Shorter Time to Full Preterm Feeding Using Intact Protein Formula: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Randomization and Study Group Allocation
2.3. Feeding Protocol
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
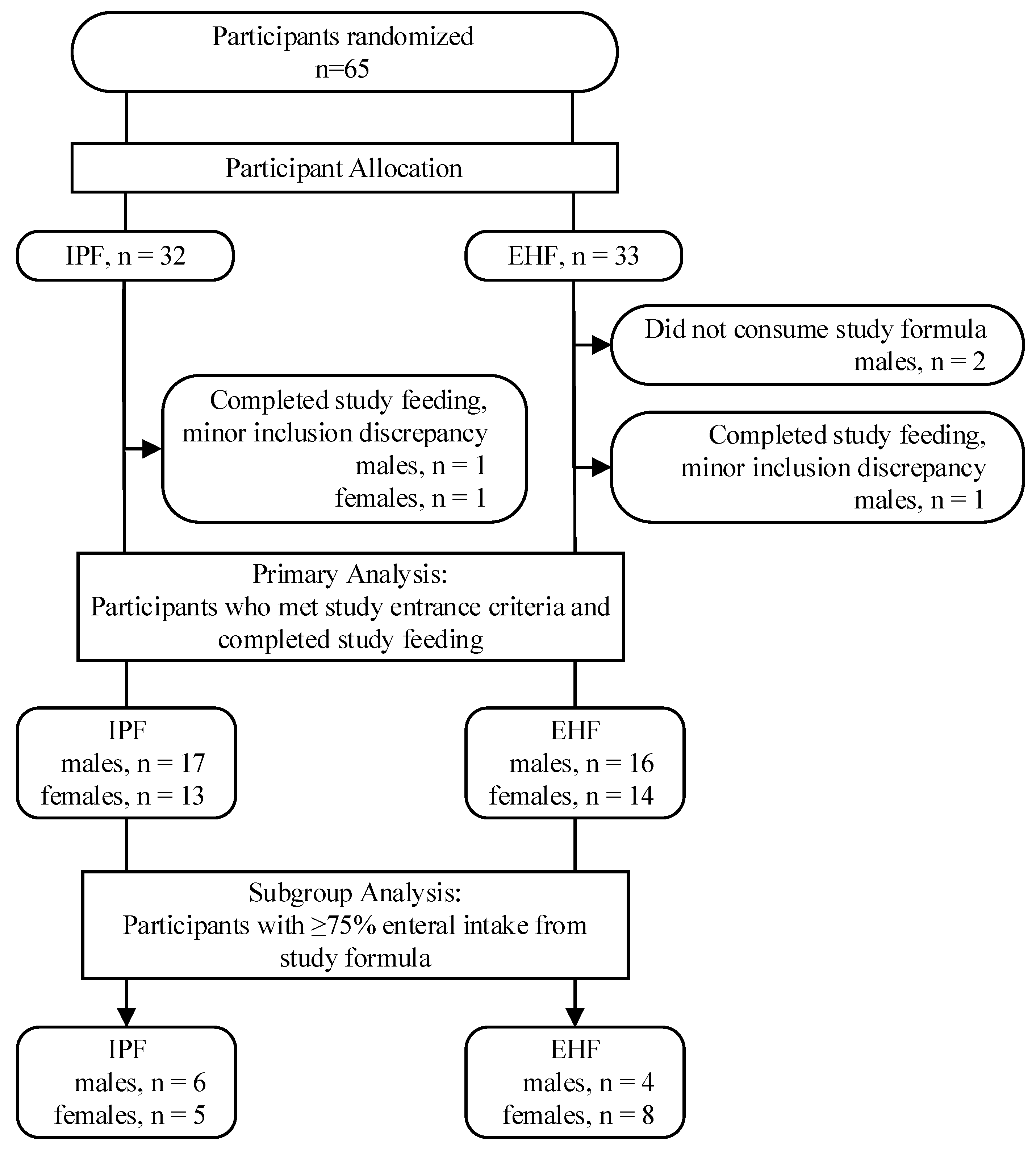
3.1. Participants
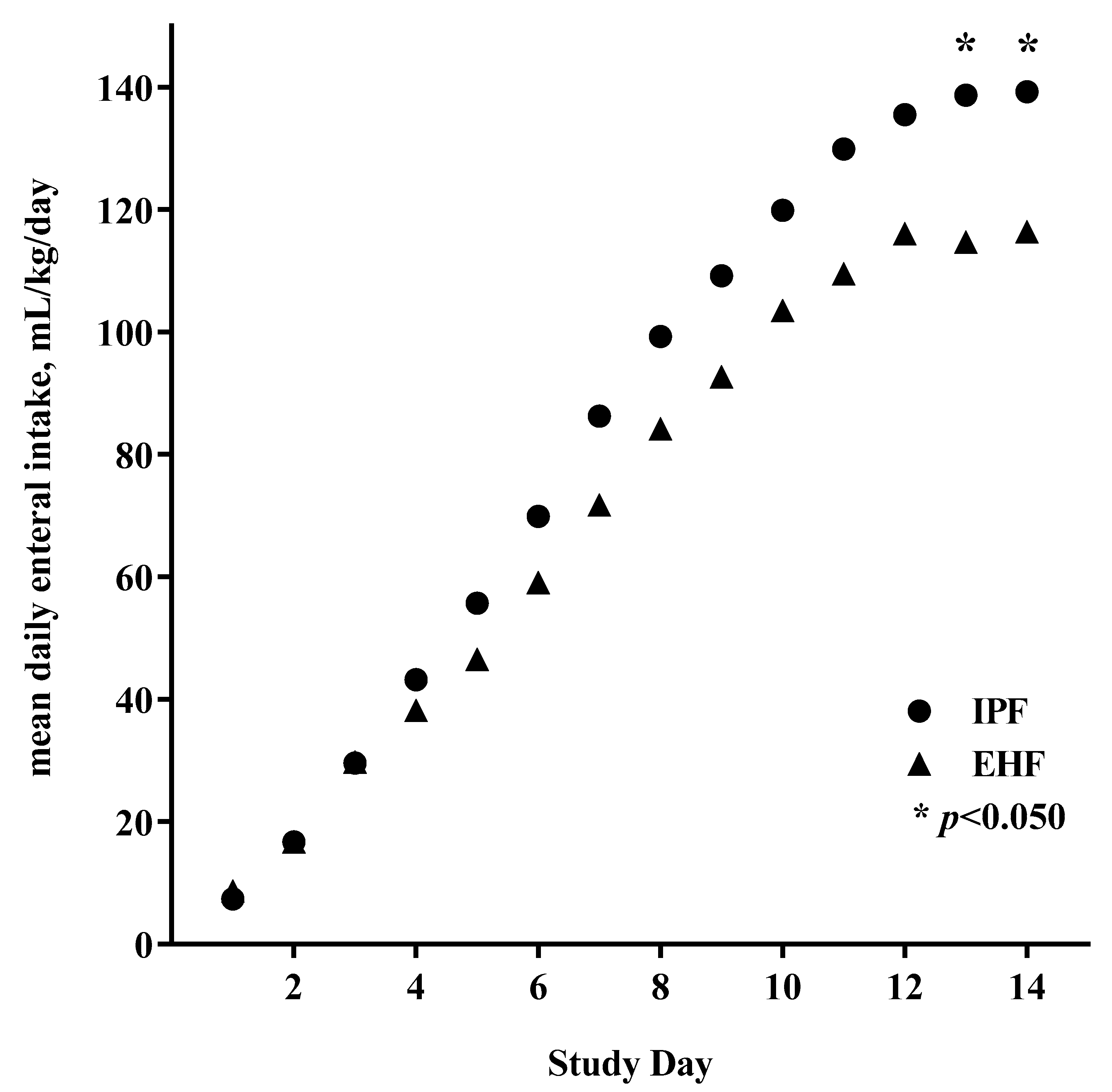
3.2. Feeding Advancement
3.3. Growth
3.4. Tolerance Measures, Indicators of Respiratory Status, and Global Morbidity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Day of Life | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Max. |
|---|---|---|---|---|---|---|---|---|
| Calories (k/kg/day) | 40 | 50 | 65 | 75 | 85 | 90 | 100 | 110 |
| Glucose (g/kg/day) | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 14 |
| Lipids (g/kg/day) | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.0 | 3.5 |
| Proteins (g/kg/day) | 2.0 | 2.5 | 3.0 | 3.5 | 3.5 | 3.5 | 3.5 | 4.0 |
| Calcium (mg/kg/day) | 40 | 50 | 60 | 65 | 65 | 65 | 65 | 70 |
| Phosphorus (mg/kg/day) | 20 | 30 | 40 | 45 | 45 | 45 | 55 | |
| Magnesium(mg/kg/day) | 5 | 5 | 5 | 5 | 5 | 10 |
References
- Indrio, F.; Riezzo, G.; Cavallo, L.; Di Mauro, A.A.; Francavilla, R. Physiological basis of food intolerance in VLBW. J. Matern. Fetal Neonatal Med. 2011, 24, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Pietz, J. Feeding and Fasting in the Neonatal Intensive Care Unit. J. Parenter. Enteral Nutr. 2014, 39, 621. