Glyphosate’s Synergistic Toxicity in Combination with Other Factors as a Cause of Chronic Kidney Disease of Unknown Origin
Abstract
:1. Introduction
2. Environmental Condition of People with Chronic Kidney Disease of Unknown Etiology
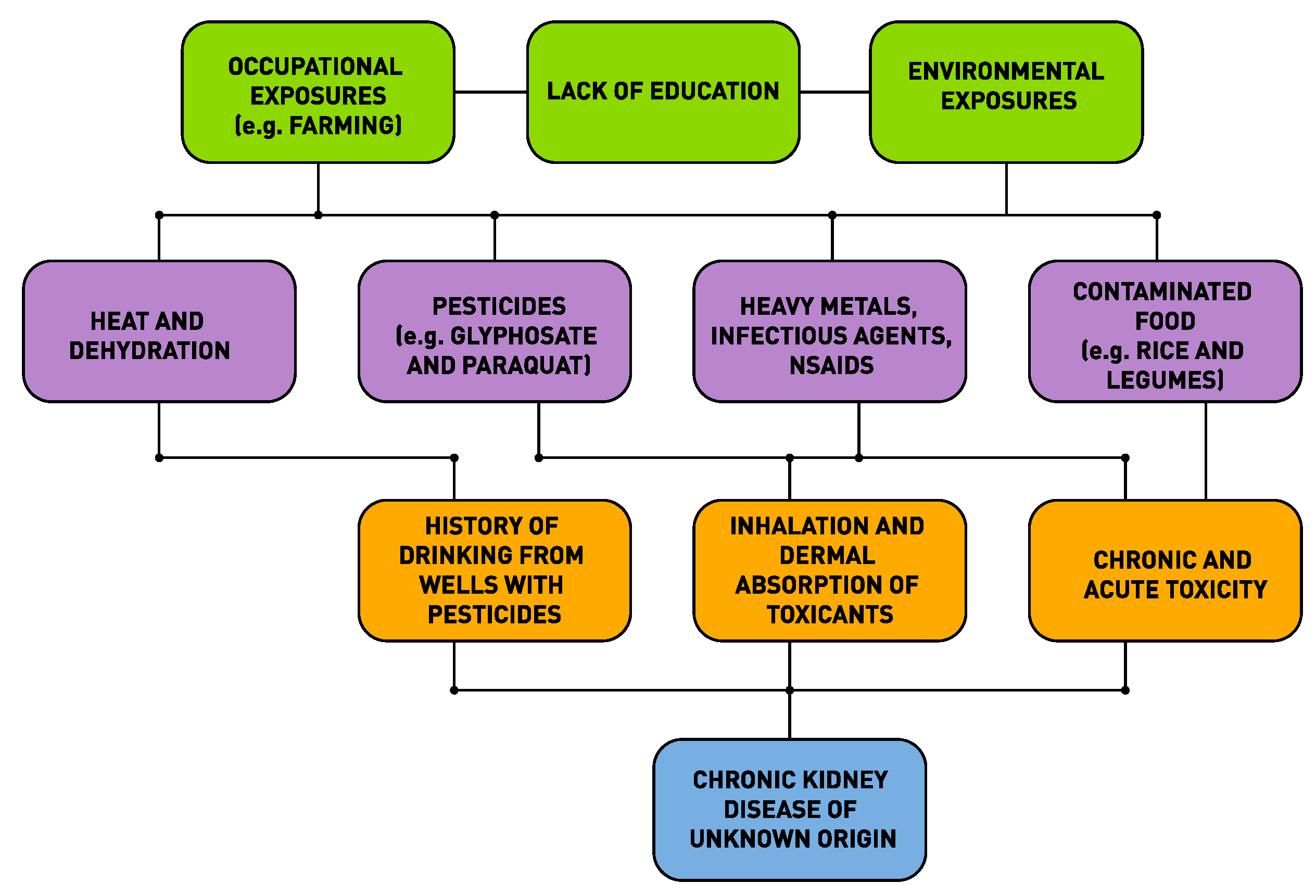
3. The Web of Causation of CKDu
4. Glyphosate as a Glycine Analogue
5. Glyphosate and Phosphate Binding
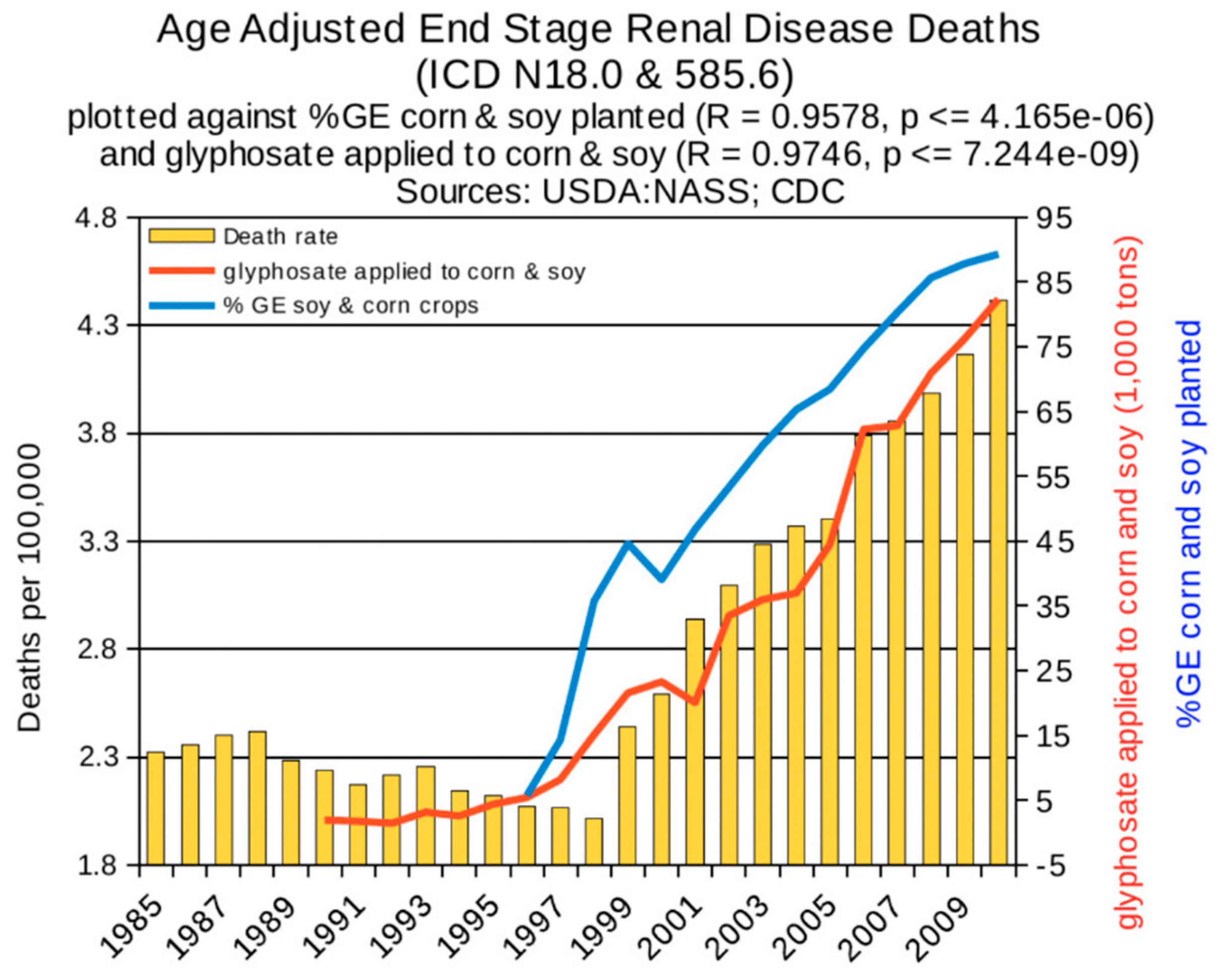
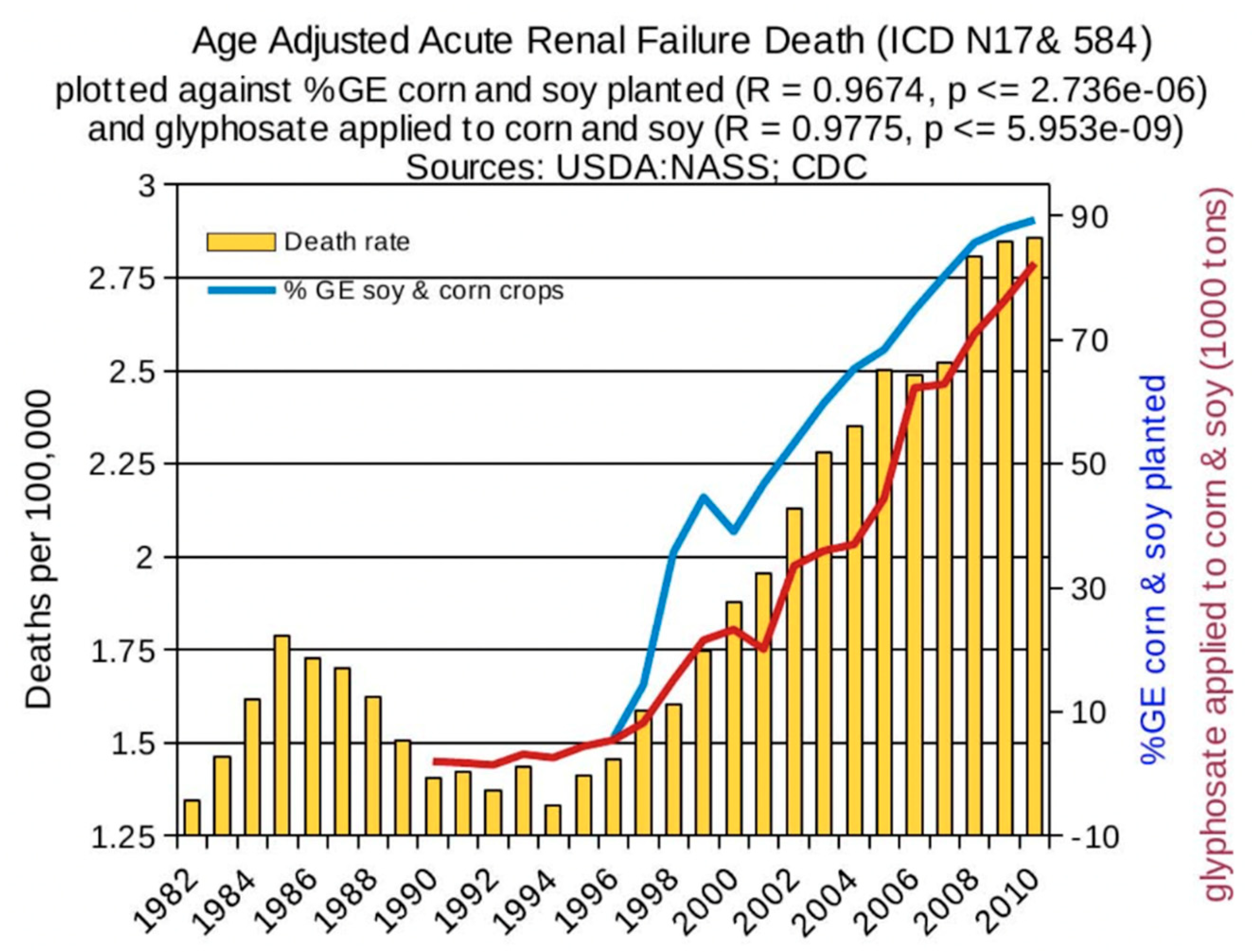
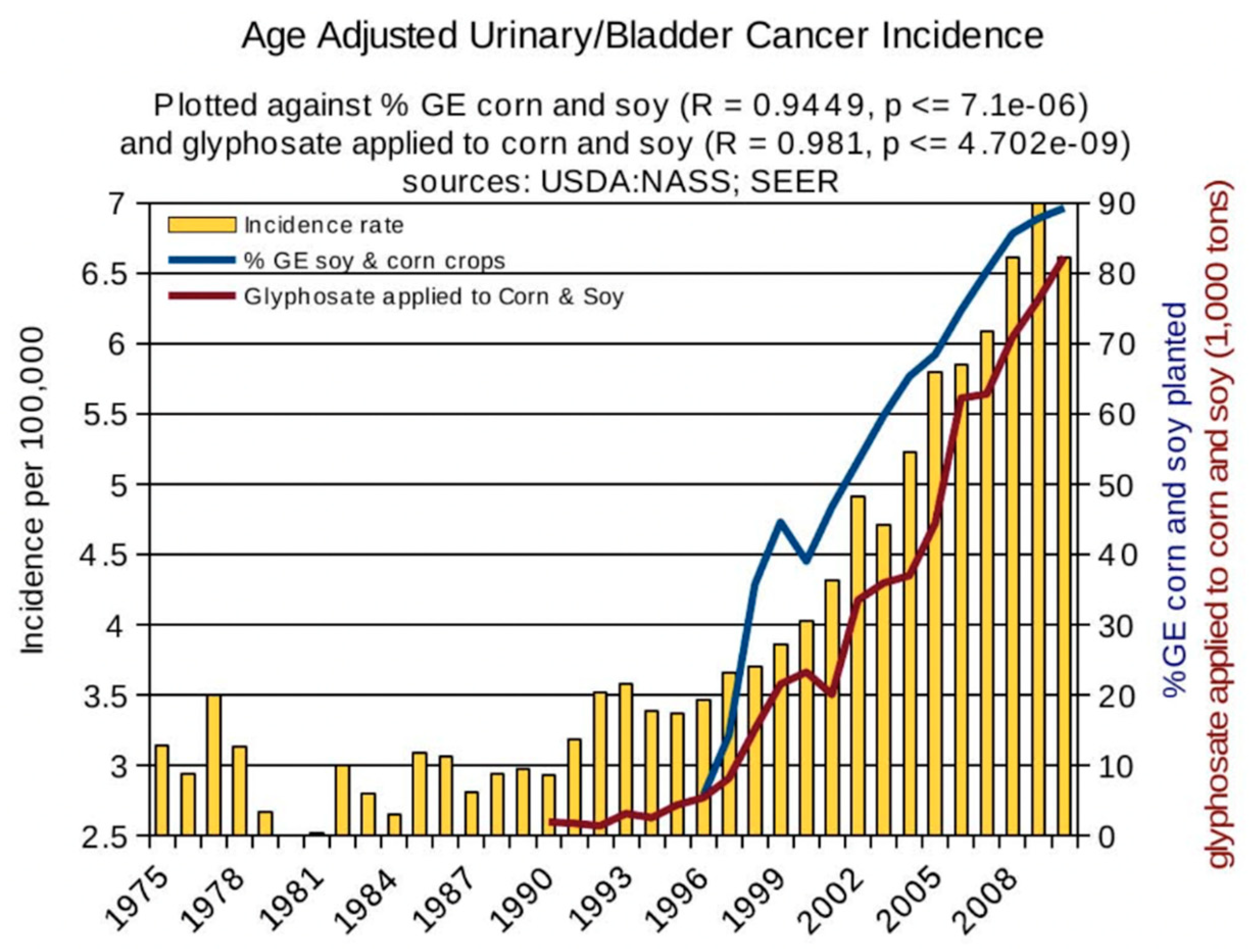
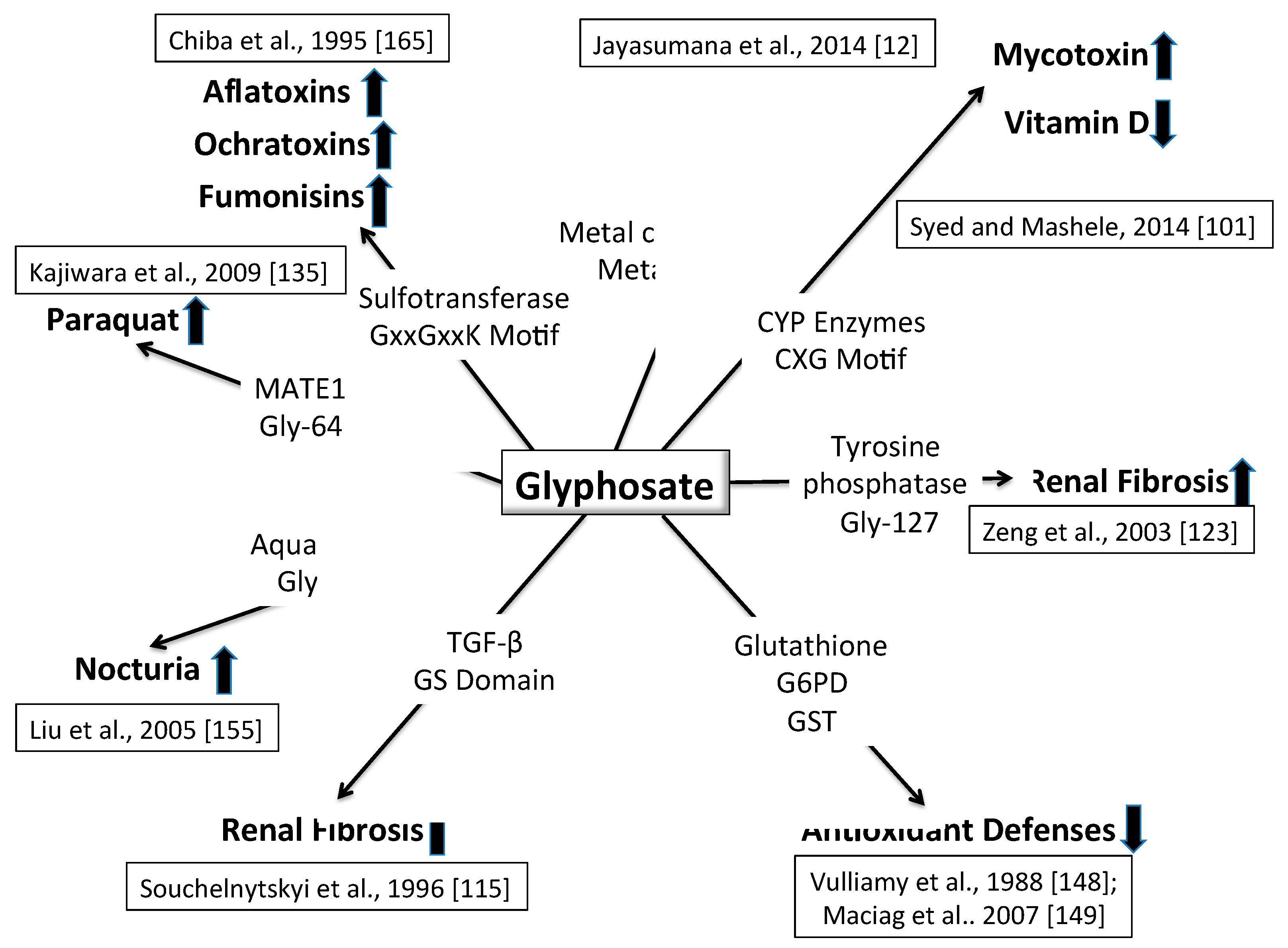
6. Synergy between Glyphosate and Other Toxic Elements
6.1. Glyphosate and Cytochrome P450 Impairment
6.2. Glyphosate and Toxic Metals
6.3. Hyperphosphorylation and Tubular Fibrosis Following Injury
6.4. Paraquat and MATE1
6.5. Impaired Antioxidant Defenses
6.6. Aquaporins and Dehydration
6.7. Cyanobacteria and Glyphosate
6.8. Mycotoxins and Sulfotransferases
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jayasekara, J.M.; Dissanayake, D.M.; Adhikari, S.B.; Bandara, P. Geographical distribution of chronic kidney disease of unknown origin in North Central Region of Sri Lanka. Ceylon Med. J. 2013, 58, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Jayasekara, J.M.; Dissanayake, D.M.; Gunaratne, N.; Ramiah, S.; Dissanayake, D.M. Prevalence of G6PD deficiency in patients with chronic kidney disease of unknown origin in North Central region of Sri Lanka: Case control study. Int. J. Recent Sci. Res. 2013, 4, 455–458. [Google Scholar]
- Wimalawansa, S.J. Agrochemicals and Chronic Kidney Disease of Multi-Factorial Origin: Environmentally Induced Occupational Exposure an Occupational Exposure Disease. Int. J. Nephrol. Kidney Fail. 2015, 1. [Google Scholar] [CrossRef]
- Rajapakse, S.; Shivanthan, M.C.; Selvarajah, M. Chronic kidney disease of unknown etiology in Sri Lanka. Int. J. Occup. Environ. Health 2016, 22, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute for Health Metrics and Evaluation. Available online: http://www.healthdata.org/el-salvador (accessed on 2 December 2018).
