1. Introduction
Catchment and river-bed transformations cause changes in the river regime, resulting in irreversible alterations of the physicochemical and biological variables [
1,
2]. Different catchment land uses play a paramount role in the functioning of river ecosystems, and particularly trophic network structures [
3]. More extensive agriculture in a catchment area has a significant impact on the organic and inorganic nutrients, especially the nitrogen and phosphorus compounds [
4]. It undoubtedly increases the amount of live and dead organic matter in a river, the size of the macrophyte-covered area of river bed, and the abundance of aquatic organisms.
Anthropogenic changes in the river bed, for example dams or reservoirs, are another factor that positively affects the increase of nutrients in a river. These changes include larger built-up areas and river-bed maintenance practices that secure flood control systems and energy needs, and allow water-retention for agglomeration, crops, and forestry [
2,
5,
6]. The above-mentioned needs are secured by building water-impounding structures that create reservoirs altering the hydrological and biological regime of rivers. Also, long water-retention time and low current velocity in the reservoirs lead to phenomena typical of flow-through lakes and not fast-flowing rivers. Consequently, significant amounts of organic sediments accumulate, aquatic vegetation grows (including phytoplankton and macrophytes), and the river ichthyofauna is replaced by lake ichthyofauna [
2,
7,
8,
9].
Due to these alterations, typical lake organisms, which usually are not present or expected to be present in a high biomass, can develop and maintain their populations. Zooplankton normally occurs in scarce quantities in small rivers and its abundance is an indicator of such alterations [
10,
11,
12]. However, hydrological changes, such as lower current velocity or an increase of nutrient content, and higher temperatures in small rivers provide atypical yet favorable conditions for zooplankton growth. Zooplankton reacts rapidly to the hydrological changes in rivers, increasing its richness and abundance [
13,
14]. This certainly depends on the reservoir characteristics, and specifically its hydrological conditions, which consequently can change biological conditions and favor the development of planktonic organisms that cannot resist stronger water currents. Even though zooplankton has been proven to show good indicative properties for evaluating hydrological changes in rivers [
15], it has never been used as such an indicator. The authors of the present work attempted to show how zooplankton can be an indicator of hydrological changes in rivers as well as catchment alterations.
According to the River Continuum Concept (RCC) [
3], in large rivers, zooplankton is visible in larger amounts only in the lower section, where the current is the slowest, and stagnant water basins supply the river with zooplankton. It is commonly known that any human-induced alterations to environmental conditions have a transforming effect on the RCC in the downstream sections of a river. The more severe the changes upstream, the stronger the effects downstream [
2]. Zooplankton was one of the indicators for these changes [
6,
14]. However, the functioning of a small lowland river depends on different values of hydrological factors than that of mountain rivers. In lowland rivers, slower current velocity and weaker turbulence can foster a greater reduction in the zooplankton abundance through the sedimentation phenomena and fish predation [
13,
16]. Moreover, similar to lowland reservoirs, the retention time needed for zooplankton development and maintenance of its great abundance may be too short in mountain reservoirs. Until now, studies showing spatial changes in the zooplankton composition in small mountain rivers in regard to environmental changes in the catchment and river-bed transformations have not been conducted. Besides, zooplankton communities in small and upstream rivers is still poorly documented [
17].