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.; Embleton, N.D.; Jacobs, S.E.; O’Connell, L.A.; Kuschel, C.A. Enteral feeding practices in very preterm infants: An international survey. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F56–F61. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellof, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, R.E. (Ed.) Nutritional Needs of the Preterm Infant. In Pediatric Nutrition, 7th ed.; American Academy of Pediatrics: Itasca, IL, USA, 2014. [Google Scholar]
- Ng, D.H.; Embleton, N.D.; McGuire, W. Hydrolyzed Formula Compared with Standard Formula for Preterm Infants. JAMA 2018, 319, 1717–1718. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, W.A.; Franz, A.R.; Högel, J.; Pohlandt, F. Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics 2002, 110, 1199–1203. [Google Scholar] [CrossRef]
- Zuppa, A.A.; Visintini, F.; Cota, F.; Maggio, L.; Romagnoli, C.; Tortorolo, G. Hydrolysed milk in preterm infants: An open problem. Acta Paediatr. 2005, 94, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, A.; Matar, M.; Adleff, A.; Chbihi, M.; Kermorvant-Duchemin, E.; Campeotto, F. Use of extensively hydrolysed formula for refeeding neonates postnecrotising enterocolitis: A nationwide survey-based, cross-sectional study. BMJ Open 2016, 6, e008613. [Google Scholar] [CrossRef]
- Szajewska, H. Extensive and partial protein hydrolysate preterm formulas. J. Pediatr. Gastroenterol. Nutr. 2007, 45, S183–S187. [Google Scholar] [CrossRef]