- Almaguer, M.; Herrera, R.; Orantes, C.M. Chronic Kidney Disease of Unknown Etiology in Agricultural Communities. MEDICC Rev. 2014, 16, 9–15. [Google Scholar] [PubMed]
- Perez-Gomez, M.V.; Martin-Cleary, C.; Fernandez-Fernandez, B.; Ortiz, A. Meso-American nephropathy: What we have learned about the potential genetic influence on chronic kidney disease development. Clin. Kidney J. 2018, 11, 491–495. [Google Scholar] [CrossRef]
- López-Marín, L.; Chávez, Y.; García, X.A.; Flores, W.M.; García, Y.M.; Herrera, R.; Almaguer, M.; Orantes, C.M.; Calero, D.; Bayarre, H.D.; et al. Histopathology of chronic kidney disease of unknown etiology in Salvadoran agricultural communities. MEDICC Rev. 2014, 16, 49–54. [Google Scholar] [PubMed]
- Weaver, V.M.; Fadrowski, J.J.; Jaar, B.G. Global dimensions of chronic kidney disease of unknown etiology (CKDu): A modern era environmental and/or occupational nephropathy? BMC Nephrol. 2015, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Orlando, L.F. Is glyphosate a key factor in Mesoamerican nephropathy? J. Environ. Anal. Toxicol. 2018, 8, 542. [Google Scholar] [CrossRef]
- Jayasumana, C.; Gunatilake, S.; Senanayake, P. Glyphosate, hard water and nephrotoxic metals: Are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? Int. J. Environ. Res. Public Health 2014, 11, 2125–2147. [Google Scholar] [CrossRef]
- Jayasumana, C.; Gajanayake, R.; Siribaddana, S. Importance of Arsenic and pesticides in epidemic chronic kidney disease in Sri Lanka. BMC Nephrol. 2014, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Wijkström, J.; Jayasumana, C.; Dassanayake, R.; Priyawardane, N.; Godakanda, N.; Siribaddana, S.; Ring, A.; Hultenby, K.; Söderberg, M.; Elinder, C.G.; et al. Morphological and clinical findings in Sri Lankan patients with chronic kidney disease of unknown cause (CKDu): Similarities and differences with Mesoamerican Nephropathy. PLoS ONE 2018, 13, e0193056. [Google Scholar] [CrossRef] [PubMed]
