan, M.N.H.; Sack, R.B.; Albert, M.J.; Huq, A.; Colwell, R.R. Survival of Shigella dysenteriae Type 1 on fomites. J. Health Popul. Nutr. 2001, 19, 177–182. [Google Scholar]
- Trevett, A.F.; Carter, R.C.; Tyrrel, S.F. The importance of domestic water quality management in the context of faecal-oral disease transmission. J. Water Health 2005, 3, 221–228. [Google Scholar] [CrossRef]
- Peter, G. Impact of rural water projects on hygienic behaviour in Swaziland. Phys. Chem. Earth 2010, 35, 772–779. [Google Scholar] [CrossRef]
- Blum, D.; Emeh, R.N.; Huttly, S.R.A.; Dosunmu-Ogunbi, O.; Okeke, N.; Ajala, M.; Okoro, J.I.; Akujobi, C.; Kirkwood, B.R.; Feachem, R.G. The Imo State (Nigeria) drinking water supply and sanitation project, 1. Description of the project, evaluation methods, and impact on intervening variables. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 309–315. [Google Scholar] [CrossRef]
- Singh, U.; Lutchmanariyan, R.; Wright, J.; Knight, S.; Jackson, S.; Langmark, J.; Voslooand, D.; Rodda, N. Microbial quality of drinking water from ground tanks and tankers at source and point-of-use in eThekwini Municipality, South Africa, and its relationship to health outcomes. Water SA 2013, 39, 663–674. [Google Scholar] [CrossRef]
- Trevett, A.F.; Carter, R.C.; Tyrrel, S.F. Water quality deterioration: A study of household drinking-water quality in rural Honduras. Int. J. Environ. Health 2004, 14, 273–283. [Google Scholar] [CrossRef]
- Clasen, T.; Roberts, I.; Rabie, T.; Schmidt, W.; Cairncross, S. Interventions to Improve Water Quality for Preventing Infectious Diarrhoea; The Cochrane Library: Oxford, UK, 2005. [Google Scholar]
- Yang, Z.; Wu, X.; Li, T.; Li, M.; Zhong, Y.; Liu, Y.; Deng, Z.; Di, B.; Huang, C.; Liang, H.; et al. Epidemiological survey and analysis on an outbreak of gastroenteristis due to water contamination. Biomed. Environ. Sci. 2011, 24, 275–283. [Google Scholar]
- Block, J.C.; Haudidier, K.; Paquin, J.L.; Miazga, J.; Levi, Y. Biofilm accumulation in drinking water distribution systems. Biofouling 1993, 6, 333–343. [Google Scholar] [CrossRef]
- Camper, A.K. Coliform regrowth and biofilm accumulation in drinking water systems: A review. In Biofouling and Biocorrosion in Industrial Water Systems; Geesey, G.G., Lewandowski, Z., Flemming, H.-C., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 91–105. [Google Scholar]
- Costerton, J.W.; Lewandowski, Z.; De Beer, D.; Caldwell, D.; Korber, D.; James, G. Biofilms, the customised microniche. J. Bacteriol. 1994, 176, 2137–2142. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Guidelines for Drinking-Water Quality, Incorporating 1st and 2nd Addenda, Recommendations, 3rd ed.; WHO: Geneva, Switzerland, 2008; Volume 1. [Google Scholar]
- Department of Water Affairs & Forestry (DWAF). South African Water Quality Guidelines—Volume 1: Domestic Water Use; Department of Water Affairs and Forestry: Pretoria, South Africa, 1996; pp. 86–87.