- Ng, D.H.C.; Klassen, J.; Embleton, N.D.; McGuire, W. Protein hydrolysate versus standard formula for preterm infants. Cochrane Database Syst. Rev. 2017, 10, CD012412. [Google Scholar] [CrossRef]
- Dutta, S.; Singh, B.; Chessell, L.; Wilson, J.; Janes, M.; McDonald, K.; Watson, J.; Shahid, S.; Gardner, V.A.; Hjartarson, A.; et al. Guidelines for feeding very low birth weight infants. Nutrients 2015, 7, 423–442. [Google Scholar] [CrossRef] [PubMed]
- Flidel-Rimon, O.; Friedman, S.; Lev, E.; Juster-Reicher, A.; Amitay, M.; Shinwell, E.S. Early enteral feeding and nosocomial sepsis in very low birthweight infants. Arch Dis. Child. Fetal Neonatal Ed. 2004, 89, F289–F292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Härtel, C.; Haase, B.; Browning-Carmo, K.; Gebauer, C.; Kattner, E.; Kribs, A.; Herting, E.; Segerer, H.; Teig, N.; Wense, A.; et al. Does the enteral feeding advancement affect short-term outcomes in very low birth weight infants? J. Pediatr. Gastroenterol. Nutr. 2009, 48, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Rochow, N.; Fusch, G.; Mühlinghaus, A.; Niesytto, C.; Straube, S.; Utzig, N.; Fusch, C. A nutritional program to improve outcome of very low birth weight infants. Clin. Nutr. 2012, 31, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zecca, E.; Costa, S.; Barone, G.; Giordano, L.; Zecca, C.; Maggio, L. Proactive enteral nutrition in moderately preterm small for gestational age infants: A randomized clinical trial. J. Pediatr. 2014, 165, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/show/NCT01987154 (accessed on 13 August 2019).