- Bandara, J.M.; Senevirathna, D.M.; Dasanayake, D.M.; Herath, V.; Bandara, J.M.; Abeysekara, T.; Rajapaksha, K.H. Chronic renal failure among farm families in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and freshwater fish (Tilapia). Environ. Geochem. Health 2008, 30, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Wanigasuriya, K.P.; Peiris-John, R.J.; Wickremasinghe, R. Chronic kidney disease of unknown aetiology in Sri Lanka: Is cadmium a likely cause? BMC Nephrol. 2011, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Chandrajith, R.; Nanayakkara, S.; Itai, K.; Aturaliya, T.N.; Dissanayake, C.B.; Abeysekera, T.; Harada, K.; Watanabe, T.; Koizumi, A. Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: Geographic distribution and environmental implications. Environ. Geochem. Health 2011, 33, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Redmon, J.H.; Elledge, M.F.; Womack, D.S.; Wickremashinghe, R.; Wanigasuriya, K.P.; Peiris-John, R.J.; Lunyera, J.; Smith, K.L.; Raymer, J.H.; Levine, K. Additional perspectives on chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka–lessons learned from the WHO CKDu population prevalence study. BMC Nephrol. 2014, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, E.A.; Perera, P.A.; Sivakanesan, R.; Abeysekara, T.; Nugegoda, D.B.; Weerakoon, K.G. Is the staple diet eaten in Medawachchiya, Sri Lanka, a predisposing factor in the development of chronic kidney disease of unknown etiology? A comparison based on urinary beta2-microglobulin measurements. BMC Nephrol. 2014, 15, 103. [Google Scholar] [CrossRef]
- Kumares, J.; Seneviratne, R. Beginning of a journey: Unraveling the mystery of chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka. Glob. Health 2017, 13, 43. [Google Scholar] [CrossRef]
- Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef]
- Avramova, N. Austrian Lawmakers Vote to Ban Weed Killer Glyphosate. CNN. 3 July 2019. Available online: https://www.cnn.com/2019/07/03/health/austria-glyphosate-ban-weed-killer-bayer-intl/index.html (accessed on 26 July 2019).