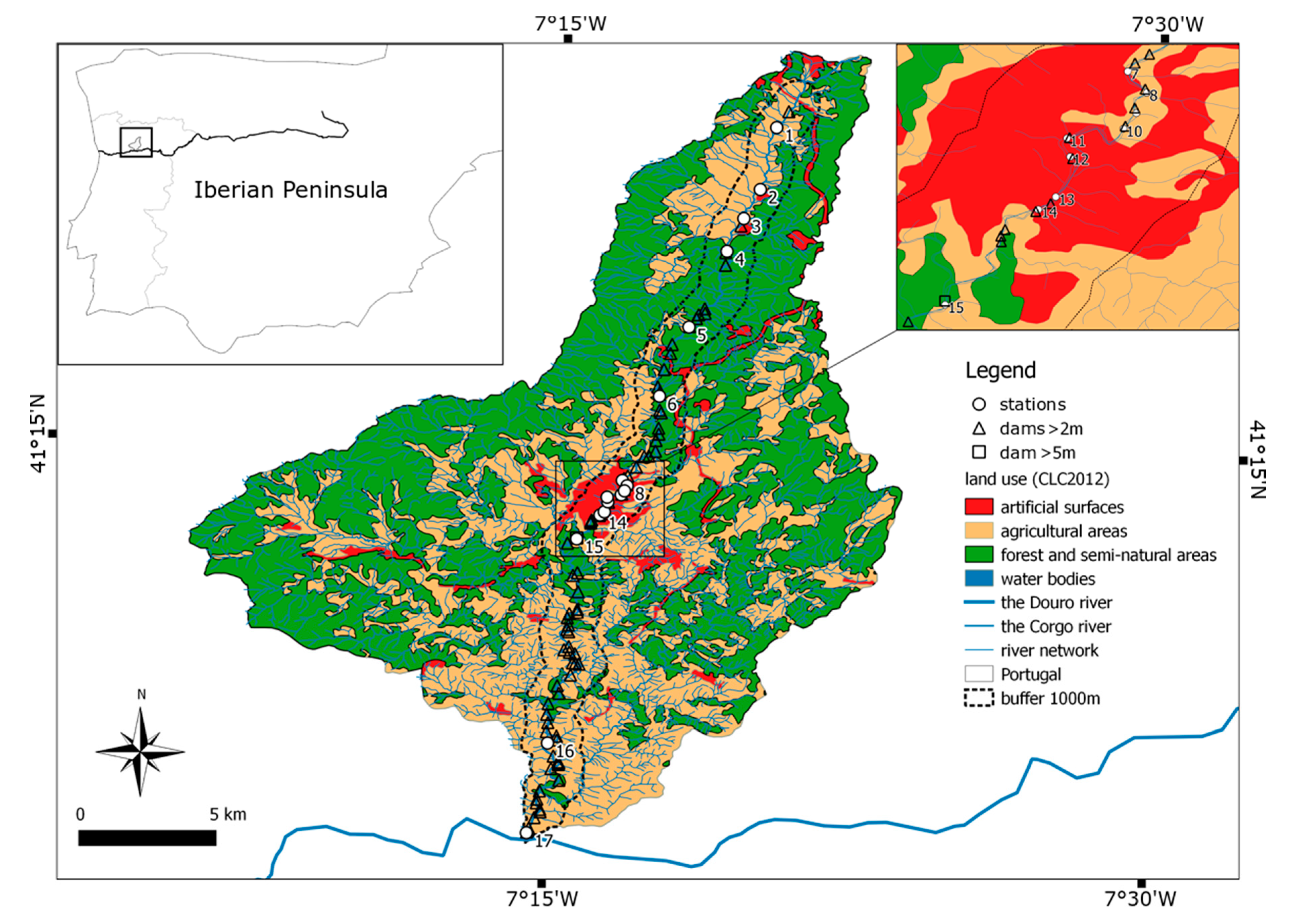
This study aimed to examine spatial distribution of zooplankton communities in a small mountain river subjected to anthropogenic changes. To achieve the objective, the authors attempted to answer the following questions: (1) What type of catchment use has a greater influence on the crustacean and Rotifera communities in a river? (2) How do dam impoundments influence the zooplankton communities downstream?