- Bertino, E.; Spada, E.; Occhi, L.; Coscia, A.; Giuliani, F.; Gagliardi, L.; Milani, S.; Gilli, G.; Bona, G.; Fabris, C.; et al. Neonatal anthropometric charts: The Italian neonatal study compared with other European studies. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, R.; Bizzarri, B.; Giampietro, S.; De Curtis, M. Feeding intolerance in preterm infants. How to understand the warning signs. J. Matern. Fetal Neonatal Med. 2011, 24, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, D.W.; Brady, M.T.; Jackson, M.; Long, S.S. (Eds.) 2015 Group B Streptococcal Infections. In Red Book, 30th ed.; American Academy of Pediatrics: Itasca, IL, USA, 2015; p. 1064. [Google Scholar]
- Neu, J. Necrotizing enterocolitis: The search for a unifying pathogenic theory leading to prevention. Pediatr. Clin. N. Am. 1996, 43, 409–432. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Walsh, M.C.; Vohr, B.R.; Jobe, A.H.; Wright, L.L.; Fanaroff, A.A.; Wrage, L.A.; Poole, K. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005, 116, 1353–1360. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. An international classification of retinopathy of prematurity. Pediatrics 1984, 74, 127–133. [Google Scholar]
- Volpe, J.J. (Ed.) Intracranial Hemorrhage: Germinal matrix-intraventricular hemorrhage of the premature infant. In Neurology of the Newborn; Saunders Elsevier: Philadelphia, PA, USA, 2008; p. 1120. [Google Scholar]
- Clark, R.H.; Thomas, P.; Peabody, J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003, 111, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.E.; Di Mauro, A.; Montagna, O.; Fanelli, M.; Capozza, M.; Wampler, J.L.; Cooper, T.; Laforgia, N. Faster Gastric Emptying Is Unrelated to Feeding Success in Preterm Infants: Randomized Controlled Trial. Nutrients 2019, 11, 1670. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Pan, J.H. Clinical effect of extensively hydrolyzed formula in preterm infants: An analysis of 327 cases. Chin. J. Contemp. Pediatr. 2017, 19, 856–860. [Google Scholar]
- Florendo, K.N.; Bellflower, B.; Van Zwol, A.; Cooke, R.J. Growth in preterm infants fed either a partially hydrolyzed whey or an intact casein/whey preterm infant formula. J. Perinatol. 2009, 29, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.X.; Zhuang, S.Q.; Wang, D.H.; Zhou, X.Y.; Liu, X.H.; Shi, L.P.; Sun, J.H.; Yue, S.J.; Qian, J.H. Effects of extensively hydrolyzed protein formula on feeding and growth in preterm infants: A multicenter controlled clinical study. Chin. J. Contemp. Pediatr. 2014, 16, 684–690. [Google Scholar]
- Rigo, J.; Salle, B.L.; Picaud, J.C.; Putet, G.; Senterre, J. Nutritional evaluation of protein hydrolysate formulas. Eur. J. Clin. Nutr. 1995, 49, S26–S38. [Google Scholar]
- Mihatsch, W.A.; Pohlandt, F. Protein hydrolysate formula maintains homeostasis of plasma amino acids in preterm infants. J. Pediatr. Gastroenterol. Nutr. 1999, 29, 406–410. [Google Scholar] [CrossRef]
- Szajewska, H.; Albrecht, P.; Stoitiska, B.; Prochowska, A.; Gawecka, A.; Laskowska-Klita, T. Extensive and partial protein hydrolysate preterm formulas: The effect on growth rate, protein metabolism indices, and plasma amino acid concentrations. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 303–309. [Google Scholar] [CrossRef]
- Picaud, J.C.; Rigo, J.; Normand, S.; Lapillonne, A.; Reygrobellet, B.; Claris, O.; Salle, B.L. Nutritional efficacy of preterm formula with a partially hydrolyzed protein source: A randomized pilot study. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 555–561. [Google Scholar] [CrossRef]
- Maggio, L.; Zuppa, A.A.; Sawatzki, G.; Valsasina, R.; Schubert, W.; Tortorolo, G. Higher urinary excretion of essential amino acids in preterm infants fed protein hydrolysates. Acta Paediatr. 2005, 94, 75–84. [Google Scholar] [CrossRef]
- Rochow, N.; Landau-Crangle, E.; Fusch, C. Challenges in breast milk fortification for preterm infants. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 276–284. [Google Scholar] [CrossRef]