- Jayasumana, C.; Orantes, C.; Herrera, R.; Almaguer, M.; Lopez, L.; Silva, L.C.; Ordunez, P.; Siribaddana, S.; Gunatilake, S.; De Broe, M.E. Chronic interstitial nephritis in agricultural communities: A worldwide epidemic with social, occupational and environmental determinants. Nephrol. Dial. Transpl. 2017, 32, 234–241. [Google Scholar] [CrossRef]
- Glaser, J.; Lemery, J.; Rajagopalan, B.; Diaz, H.F.; García-Trabanino, R.; Taduri, G.; Madero, M.; Amarasinghe, M.; Abraham, G.; Anutrakulchai, S.; et al. Climate Change and the Emergent Epidemic of CKD from Heat Stress in Rural Communities: The Case for Heat Stress Nephropathy. Clin. J. Am. Soc. Nephrol. 2016, 11, 1472–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandara, N.J. Water and wastewater related issues in Sri Lanka. Water Sci. Technol. 2003, 47, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Sagasta, J.; Marjani Zadeh, S.; Turral, H. Water Pollution from Agriculture: A Global Review. The Food and Agricultural Organization 2017. Available online: http://www.fao.org/3/a-i7754e.pdf (accessed on 2 December 2018).
- Agampodi, S.B.; Amarasinghe, G.S.; Naotunna, P.G.C.R.; Jayasumana, C.S.; Siribaddana, S.H. Early renal damage among children living in the region of highest burden of chronic kidney disease of unknown etiology (CKDu) in Sri Lanka. BMC Nephrol. 2018, 19, 115. [Google Scholar] [CrossRef] [PubMed]
- Wanigasuriya, K. Update on uncertain etiology of chronic kidney disease in Sri Lankas north-central dry zone. MEDICC Rev. 2014, 16, 61–65. [Google Scholar] [PubMed]
- Noble, A.; Amerasinghe, P.; Manthrithilake, H.; Sutharsiny, A. Review of Literature on Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka. Colombo, Sri Lanka. IWMI 2014. [Google Scholar] [CrossRef]
- Jayasumana, C.; Fonseka, S.; Fernando, A.; Jayalath, K.; Amarasinghe, M.; Siribaddana, S.; Gunatilake, S.; Paranagama, P. Phosphate fertilizer is a main source of arsenic in areas affected with chronic kidney disease of unknown etiology in Sri Lanka. Springerplus 2015, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- Orantes-Navarro, C.M.; Herrera-Valdés, R.; Almaguer-López, M.; Elsy, G.; Brizuela-Díaz, E.G.; Nelly, P.; Alvarado-Ascencio, N.P.; Fuentes-de Morales, E.J.; Bayarre-Vea, H.D.; Calero-Brizuela, D.L.; et al. Chronic kidney disease in children and adolescents in Salvadoran farming communities: NefroSalva pediatric study (2009–2011). MEDICC Rev. 2016, 18, 15–21. [Google Scholar]
- Bandarage, A. Political Economy of Epidemic Kidney Disease in Sri Lanka. SAGE Open 2013, 1–13. [Google Scholar] [CrossRef]
- Gunatilake, S.; Illangasekere, T. Hydro-epidemiology of chronic kidney disease (CKD) in Sri Lanka and its similarities to the CKD epidemic in Meso-America. In Proceedings of the AGU Fall Meeting, San Francisco, CA, USA, 14–18 December 2015. [Google Scholar]
- Jayasumana, C.; Paranagama, P.; Agampodi, S.; Wijewardane, C.; Gunatilake, S.; Siribaddana, S. Drinking well water and occupational exposure to herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ. Health 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Abeysingha, N.S.; Dassanayake, K.B.; Weerarathna, C.S. Will restoration of ecological functions of tank cascade system contribute to reduce CKDu in Sri Lanka? A review. Environ. Manag. Sustain. Dev. 2018, 7, 60–81. [Google Scholar] [CrossRef]
- De Silva, M.W.A. Drinking water and chronic kidney disease of unknown aetiology in Anuradhapura, Sri Lanka. Anthr. Med. 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jayatilake, N.; Mendis, S.; Maheepala, P.; Mehta, F.R.; CKDu National Research Project Team. CKDu National Research Project Team. Chronic kidney disease of uncertain aetiology: Prevalence and causative factors in a developing country. BMC Nephrol. 2013, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Chandrajith, R.; Dissanayake, C.B.; Ariyarathna, T.; Herath, H.M.; Padmasiri, J.P. Dose-dependent Na and Ca in fluoride-rich drinking water—another major cause of chronic renal failure in tropical arid regions. Sci. Total Environ. 2011, 409, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Dharma-Wardana, M.W.; Amarasiri, S.L.; Dharmawardene, N.; Panabokke, C.R. Chronic kidney disease of unknown aetiology and ground-water ionicity: Study based on sri Lanka. Environ. Geochem. Health 2015, 37, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Jayasumana, C.; Gunatilake, S.; Siribaddana, S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 2015, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Lakshani, P.W.Y.; Rajapaksha, M.K.L.K.; Sendthuran, K. Pesticide residues in selected vegetables in several growing areas by GC/MS using QuEChERS Technique. Ann. Sri Lanka Dep. Agric. 2017, 19, 188–208. [Google Scholar]
- Gunarathna, S.; Gunawardana, B.; Jayaweera, M.; Manatunge, J.; Zoysa, K. Glyphosate and AMPA of agricultural soil, surface water, groundwater and sediments in areas prevalent with chronic kidney disease of unknown etiology, Sri Lanka. J. Environ. Sci. Health Part B 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.L.; Demetrio, P.M.; Etchegoyen, M.A.; Marino, D.J. Glyphosate and atrazine in rainfall and soils in agroproductive areas of the pampas region in Argentina. Sci. Total Environ. 2018, 645, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Thavarajah, D.; Thavarajah, P.; Wejesuriya, A.; Rutzke, M.A.; Glahn, R.P.; Combs, G.; Vandenberg, A. The potential of lentil (Lens culinaris L.) as a whole food for increased selenium, iron, and zinc intake: Preliminary results from a 3 year study. Euphytica 2011, 180, 123–128. [Google Scholar] [CrossRef]
- Sudhir, S.N.F. Imported Lentils Laced with Weed Killer. Deccan Chronicle. 19 July 2018. Available online: https://deccanchronicle.com/nation/current-affairs/190718/imported-lentils-laced-with-weed-killer.html (accessed on 10 September 2018).