4. Discussion
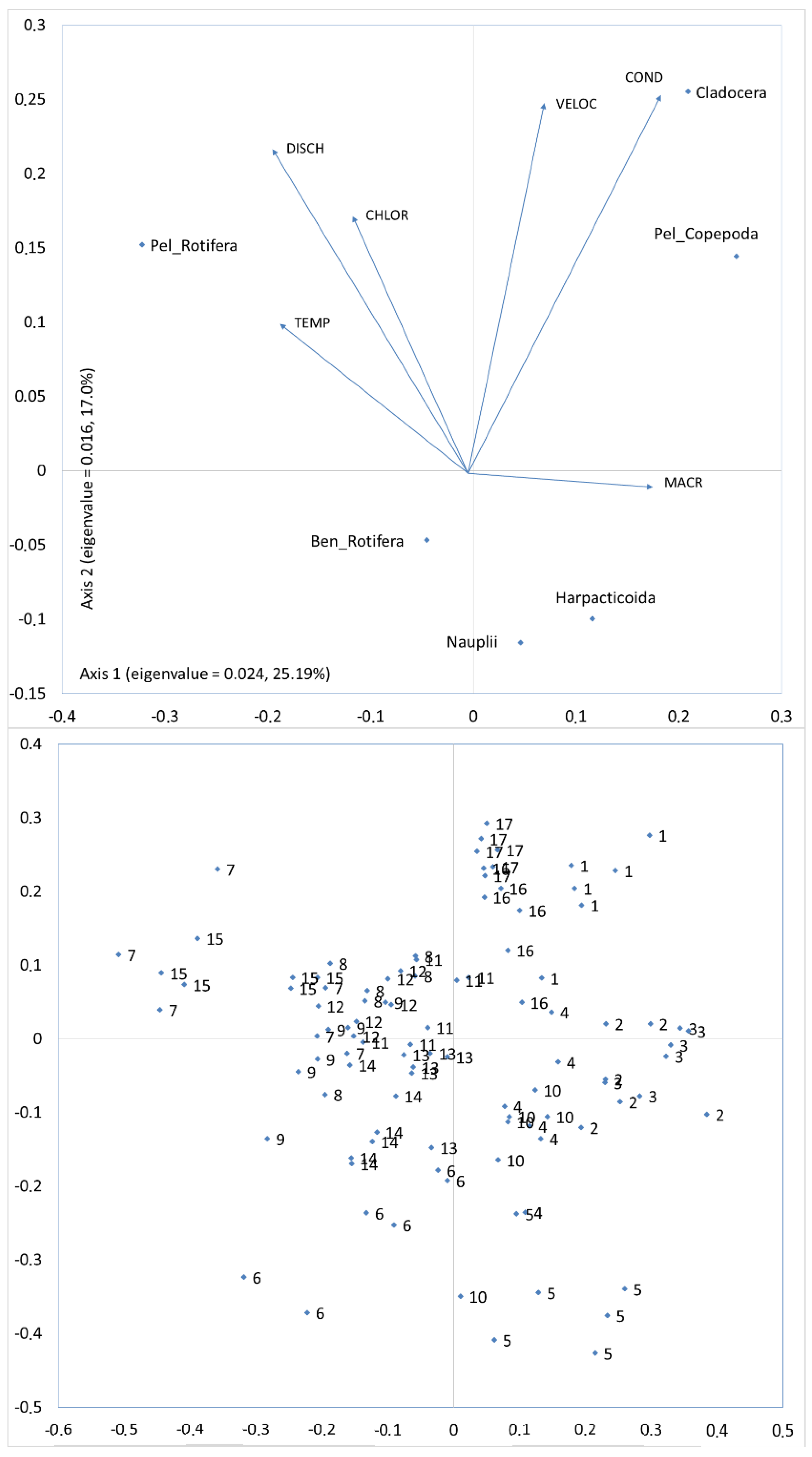
Zooplankton communities in large and small rivers are shaped by local conditions of the riverbed, which has been demonstrated in numerous papers [
23,
24,
25,
26,
27,
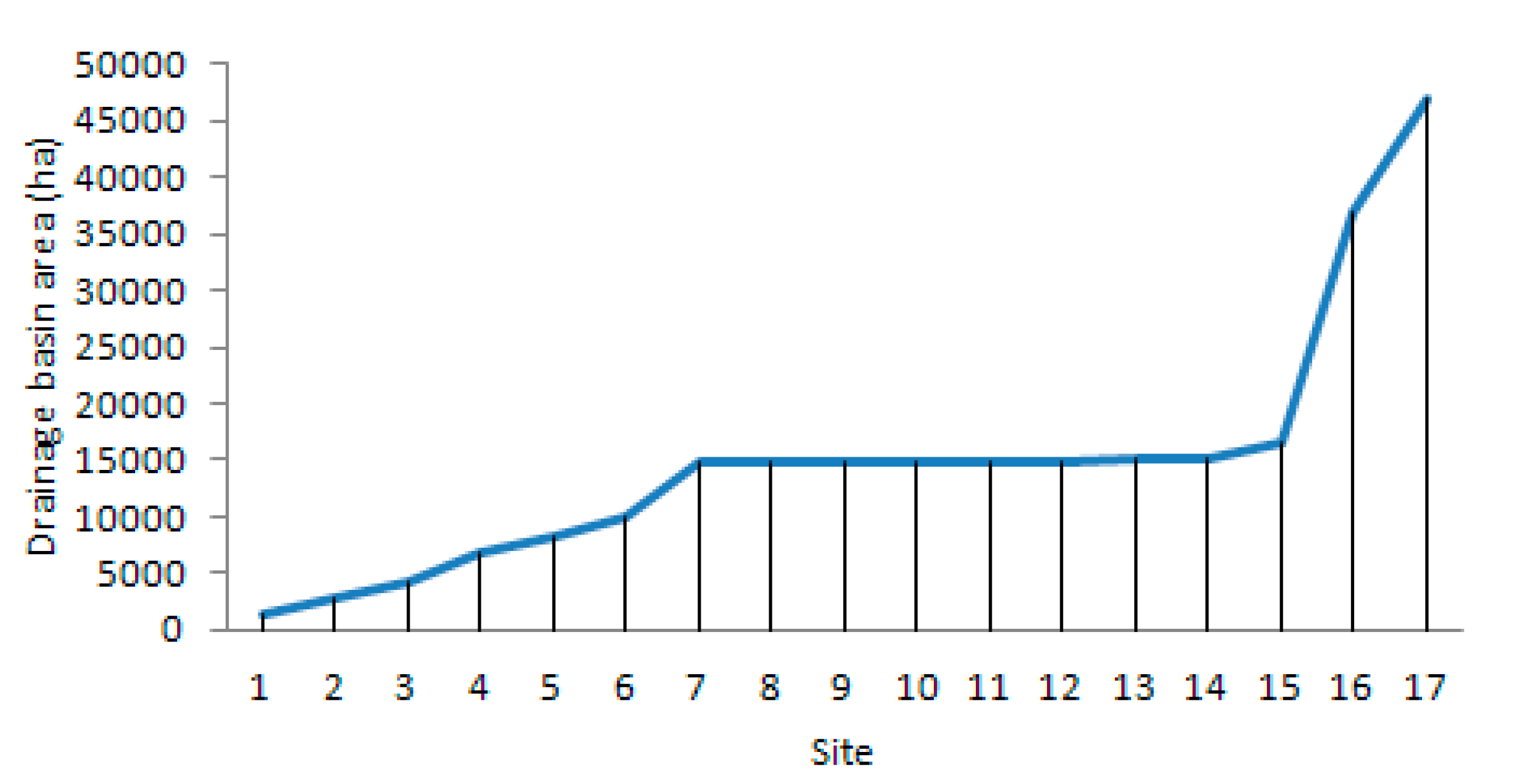
28]. Due to the greatest abundance of zooplankton being in the downstream section of the Corgo, we could not examine the influence of the entire catchment area on the zooplankton communities because it is evident that the zooplankton density increases with the catchment area size. The above phenomenon is well-known, and it has been defined by the RCC [
3]. Therefore, it is more noteworthy to focus on the local conditions [
17,
29] (such as catchment and river-bed use) and the buffer zone where the influence of the catchment on the nutrient contents [
4], and consequently on each zooplankton group, is best seen. Focusing on the entire catchment area seems rather pointless because it is quite unlikely that the upstream environmental conditions would affect the zooplankton communities downstream. Because the rivers have the ability to self-purify, over a few hundred meters there is a significant reduction of plankton observed in lake outflows [
13,
16].