| Characteristics | Study Formula * | |
|---|---|---|
| IPF | EHF | |
| Nutrient density (cal/fl oz) | 24 | 24 |
| Nutrient composition per 100 kcal | ||
| Total Protein (g, % calories) † | 3.0, 12% | 2.8, 11% |
| Total Carbohydrate (g, % calories) ‡ | 11, 44% | 10.2, 41% |
| Total Fat (g, % calories) § | 5.1, 44% | 5.6, 48% |
| Arachidonic acid (ARA), mg | 34 | 34 |
| Docosahexaenoic acid (DHA), mg | 17 | 17 |
| Potential Renal Solute Load (mOsm/100 Cal) | 27 | 25 |
| Potential Renal Solute Load (mOsm/100 mL) | 21 | 21 |
| Osmolality (mOsm/kg H2O) | 310 | 340 |
| Osmolarity (mOsm/L) | 270 | 300 |
| Infant Characteristics | Study Group | p | |
|---|---|---|---|
| IPF | EHF | ||
| Gender, n (%) | |||
| Male | 17 (56.7) | 16 (53.3) | 0.795 |
| Female | 13 (43.3) | 14 (46.7) | |
| Birth type, n (%) | |||
| Singleton | 15 (50) | 17 (56.7) | 0.605 |
| Twin | 15 (50) | 13 (43.3) | |
| Caesarean Section, n (%) | 26 (86.7) | 26 (86.7) | 1.000 |
| Apgar score, n (%) | |||
| 6–7 | 3 (10.0) | 6 (20) | 0.760 |
| 8 | 6 (20.0) | 6 (20) | |
| 9 | 16 (53.3) | 12 (40) | |
| 10 | 5 (16.7) | 6 (20) | |
| Gestational age (days) * | 30.1 (1.6) | 30.9 (1.9) | 0.803 |
| Birth anthropometrics * | |||
| Weight (g) | 1278.7 (259.7) | 1301 (293.2) | 0.756 |
| Length (cm) | 38.1 (2.8) | 38.1 (3.3) | 0.973 |
| Head circumference (cm) | 27.3 (1.7) | 27.7 (2.4) | 0.481 |
| Tolerance Outcomes | Primary Analysis | p | Subset Analysis | p | ||
|---|---|---|---|---|---|---|
| IPF, n = 30 | EHF, n = 30 | IPF, n = 11 | EHF, n = 12 | |||
| Number of stools/day * | 2 (0.8) | 2 (0.9) | 0.525 | 1 (0.5) | 2 (0.9) | 0.160 |
| Abdominal distention ** | 0 | 0 | 1 | 0 | 0 | 1 |
| Regurgitation/emesis ** | 21 | 18 | 1 | 29 | 18 | 0.684 |
| Feedings withheld ≥4 h ** | 14 | 25 | 0.060 | 14 | 25 | 0.400 |
| Bloody stools ** | 0 | 0 | 0.472 | 0 | 0 | 1 |
| Parenteral nutrition, days * | 11 (7.1) | 15 (12.3) | 0.181 | 10 (6.5) | 15 (12.6) | 0.315 |
| Global morbidities, n (%) | ||||||
| Early-onset sepsis | 2 (6.7) | 3 (10) | 0.640 | 1 (9.1) | 1(8.3) | 0.739 |
| Late-onset sepsis | 6 (20) | 6 (20) | 1.000 | 2 (18.2) | 3 (25) | 0.545 |
| BDP | 0 (0) | 3 (10) | 0.119 | 0 (0) | 1 (8.3) | 0.522 |
| IVH | 1(3.3) | 4 (13.3) | 0.177 | 0 (0) | 0 (0) | 1.000 |
| ROP | 5 (16.7) | 7 (23.3) | 0.519 | 1 (9.1) | 4 (33.3) | 0.185 |
| EUGR | 18 (60) | 16 (53.3) | 0.602 | 8 (72.7) | 9 (75) | 0.635 |
| Hospitalization, days † | 44 (38–50) | 49 (34–64) | 0.236 | 49 (36–62) | 44 (28–60) | 0.871 |
| Postmenstrual age at discharge, weeks * | 37 (2.1) | 38 (2.8) | 0.229 | 37 (2.1) | 38 (1.9) | 0.740 |
| Weight at discharge, g * | 2141 (391) | 2140 (283) | 0.996 | 2176 (293) | 2128 (204) | 0.704 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldassarre, M.E.; Di Mauro, A.; Fanelli, M.; Capozza, M.; Wampler, J.L.; Cooper, T.; Laforgia, N. Shorter Time to Full Preterm Feeding Using Intact Protein Formula: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2019, 16, 2911. https://doi.org/10.3390/ijerph16162911
Baldassarre ME, Di Mauro A, Fanelli M, Capozza M, Wampler JL, Cooper T, Laforgia N. Shorter Time to Full Preterm Feeding Using Intact Protein Formula: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2019; 16(16):2911. https://doi.org/10.3390/ijerph16162911
Chicago/Turabian StyleBaldassarre, Maria Elisabetta, Antonio Di Mauro, Margherita Fanelli, Manuela Capozza, Jennifer L. Wampler, Timothy Cooper, and Nicola Laforgia. 2019. "Shorter Time to Full Preterm Feeding Using Intact Protein Formula: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 16, no. 16: 2911. https://doi.org/10.3390/ijerph16162911
APA StyleBaldassarre, M. E., Di Mauro, A., Fanelli, M., Capozza, M., Wampler, J. L., Cooper, T., & Laforgia, N. (2019). Shorter Time to Full Preterm Feeding Using Intact Protein Formula: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 16(16), 2911. https://doi.org/10.3390/ijerph16162911