- Mitra, T. Poison Foods of North America: Guide to Navigating the Glyphosate Mine Field in Our Food Web; Amazon Digital Services LLC: Seattle, WA, USA, 2017. [Google Scholar]
- Bandara, J.M.; Wijewardena, H.V.; Bandara, Y.M.; Jayasooriya, R.G.; Rajapaksha, H. Pollution of River Mahaweli and farmlands under irrigation by cadmium from agricultural inputs leading to a chronic renal failure epidemic among farmers in NCP, Sri Lanka. Environ. Geochem. Health 2011, 33, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Wanigasuriya, K. Aetiological Factors of Chronic Kidney Disease in the North Central Province of Sri Lanka: A Review of Evidence to-Date. J. Coll Community Physicians. Sri Lanka 2012, 17, 21–42. [Google Scholar] [CrossRef]
- Jayasumana, M.A.C.S.; Paranagama, P.A.; Amarasinghe, M.D.; Wijewardane, K.M.R.C.; Dahanayake, K.S.; Fonseka, S.I.; Rajakaruna, K.D.L.M.P.; Mahamithawa, A.M.P.; Samarasinghe, U.D.; Senanayake, V.K. Possible link of chronic arsenic toxicity to chronic kidney disease of unknown etiology in Sri Lanka. JNSR 2013, 3, 64–73. [Google Scholar]
- Correa-Rotter, R.; Wesseling, C.; Johnson, R.J. CKD of unknown origin in Central America: The case for a Mesoamerican nephropathy. Am. J. Kidney Dis. 2014, 63, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Campese, V.M. Con: Mesoamerican nephropathy: Is the problem dehydration or rehydration? Nephrol. Dial. Transplant. 2017, 32, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Laws, R.L.; Brooks, D.R.; Amador, J.J.; Laws, R.L.; Brooks, D.R.; Amador, J.J.; Weiner, D.E.; Kaufman, J.S.; Ramírez-Rubio, O.; Riefkohl, A.; et al. Changes in kidney function among Nicaraguan sugarcane workers. Int. J. Occup. Environ. Health 2015, 21, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mix, J.; Elon, L.; Mac, V.; Flocks, J.; Economos, E.; Tovar-Aguilar, A.J.; Stover Hertzberg, V.; McCauley, L.A. Hydration Status, Kidney Function and Kidney Injury in Florida Agricultural Workers. J. Occup. Environ. Med. 2017, 60, e253–e260. [Google Scholar] [CrossRef] [PubMed]
- García-Trabanino, R.; Jarquín, E.; Wesseling, C.; Johnson, R.J.; González-Quiroz, M.; Weiss, I.; Glaser, J.; José Vindell, J.; Stockfelt, L.; Roncal, C.; et al. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador—A cross-shift study of workers at risk of Mesoamerican nephropathy. Environ. Res. 2015, 142, 746–755. [Google Scholar] [CrossRef]
- Nerbass, F.B.; Pecoits-Filho, R.; Clark, W.F.; Sontrop, J.M.; McIntyre, C.W.; Moist, L. Occupational heat stress and kidney health: From farms to factories. Kidney Int. Rep. 2017, 2, 998–1008. [Google Scholar] [CrossRef]
- Xiang, W.-S.; Wang, X.-J.; Ren, T.-R.; Ju, X.L. Expression of a wheat cytochrome P450 monooxygenase in yeast and its inhibition by glyphosate. Pest Manag. Sci. 2005, 61, 402–406. [Google Scholar] [CrossRef]
- Samsel, A.; Seneff, S. Glyphosates suppression of cytochrome P450 enzymes and amino acid biosynthesis by the gut microbiome: Pathways to modern diseases. Entropy 2013, 15, 1416–1463. [Google Scholar] [CrossRef]
- MacMahon, B.; Pugh, T.F. Epidemiology: Principles and Methods; Little Brown and Co.: Boston, MA, USA, 1960. [Google Scholar]
- Kitchen, L.M.; Witt, W.W.; Rieck, C.E. Inhibition of δ-aminolevulinic acid synthesis by glyphosate. Weed Sci. 1981, 29, 571–577. [Google Scholar] [CrossRef]
- Cattani, D.; de Liz Oliveira Cavalli, V.L.; Rieg, C.E.H.; Domingues, J.T.; Dal-Cim, T.; Tasca, C.; Mena Barreto Silva, F.R.; Zamoner, A. Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: Involvement of glutamate excitotoxicity. Toxicology 2014, 320, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samsel, A.; Seneff, S. Glyphosate, pathways to modern diseases V: Amino acid analogue of glycine in diverse proteins. J. Biol. Phys. Chem. 2016, 16, 9–46. [Google Scholar] [CrossRef]
- Swanson, N.; Leu, A.; Abrahamson, J.; Wallet, B. Genetically engineered crops, glyphosate and the deterioration of health in the United States of America. J. Org. Syst. 2014, 9, 6–37. [Google Scholar]
- Hoy, J.; Swanson, N.; Seneff, S. The high cost of pesticides: Human and animal diseases. Poult. Fish Wildl. Sci. 2015, 3, 132. [Google Scholar] [CrossRef]
- Seneff, S.; Morley, W.; Hadden, M.J.; Michener, M. Does glyphosate acting as a glycine analogue contribute to ALS? J. Bioinfo. Proteom. Rev. 2016, 2, 1–21. [Google Scholar] [CrossRef]
- Samsel, A.; Seneff, S. Glyphosate pathways to modern diseases VI: Prions, amyloidoses and autoimmune neurological diseases. J. Biol. Phys. Chem. 2017, 17, 8–32. [Google Scholar] [CrossRef]
- Seneff, S.; Nigh, G. Glyphosate and anencephaly: Death by a thousand cuts. J. Neurol. Neurobiol. 2017, 3, 2. [Google Scholar] [CrossRef]
- Seneff, S.; Orlando, L. Glyphosate substitution for glycine during protein synthesis as a causal factor in Mesoamerican Nephropathy. J. Environ. Anal. Toxicol. 2018, 8, 541. [Google Scholar] [CrossRef]
- Seneff, S.; Causton, N.J.; Nigh, G.L.; Koenig, G.; Avalon, D. Can glyphosates disruption of the gut microbiome and induction of sulfate deficiency explain the epidemic in gout and associated diseases in the industrialized world? J. Biol. Phys. Chem. 2017, 17, 53–76. [Google Scholar] [CrossRef]
- Ridley, W.P.; Chott, K.A. Uptake, Depuration and Bioconcentration of C-14 Glyphosate to Bluegill Sunfish (Lepomis machrochirus) Part II: Characterization and Quantitation of Glyphosate and its Metabolites; Monsanto Agricultural Company: St Louis, MI, USA, 1989; unpublished study. [Google Scholar]
- Eschenburg, S.; Healy, M.L.; Priestman, M.A.; Lushington, G.H.; Schönbrunn, E. How the mutation glycine 96 to alanine confers glyphosate insensitivity to 5-enolpyruvyl shikimate-3-phosphate synthase from Escherichia coli. Planta 2002, 2016, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Funke, T.; Han, H.; Healy-Fried, M.L.; Fischer, M.; Schönbrunn, E. Molecular basis for the herbicide resistance of Roundup Ready crops. Proc. Natl. Acad. Sci. USA 2006, 103, 13010–13015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikorski, J.A.; Gruys, K.J. Understanding glyphosate’s molecular mode of action with EPSP synthase: Evidence favoring an allosteric inhibitor model. Acc. Chem. Res. 1997, 30, 2–8. [Google Scholar] [CrossRef]
- Franz, J.E.; Mao, M.K.; Sikorski, J.A. Glyphosate: A Unique Global Herbicide. Am. Chem. Soc. 1977, 12, 564–565. [Google Scholar] [CrossRef]
- Dong, Y.; Ng, E.C.; Lu, J.; Fenwick, T.; Tao, Y.; Bertain, S.; Sandoval, M.; Bermudez, E.; Hou, Z.; Patten, P.; et al. Desensitizing plant EPSP synthase to glyphosate: Optimized global sequence context accommodates a glycine-to-alanine change in the active site. J. Biol. Chem. 2019, 294, 716–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priestman, M.A.; Funke, T.; Singh, I.M.; Crupper, S.S.; Schönbrunn, E. 5-Enolpyruvylshikimate-3-phosphate synthase from Staphylococcus aureus is insensitive to glyphosate. FEBS Lett. 2005, 579, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Padgette, S.R.; Re, D.B.; Gasser, C.S.; Eichholtz, D.A.; Frazier, R.B.; Hironaka, C.M.; Levine, E.B.; Shah, D.M.; Fraley, R.T.; Kishore, G.M. Site-directed mutagenesis of a conserved region of the 5-enolpyruvylshikimate-3-phosphate synthase active site. J. Biol. Chem. 1991, 266, 22364–22369. [Google Scholar]
- Herzine, A.; Laugeray, A.; Feat, J.; Menuet, A.; Quesniaux, V.; Richard, O.; Pichon, J.; Montécot-Dubourg, C.; Perche, O.; Mortaud, S. Perinatal exposure to glufosinate ammonium herbicide impairs neurogenesis and neuroblast migration through cytoskeleton destabilization. Front. Cell Neurosci. 2016, 10, 191. [Google Scholar] [CrossRef]