| E. coli Strain | Designation | Sequence (5′ to 3′) | Target Gene | Product Size (bp) | Reference |
|---|---|---|---|---|---|
| EPEC | SK1 | CCCGAATTCGGCACAAGCATAAGC | Eae | 881 | [31] |
| SK2 | CCCGGATCCGTCTCGCCAGTATTCG | ||||
| EHEC | VTcom-u | GAGCGAAATAATTTATATGTG | Stx | 518 | [32] |
| VTcom-d | TGATGATGGCAATTCAGTAT | ||||
| EIEC | ipaIII | GTTCCTTGACCGCCTTTCCGATACCGTC | ipaH | 619 | [33] |
| ipaIV | GCCGGTCAGCCACCCTCTGAGAGTAC | ||||
| EAEC | aggRks1 | GTATACACAAAAGAAGGAAGC | aggR | 254 | [34] |
| aggRks2 | ACAGAATCGTCAGCATCAGC |
| Socio-Demographic Characteristics | Rural Areas | Urban Areas | |||
|---|---|---|---|---|---|
| Frequency (n = 220) | Percentage (%) | Frequency (n = 108) | Percentage (%) | ||
| Water supply | Inside houses | 0 | 0 | 108 | 100 |
| Within the yard (1 m–2.5 m) | 20 | 9.1 | 0 | 0 | |
| Communal tap (≥ 200 m away) | 200 | 90.9 | 0 | 0 | |
| No access | 0 | 0 | 0 | 0 | |
| Sanitation facilities | Flush toilets | 0 | 0 | 108 | 100 |
| Pit latrines | 216 | 98.2 | 0 | 0 | |
| Open defecation | 4 | 1.8 | 0 | 0 | |
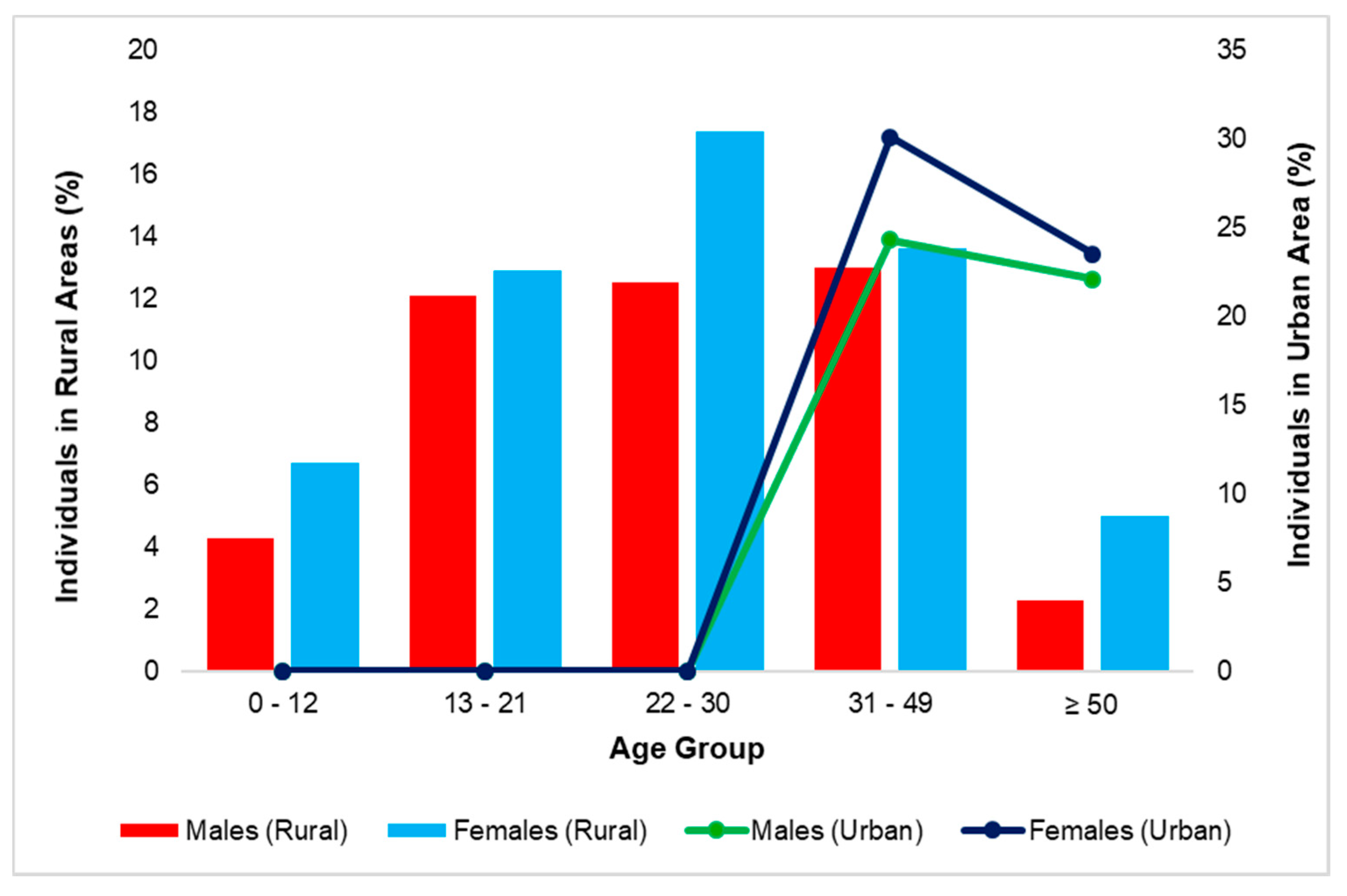
| Diarrhoeal episodes | Yes | 75 | 34.1 | 0 | 0 |
| No | 145 | 65.9 | 108 | 0 | |
| Socio-Demographic Characteristics | Rural Areas | Urban Areas | |||
|---|---|---|---|---|---|
| Frequency (n = 1533) | Percentage (%) | Frequency (n = 226) | Percentage (%) | ||
| Employment rate | Unemployed | 890 | 58.1 | 0 | 0 |
| Domestic | 177 | 11.5 | 0 | 0 | |
| Security job | 209 | 13.6 | 0 | 0 | |
| Professional | 189 | 12.3 | 126 | 55.8 | |
| Retired/pensioners | 68 | 4.5 | 100 | 44.2 | |
| Education level | Primary | 130 | 8.5 | 0 | 0 |
| Completed secondary school | 209 | 13.6 | 159 | 70.4 | |
| Tertiary education | 278 | 18.1 | 67 | 29.6 | |
| Drop-out | 916 | 59.8 | 0 | 0 | |
| Rural Area | ||||||||||||
| Parameter | Point of Treatment | Reservoir | Standpipe | Container-Stored Water | ||||||||
| BY | BM | Gam | BY | BM | Gam | BY | BM | Gam | BY | BM | Gam | |
| Turbidity (NTU) | 0.8 | 0.8 | 0.8 | 2.5 | 1.9 | 2.4 | 3.3 | 2.4 | 2.9 | 4.7 | 3.9 | 2.8 |
| Temperature (°C) | 26.4 | 26.4 | 26.4 | 25 | 25.6 | 25.3 | 25.4 | 24.8 | 25 | 23.2 | 22.7 | 22.6 |
| pH | 6.4 | 6.4 | 6.4 | 6.8 | 6.5 | 7.0 | 7.5 | 6.8 | 7.3 | 7.5 | 6.8 | 7.3 |
| Residual chlorine (mg/L) | 0.36 | 0.34 | 0.38 | 0.45 | 0.77 | 0.08 | 0.09 | 0.13 | 0.57 | 0.77 | 0.86 | 0.90 |
| Urban Area | ||||||||||||
| Parameter | Point of Treatment | Reservoir | Tap Water in Dwellings | |||||||||
| ANN | HIB | MG | PS | ANN | HIB | MG | PS | ANN | HIB | MG | PS | |
| Turbidity (NTU) | 0.8 | 0.8 | 0.8 | 0.8 | 1.5 | 1.9 | 1.6 | 1.5 | 1.5 | 1.8 | 1.6 | 1.5 |
| Temperature (°C) | 26.4 | 26.4 | 26.4 | 26.4 | 24.4 | 24.6 | 24.5 | 24.0 | 23 | 23.2 | 23.4 | 22.7 |
| pH | 6.4 | 6.4 | 6.4 | 6.8 | 6.7 | 6.7 | 6.8 | 6.6 | 6.8 | 6.8 | 6.9 | 6.6 |
| Residual chlorine (mg/L) | 0.14 | 0.36 | 0.1 | 0.36 | 0.33 | 0.32 | 0.38 | 0.41 | 0.31 | 0.40 | 0.37 | 0.81 |
| Rural Areas | ||||||||||||
| Point of Treatment | Reservoir | Standpipe | Stored Water | |||||||||
| Thermo-Tolerant Coliforms | Faecal Coliforms | E. coli | Thermo-Tolerant Coliforms | Faecal Coliforms | E. coli | Thermo-Tolerant Coliforms | Faecal Coliforms | E. coli | Thermo-Tolerant Coliforms | Faecal Coliforms | E. coli | |
| Boboyi | NG | NG | NG | NG | NG | NG | 1.079 | 0.954 | 0.903 | 1.653 | 1.544 | 1.255 |
| Bomela | NG | NG | NG | NG | NG | NG | 0.954 | 0.778 | 0.602 | 1.477 | 1.301 | 1.176 |
| Gamalakhe | NG | NG | NG | NG | NG | NG | 0.602 | 0.477 | 0.301 | 1.556 | 1.342 | 1.230 |
| Urban Areas | ||||||||||||
| Point of Treatment | Reservoir | Tap Water in Dwellings | ||||||||||
| Thermo-Tolerant Coliform | Faecal Coliforms | E. coli | Thermos-Tolerant Coliforms | Faecal Coliforms | E. coli | Thermo-Tolerant Coliforms | Faecal Coliforms | E. coli | ||||
| Annelin | NG | NG | NG | NG | NG | NG | NG | NG | NG | |||
| Hibberdene | NG | NG | NG | NG | NG | NG | NG | NG | NG | |||
| Margate | NG | NG | NG | NG | NG | NG | NG | NG | NG | |||