- Bertin, C.; Weston, L.A.; Huang, T.; Jander, G.; Owens, T.; Meinwald, J.; Schroeder, F.C. Grass roots chemistry: Meta-Tyrosine, an herbicidal nonprotein amino acid. Proc. Natl. Acad. Sci. USA 2007, 104, 6964–16969. [Google Scholar] [CrossRef]
- Rubenstein, E. Misincorporation of the proline analog azetidine-2-carboxylic acid in the pathogenesis of multiple sclerosis: A hypothesis. J. Neuropathol. Exp. Neurol. 2008, 67, 1035–1040. [Google Scholar] [CrossRef]
- Dunlop, R.A.; Cox, P.A.; Banack, S.A.; Rogers, K.J. The non-protein amino acid BMAA is misincorporated into human proteins in place of L-serine causing protein misfolding and aggregation. PLoS ONE 2013, 8, e75376. [Google Scholar] [CrossRef] [PubMed]
- Main, B.J.; Dunlop, R.A.; Rodgers, K.J. The use of L-serine to prevent β-methylamino-L- alanine (BMAA)-induced proteotoxic stress in vitro. Toxicon 2016, 109, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, J.; Long, Y.; Kolbert, A.; Thanedar, S.; Southard, J. Presence of L-canavanine in Hedysarum alpinum seeds and its potential role in the death of Chris McCandless. Wilderness Environ. Med. 2015, 26, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, J.S.; Mishra, N.K.; Raghava, G.P. Identification of ATP binding residues of a protein from its primary sequence. BMC Bioinform. 2009, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Huynh, Q.K. Mechanism of inactivation of Escherichia coli 5-enolpyruvoylshikimate-3-phosphate synthase by o-phthalaldehyde. J. Biol. Chem. 1990, 265, 6700–6704. [Google Scholar] [PubMed]
- Sagong, H.-Y.; Son, H.F.; Kim, S.; Kim, Y.H.; Kim, I.K.; Kim, K.J. Crystal Structure and Pyridoxal 5′-Phosphate Binding Property of Lysine Decarboxylase from Selenomonas ru- minantium. PLoS ONE 2016, 11, e0166667. [Google Scholar] [CrossRef] [PubMed]
- Bergonia, H.A.; Franklin, M.R.; Kushner, J.P.; Phillips, J.D. A method for determining δ-aminolevulinic acid synthase activity in homogenized cells and tissues. Clin. Biochem. 2015, 48, 788–795. [Google Scholar] [CrossRef]
- Lu, W.; Li, L.; Chen, M.; Zhou, Z.; Zhang, W.; Ping, S.; Yan, Y.; Wang, J.; Lin, M. Genome-wide transcriptional responses of Escherichia coli to glyphosate, a potent inhibitor of the shikimate pathway enzyme 5-enolpyruvylshikimate-3-phosphate synthase. Mol. Biosyst. 2013, 9, 522–530. [Google Scholar] [CrossRef]
- Nikaidok, H. How are the ABC transporters energized? Proc. Natl. Acad. Sci. USA 2002, 99, 9609–9610. [Google Scholar] [CrossRef] [Green Version]
- Dym, O.; Eisenberg, D. Sequence-structure analysis of FAD-containing proteins. Protein. Sci. 2001, 10, 1712–1728. [Google Scholar] [CrossRef]
- Ugarte, R. Interaction between glyphosate and mitochondrial succinate dehydrogenase. Comput. Theor. Chem. 2014, 1043, 54–63. [Google Scholar] [CrossRef]
- Cheng, Z.Q.; McFadden, B.A. A study of conserved in-loop and out-of-loop glycine residues in the large subunit of ribulose bisphosphate carboxylase/oxygenase by directed mutagenesis. Protein. Eng. 1998, 11, 457–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, Y.; Altman-Gueta, H.; Finkler, A.; Gurevitz, M. Mutagenesis at two distinct phosphate-binding sites unravels their differential roles in regulation of rubisco activation and catalysis. J. Bacteriol. 2005, 187, 4222–4228. [Google Scholar] [CrossRef] [PubMed]
- De María, N.; Becerril, J.M.; García-Plazaola, J.I.; Hernandez, A.; de Felipe, J.R.; Fernández-Pascual, M. New insights on glyphosate mode of action in nodular metabolism: Role of shikimate accumulation. J. Agric. Food Chem. 2006, 54, 2621–2628. [Google Scholar] [CrossRef]
- Picoli, J.R.; Carbonari, G.J.; Matos, C.A.; Rodrigues, A.K.A.; Velini, L.F.O.S. Influence of glyphosate on susceptible and resistant ryegrass populations to herbicide. Planta Daninha 2017, 35, e017163391. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; de Oliveira, R.S.; Kremer, R.J.; Constantin, J.; Bonato, C.M.; Muniz, A.S. Water use efficiency and photosynthesis of glyphosate-resistant soybean as affected by glyphosate. Pestic. Biochem. Physiol. 2010, 97, 182–193. [Google Scholar] [CrossRef]
- Mesnage, R.; Arno, M.; Costanzo, M.; Séralini, G.-E.; Antoniou, M.N. Transcriptome profile analysis reflects rat liver and kidney damage following chronic ultra-low dose Roundup exposure. Environ. Health 2015, 14, 70. [Google Scholar] [CrossRef]
- Shang, S.; Jiang, J.; Deng, Y. Chicken cytochrome P450 1A5 is the key enzyme for metabolizing T-2 toxin to 3OH-T-2. Int. J. Mol. Sci. 2013, 14, 10809–10818. [Google Scholar] [CrossRef]
- Al-Badr, W.; Martin, K.J. Vitamin D and Kidney Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1555–1560. [Google Scholar] [CrossRef] [Green Version]
- Patel, T.V.; Singh, A.K. Role of vitamin D in chronic kidney disease. Semin. Nephrol. 2009, 29, 113–121. [Google Scholar] [CrossRef]
- Thadhani, R. Is calcitriol life-protective for patients with chronic kidney disease? J. Am. Soc. Nephrol. 2009, 20, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Kulie, T.; Groff, A.; Redmer, J.; Hounshell, J.; Schrager, S. Vitamin D: An evidence-based review. J. Am. Board Fam. Med. 2009, 22, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Syed, K.; Mashele, S.S. Comparative analysis of P450 signature motifs EXXR and CXG in the large and diverse kingdom of fungi: Identification of evolutionarily conserved amino acid patterns characteristic of P450 family. PLoS ONE 2014, 9, e95616. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Modi, S.; Smith, G.; Paine, M.; McDonagh, P.D.; Wolf, C.R.; Tew, D.; Lian, L.Y.; Roberts, G.C.; Driessen, H.P. Crystal structure of the FMN-binding domain of human cytochrome P450 reductase at 1.93 A resolution. Protein Sci. 1999, 8, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Fukuwatari, T.; Shibata, K. Nutritional Aspect of Tryptophan Metabolism. Int. J. Tryptophan. Res. 2013, 6, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Cetina Biefer, H.; Vasudevan, A.; Elkhal, A. Aspects of tryptophan and nicotinamide adenine dinucleotide in immunity: A new twist in an old tale. Int. J. Tryptophan. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hietanen, E.; Linnainmaa, K.; Vainio, H. Effects of phenoxyherbicides and glyphosate on the hepatic and intestinal biotransformation activities in the rat. Acta Pharmacol. Toxicol. (Copenh) 1983, 53, 103–112. [Google Scholar] [CrossRef]
- Seneff, S.; Swanson, N.; Li, C. Aluminum and glyphosate can synergistically induce pineal gland pathology: Connection to gut dysbiosis and neurological disease. Agric. Sci. 2015, 6, 42–70. [Google Scholar] [CrossRef]
- Froment, D.P.; Molitoris, B.A.; Buddington, B.; Miller, N.; Alfrey, A.C. Site and mechanism of enhanced gastrointestinal absorption of aluminum by citrate. Kidney Int. 1989, 36, 978–984. [Google Scholar] [CrossRef] [Green Version]
- Illeperuma, O.; Dharmagunawardena, H.A.; Herath, K.P. Dissolution of aluminum from sub-standard utensils under high fluoride stress. Possible risk factor for chronic renal failure in North Central Province. J. Natl. Sci. Found. Sri Lanka 2005, 37, 219–222. [Google Scholar] [CrossRef]
- Nanayakkara, S.; Komiya, T.; Ratnatunga, N.; Senevirathna, S.T.; Harada, K.H.; Hitomi, T.; Gobe, G.; Muso, E.; Abeysekera, T.; Koizumi, A. Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in North Central Province of Sri Lanka. Environ. Health Prev. Med. 2012, 17, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Couser, W.G.; Johnson, R.J. Mechanisms of progressive renal disease in glomerulonephritis. Am. J. Kidney Dis. 1994, 23, 193–198. [Google Scholar] [CrossRef]