| Port Shepstone | NG | NG | NG | NG | NG | NG | NG | NG | NG | |||
| Organisms Detected | Rural Area (Storage Containers) | Urban Area (In-House Taps) | |||||
|---|---|---|---|---|---|---|---|
| By | Bm | Gam | Ps | Mg | Ann | Hib | |
| N = 107 | N 90 | N = 90 | |||||
| E. coli | 65.4 % (70) | 66.7 % (60) | 63.3 % (57) | 0 % (0) | 0 % (0) | 0 % (0) | 0 % (0) |
| Rural Areas | Point of Treatment | Reservoir | Standpipe | Stored Water | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pathogenic E. coli | Pathogenic E. coli | EHEC | EPEC | EIEC | EAEC | EHEC | EPEC | EIEC | EAEC | |
| Boboyi | ND | ND | ND | 1 (1.5%) | ND | ND | ND | 2 (3.7%) | ND | ND |
| Bomela | ND | ND | ND | ND | ND | ND | ND | 1 (2.7%) | ND | ND |
| Gamalakhe | ND | ND | ND | ND | ND | ND | ND | 1 (1.4%) | ND | ND |
| Urban Area | ||||||||||
| Margate | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Annelin | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Hibberdene | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Port Shepstone | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khabo-Mmekoa, C.M.N.; Momba, M.N.B. The Impact of Social Disparities on Microbiological Quality of Drinking Water Supply in Ugu District Municipality of Kwazulu-Natal Province, South Africa. Int. J. Environ. Res. Public Health 2019, 16, 2972. https://doi.org/10.3390/ijerph16162972
Khabo-Mmekoa CMN, Momba MNB. The Impact of Social Disparities on Microbiological Quality of Drinking Water Supply in Ugu District Municipality of Kwazulu-Natal Province, South Africa. International Journal of Environmental Research and Public Health. 2019; 16(16):2972. https://doi.org/10.3390/ijerph16162972
Chicago/Turabian StyleKhabo-Mmekoa, C. M. N., and M. N. B. Momba. 2019. "The Impact of Social Disparities on Microbiological Quality of Drinking Water Supply in Ugu District Municipality of Kwazulu-Natal Province, South Africa" International Journal of Environmental Research and Public Health 16, no. 16: 2972. https://doi.org/10.3390/ijerph16162972