- Floege, J.; Eitner, F.; Alpers, C.E. A new look at platelet-derived growth factor in renal disease. J. Am. Soc. Nephrol. 2008, 19, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Grande, M.T.; López-Novoa, J.M. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat. Rev. Nephrol. 2009, 5, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Tang, P.M.; Li, J.; Lan, H.Y. TGF-/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Y. Diverse roles of TGF-/Smads in renal fibrosis and inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Souchelnytskyi, S.; ten Dijke, P.; Miyazono, K.; Heldin, C.H. Phosphorylation of Ser165 in TGF-beta type I receptor modulates TGF-beta1-induced cellular responses. EMBO J. 1996, 15, 6231–6240. [Google Scholar] [CrossRef]
- Dissmeyer, N.; Schnittger, A. Use of phospho-site substitutions to analyze the biological relevance of phosphorylation events in regulatory networks. Methods Mol. Biol. 2011, 779, 93–138. [Google Scholar] [CrossRef]
- Basu, U.; Wang, Y.; Alt, F.W. Evolution of phosphorylation-dependent regulation of activation-induced cytidine deaminase. Mol. Cell 2008, 32, 285–291. [Google Scholar] [CrossRef]
- Lin, X.; Duan, X.; Liang, Y.Y.; Su, Y.; Wrighton, K.H.; Long, J.; Hu, M.; Davis, C.M.; Wang, J.; Brunicardi, F.C.; et al. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell 2006, 125, 915–928. [Google Scholar] [CrossRef]
- Shi, Y. Serine/threonine phosphatases: Mechanism through structure. Cell 2009, 139, 468–484. [Google Scholar] [CrossRef]
- Liu, F.; Zhuang, S. Role of Receptor Tyrosine Kinase Signaling in Renal Fibrosis. Int. J. Mol. Sci. 2016, 17, 972. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, L.; Qi, H.; Wang, J.; Wang, Y.; Jiang, W.; Xu, L.; Liu, N.; Zhuang, S. Nintedanib, a triple tyrosine kinase inhibitor, attenuates renal fibrosis in chronic kidney disease. Clin. Sci. 2017, 131, 2125–2143. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Tolbert, E.; Pang, M.; Ponnusamy, M.; Yan, H.; Zhuang, S. Suramin inhibits renal fibrosis in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.Y.; Wang, Y.H.; Zhang, Y.C.; Yang, W.L.; Shi, Y.Y. Functional significance of conserved Glycine 127 in a human dual-specificity protein tyrosine phosphatase. Biochemistry 2003, 68, 634–638. [Google Scholar]
- Mejía, R.; Quinteros, E.; López, A.; Ribó, A.; Cedillos, H.; Orantes, C.M.; Valladares, E.; López, D.L. Pesticide-Handling Practices in Agriculture in El Salvador: An Example from 42 Patient Farmers with Chronic Kidney Disease in the Bajo Lempa Region. Occup. Dis. Environ. Med. 2014, 2, 56–70. [Google Scholar] [CrossRef] [Green Version]
- Conlong, D.E.; Campbell, P.L. Integrated weed management for sugarcane field verges: Melinis minutiflora and Cynodon dactylon encroachment. In Proceedings of the Annual Congress—South African Sugar Technologists’ Association 2010, Durban, South Africa, 25–27 August 2010; No.83. pp. 276–279. [Google Scholar]
- Gravois, K. Sugarcane Ripener Recommendations. LSU AgCenter. 14 August 2017. Available online: https://www.lsuagcenter.com/topics/crops/sugarcane/harvesting_and_processing/sugarcane-ripener-recommendations--glyphosate (accessed on 15 October 2018).
- Mølck, A.M.; Friis, C. The cytotoxic effect of paraquat to isolated renal proximal tubular segments from rabbits. Toxicology 1997, 122, 123–132. [Google Scholar] [CrossRef]
- Li, Q.; Peng, X.; Yang, H.; Wang, H.; Shu, Y. Deficiency of multidrug and toxin extrusion 1 enhances renal accumulation of paraquat and deteriorates kidney injury in mice. Mol. Pharm. 2011, 8, 2476–2483. [Google Scholar] [CrossRef]
- Buvall, L.; Hedman, H.; Khramova, A.; Najar, D.; Bergwall, L.; Ebefors, K.; Sihlbom, C.; Lundstam, S.; Herrmann, A.; Wallentin, H.; et al. Orellanine specifically targets renal clear cell carcinoma. Oncotarget 2017, 8, 91085–91098. [Google Scholar] [CrossRef] [Green Version]
- Chan, B.; Lazzaro, V.; Seale, J.; Duggin, G.G. Characterisation and Uptake of Paraquat by Rat Renal Proximal Tubular Cells in Primary Culture. Hum. Exp. Toxicol. 1996, 15, 949–956. [Google Scholar] [CrossRef]
- Holmdahl, J. Mushroom Poisoning: Cortinarius Speciosissimus Nephrotoxicity; University of Gothenburg: Gothenburg, Sweden, 2001. [Google Scholar]
- Lock, E.A.; Ishmael, J. The acute toxic effects of paraquat and diquat on the rat kidney. Toxicol. Appl. Pharmacol. 1979, 50, 67–76. [Google Scholar] [CrossRef]
- Mølck, A.M.; Friis, C. Transport of paraquat by isolated renal proximal tubular segments from rabbits. Pharmacol. Toxicol. 1998, 83, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, X.; Baker, J.; Tama, F.; Chang, G.; Wright, S.H. Twelve transmembrane helices form the functional core of mammalian MATE1 (multidrug and toxin extruder 1) protein. J. Biol. Chem. 2012, 287, 27971–27982. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, M.; Terada, T.; Ogasawara, K.; Iwano, J.; Katsura, T.; Fukatsu, A.; Doi, T.; Inui, K. Identification of multidrug and toxin extrusion (MATE1 and MATE2-K) variants with complete loss of transport activity. J. Hum. Genet. 2009, 54, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Gibson, C.J.; Yang, I.; Buckley, B.; Goedken, M.J.; Richardson, J.R.; Aleksunes, L.M. MDR1 transporter protects against paraquat-induced toxicity in human and mouse proximal tubule cells. Toxicol. Sci. 2014, 141, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Sousa, E.; Carmo, H.; Palmeira, A.; Barbosa, D.J.; Gameiro, M.; Pinto, M.; Bastos Mde, L.; Remião, F. Induction and activation of P-glycoprotein by dihydroxylated xanthones protect against the cytotoxicity of the P-glycoprotein substrate paraquat. Arch. Toxicol. 2014, 88, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Seigneuret, M.; Garnier-Suillerot, A. A structural model for the open conformation of the mdr1 P-glycoprotein based on the MsbA crystal structure. J. Biol. Chem. 2003, 278, 30115–30124. [Google Scholar] [CrossRef]
- Sayanthooran, S.; Gunerathne, L.; Abeysekera, T.D.J.; Magana-Arachchi, D.N. Transcriptome analysis supports viral infection and fluoride toxicity as contributors to chronic kidney disease of unknown etiology (CKDu) in Sri Lanka. Int. Urol. Nephrol. 2018, 50, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Guilford, F.T.; Hope, J. Deficient glutathione in the pathophysiology of mycotoxin-related illness. Toxins 2014, 6, 608–623. [Google Scholar] [CrossRef]
- De Liz Oliveira Cavalli, V.L.; Cattani, D.; Heinz Rieg, C.E.; Pierozan, P.; Zanatta, L.; Benedetti Parisotto, E.; Wilhelm Filho, D.; Mena Barreto Silva, F.R.; Pessoa-Pureur, R.; Zamoner, A. Roundup disrupts male reproductive functions by triggering calcium mediated cell death in rat testis and Sertoli cells. Free Radic. Biol. Med. 2013, 65, 335–346. [Google Scholar] [CrossRef]
- Fine, A.; McIntosh, W.B. Elevation of serum gamma-glutamyl transpeptidase in end-stage chronic renal failure. Scott. Med. J. 1975, 20, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Caravaca-Fontán, F.; Azevedo, L.; Bayo, M.Á.; Gonzales-Candia, B.; Luna, E.; Caravaca, F. High levels of both serum gamma-glutamyl transferase and alkaline phosphatase are independent predictors of mortality in patients with stage 4-5 chronic kidney disease. Nefrologia 2017, 37, 267–275. [Google Scholar] [CrossRef]
- Ketterer, B.; Coles, B.; Meyer, D.J. The role of glutathione in detoxication. Environ. Health Perspect. 1983, 49, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.K.; Polekhina, G.; McKinstry, W.J.; Parker, M.W.; Dragani, B.; Aceto, A.; Paludi, D.; Principe, D.R.; Mannervik, B.; Stenberg, G. Contribution of glycine 146 to a conserved folding module affecting stability and refolding of human glutathione transferase p1-1. J. Biol. Chem. 2003, 278, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Chan, T.F.; Lam, V.M.; Engel, P.C. What is the role of the second “structural” NADP+-binding site in human glucose 6-phosphate dehydrogenase? Protein Sci. 2008, 17, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Kotaka, M.; Gover, S.; Vandeputte-Rutten, L.; Au, S.W.; Lam, V.M.; Adams, M.J. Structural studies of glucose-6-phosphate and NADP+ binding to human glucose-6-phosphate dehydrogenase. Acta Crystallogr. D 2005, 61, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Vulliamy, T.J.; D’Urso, M.; Battistuzzi, G.; Estrada, M.; Foulkes, N.S.; Martini, G.; Calabro, V.; Poggi, V.; Giordano, R.; Town, M.; et al. Diverse point mutations in the human glucose-6-phosphate dehydrogenase gene cause enzyme deficiency and mild or severe hemolytic anemia. Proc. Natl. Acad. Sci. USA 1988, 85, 5171–5175. [Google Scholar] [CrossRef] [PubMed]
- Maciag, M.; Plochocka, D.; Jablonska-Skwiecinska, E.; Mendek-Czajkowska, E.; Golaszewska, E.; Strojny, W.; Balwierz, W.; Zdebska, E.; Burzynska, B. Molecular analysis of three novel G6PD variants: G6PD Pedoplis-Ckaro, G6PD Piotrkow and G6PD Krakow. Acta Biochim. Pol. 2007, 54, 877–881. [Google Scholar] [PubMed]
- Gao, H.; Chen, J.; Ding, F.; Chou, X.; Zhang, X.; Wan, Y.; Hu, J.; Wu, Q. Activation of the N-methyl-d-aspartate receptor is involved in glyphosate-induced renal proximal tubule cell apoptosis. J. Appl. Toxicol. 2019, 1–12. [Google Scholar] [CrossRef]
- Agre, P. The aquaporin water channels. Proc. Am. Thorac. Soc. 2006, 3, 5–13. [Google Scholar] [CrossRef]
- Knepper, M.A. Molecular physiology of urinary concentrating mechanism: Regulation of aquaporin water channels by vasopressin. Am. J. Physiol. 1997, 272, F3–F12. [Google Scholar] [CrossRef] [PubMed]
- Baggaley, E.; Nielsen, S.; Marples, D. Dehydration-induced increase in aquaporin-2 protein abundance is blocked by nonsteroidal anti-inflammatory drugs. Am. J. Physiol. Renal. Physiol. 2010, 298, F1051–F1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera Valdés, R.; Orantes, C.M.; Almaguer López, M.; López Marín, L.; Arévalo, P.A.; Smith González, M.J.; Morales, F.E.; Bacallao, R.; Bayarre, H.D.; Vela Parada, X.F. Clinical characteristics of chronic kidney disease of non-traditional causes in women of agricultural communities in El Salvador. Clin. Nephrol. 2015, 83, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Kozono, D.; Kato, Y.; Agre, P.; Hazama, A.; Yasui, M. Conversion of aquaporin 6 from an anion channel to a water-selective channel by a single amino acid substitution. Proc. Natl. Acad. Sci. USA 2005, 102, 2192–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulasooriya, S.A. Toxin producing freshwater cyanobacteria of Sri Lanka. Ceylon J. Sci. 2017, 46, 3–16. [Google Scholar] [CrossRef]
- Forlani, G.; Pavan, M.; Gramek, M.; Kafarski, P.; Lipok, J. Biochemical bases for a widespread tolerance of cyanobacteria to the phosphonate herbicide glyphosate. Plant Cell Physiol. 2008, 49, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Drzyzga, D.; Lipok, J. Glyphosate dose modulates the uptake of inorganic phosphate by freshwater cyanobacteria. J. Appl. Phycol. 2017, 30, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Falconer, I.R.; Hardy, S.J.; Humpage, A.R.; Froscio, S.M.; Tozer, G.J.; Hawkins, P.R. Hepatic and renal toxicity of the blue-green alga (cyanobacterium) Cylindrospermopsis raciborskii in male Swiss albino mice. Environ. Toxicol. 1999, 14, 143–150. [Google Scholar] [CrossRef]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Balogh, K.; Bázár, G.; Kocsó, D.; Ali, O.; Kovács, M. Individual and combined effects of Fumonisin B1, deoxynivalenol and zearalenone on the hepatic and renal membrane lipid integrity of rats. Toxins 2018, 10, 4. [Google Scholar] [CrossRef]
- Desalegn, B.; Nanayakkara, S.; Harada, K.H.; Hitomi, T.; Chandrajith, R.; Karunaratne, U.; Abeysekera, T.; Koizumi, A. Mycotoxin detection in urine samples from patients with chronic kidney disease of uncertain etiology in Sri Lanka. Bull. Environ. Contam. Toxicol. 2011, 87, 6–10. [Google Scholar] [CrossRef]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Andre, F.; Delaforge, M.; Lebrihi, A. Transformation of zearalenone to zearalenone-sulfate by Aspergillus spp. World Mycotoxin J. 2010, 3, 183–191. [Google Scholar] [CrossRef]
- Warth, B.; Fruhmann, P.; Wiesenberger, G.; Kluger, B.; Sarkanj, B.; Lemmens, M.; Hametner, C.; Fröhlich, J.; Adam, G.; Krska, R.; et al. Deoxynivalenol-sulfates: Identification and quantification of novel conjugated (masked) mycotoxins in wheat. Anal. Bioanal. Chem. 2015, 407, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, V.; Oestreicher, N.; Vélot, C. Multiple effects of a commercial Roundup® formulation on the soil filamentous fungus Aspergillus nidulans at low doses: Evidence of an unexpected impact on energetic metabolism. Environ. Sci. Pollut. Res. 2016, 23, 14393–14404. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Komatsu, K.; Lee, Y.C.; Tomizuka, T.; Strott, C.A. The 3′-terminal exon of the family of steroid and phenol sulfotransferase genes is spliced at the N-terminal glycine of the universally conserved GXXGXXK motif that forms the sulfonate donor binding site. Proc. Natl. Acad. Sci. USA 1995, 92, 8176–8179. [Google Scholar] [CrossRef] [PubMed]
- Yussefi, M.; Willer, H. Organic Farming Worldwide 2007: Overview & Main Statistics. In The World of Organic Agriculture—Statistics and Emerging Trends 2007; Willer, H., Yussefi, M., Eds.; International Federation of Organic Agriculture Movements IFOAM, Research Institute of Organic: Bonn, Germany, 2007; Chapter 3; pp. 9–16. [Google Scholar]
- Jayasumana, C.; Ranasinghe, O.; Ranasinghe, S.; Siriwardhana, I.; Gunatilake, S.; Siribaddana, S. Reverse osmosis plant maintenance and efficacy in chronic kidney disease endemic region in Sri Lanka. Environ. Health Prev. Med. 2016, 21, 591–596. [Google Scholar] [CrossRef] [PubMed]
| Protein | Fold Increased |
|---|---|
| D,D-dipeptide permease system, ATP-binding component | 2.83 |
| ATP-binding protein of nickel transport system | 2.24 |
| ATP-binding component of transport system for glycine, betaine and proline | 12.96 |
| Fused D-allose transporter subunits of ABC superfamily: ATP-binding components | 2.03 |
| ATP-binding component of transport system for maltose | 2.38 |
| Putative ATP-binding sugar transporter | 2.10 |
| Flagellum-specific ATP synthase | 2.07 |
| Putative ATP-binding component of a transport system | 3.04 |
| Putative part of putative ATP-binding component of a transport system | 2.31 |
| Putative ATP-binding component of a transport system | 2.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunatilake, S.; Seneff, S.; Orlando, L. Glyphosate’s Synergistic Toxicity in Combination with Other Factors as a Cause of Chronic Kidney Disease of Unknown Origin. Int. J. Environ. Res. Public Health 2019, 16, 2734. https://doi.org/10.3390/ijerph16152734
Gunatilake S, Seneff S, Orlando L. Glyphosate’s Synergistic Toxicity in Combination with Other Factors as a Cause of Chronic Kidney Disease of Unknown Origin. International Journal of Environmental Research and Public Health. 2019; 16(15):2734. https://doi.org/10.3390/ijerph16152734
Chicago/Turabian StyleGunatilake, Sarath, Stephanie Seneff, and Laura Orlando. 2019. "Glyphosate’s Synergistic Toxicity in Combination with Other Factors as a Cause of Chronic Kidney Disease of Unknown Origin" International Journal of Environmental Research and Public Health 16, no. 15: 2734. https://doi.org/10.3390/ijerph16152734