In the majority of studied rivers there is a typical spatial pattern of various zooplankton groups, and the same was observed in the Corgo. Rotifers were dominant in the lotic waters [
23,
30]. This was mainly caused by their short development cycle, the ability to rapidly colonize new habitats and to drift passively long distances because of their small specific size and weight [
13,
16,
31,
32]. The existence of small reservoirs, even with a relatively short water retention time, determines the large presence of pelagic Rotifera in rivers [
9,
14,
31,
33]. Such a pattern was also observed in the Corgo. River-bed transformations, dams, and reservoirs in this small mountain river led to an increase of zooplankton communities in certain sections. The small zooplankton reduction rate was caused by the strong current, which consequently led to a greater zooplankton community downstream than compared to the small lowland rivers [
6,
14,
30]. However, the zooplankton abundance in the Corgo was much smaller than that of small lowland rivers which are subject to anthropogenic changes. The reason for this is the high slope of the Corgo.
Crustaceans, as opposed to rotifers, occur rarely in small rivers and streams [
30,
34,
35]. Crustaceans have a longer development cycle, and they are heavier and larger in size than rotifers [
20]. The above characteristics make it difficult to maintain crustacean populations in rivers and even in small reservoirs. Crustaceans occur in rivers with suitable conditions, such as long water retention time, current velocity up to 0.1 m s
−1, high open water zones, and the presence of macrophytes [
32,
36,
37,
38,
39]. In the Corgo, crustaceans were present in sections with such conditions. In its upstream section the Corgo crosses a plateau, and it is at these sites that we recorded the slowest current velocity. Additionally, in that area, the plateau is surrounded by mountains, so there are favorable conditions for agriculture, and as a result there is more nutrient content for the crustaceans in the river. The riverbed in that section was covered mostly by submerged macrophytes. Most importantly, we acknowledge that crustaceans could have migrated to the main channel of the Corgo from the water tanks of a nearby sewage treatment plant and numerous small slackwaters covered by macrophytes [
15,
27,
28,
32]. The more extensive was the agriculture use in the catchment area, the greater was the amount of nutrient content, and consequently the more crustaceans were seen. This phenomenon can be confirmed by a high positive correlation between the crustacean abundance and the conductivity. Greater conductivity values occur in waters that are surrounded by agricultural areas [
4,
40].
Crustaceans can be seen in great abundance in small reservoirs, small slackwaters, and macrophyte-covered areas [
41,
42,
43]. Therefore, in the case of pelagic rotifer abundance, we recorded a negative correlation with the percentage of agricultural areas, and a positive correlation with the percentage of anthropogenic areas. The biggest number of impoundments with larger open-water zones was present in the areas subjected to anthropogenic changes. This must have had a positive impact on the abundance of rotifers and crustaceans. However, we believe that the impact was the greatest on the rotifer population. It should be noted that despite the fact that in lotic waters the rotifer abundances are greater than those of crustaceans, similar to the crustaceans, they occur in greater numbers in stagnant waters than in the lotic waters [
40]. However, due to short water retention time it was difficult to maintain crustacean population in the Corgo reservoirs.
In rivers, the only crustacean forms observed in greater amounts are the crustacean larvae. Such a pattern was also observed in the Corgo. At each site, Nauplius occurred in greater numbers than their adult forms. Oftentimes in rivers, larvae abundance is greater than that of adult crustaceans [
30,
32,
44]. Similar to the rotifers, and due to the same reasons, nauplii as small plankters drift much farther than adult crustaceans.
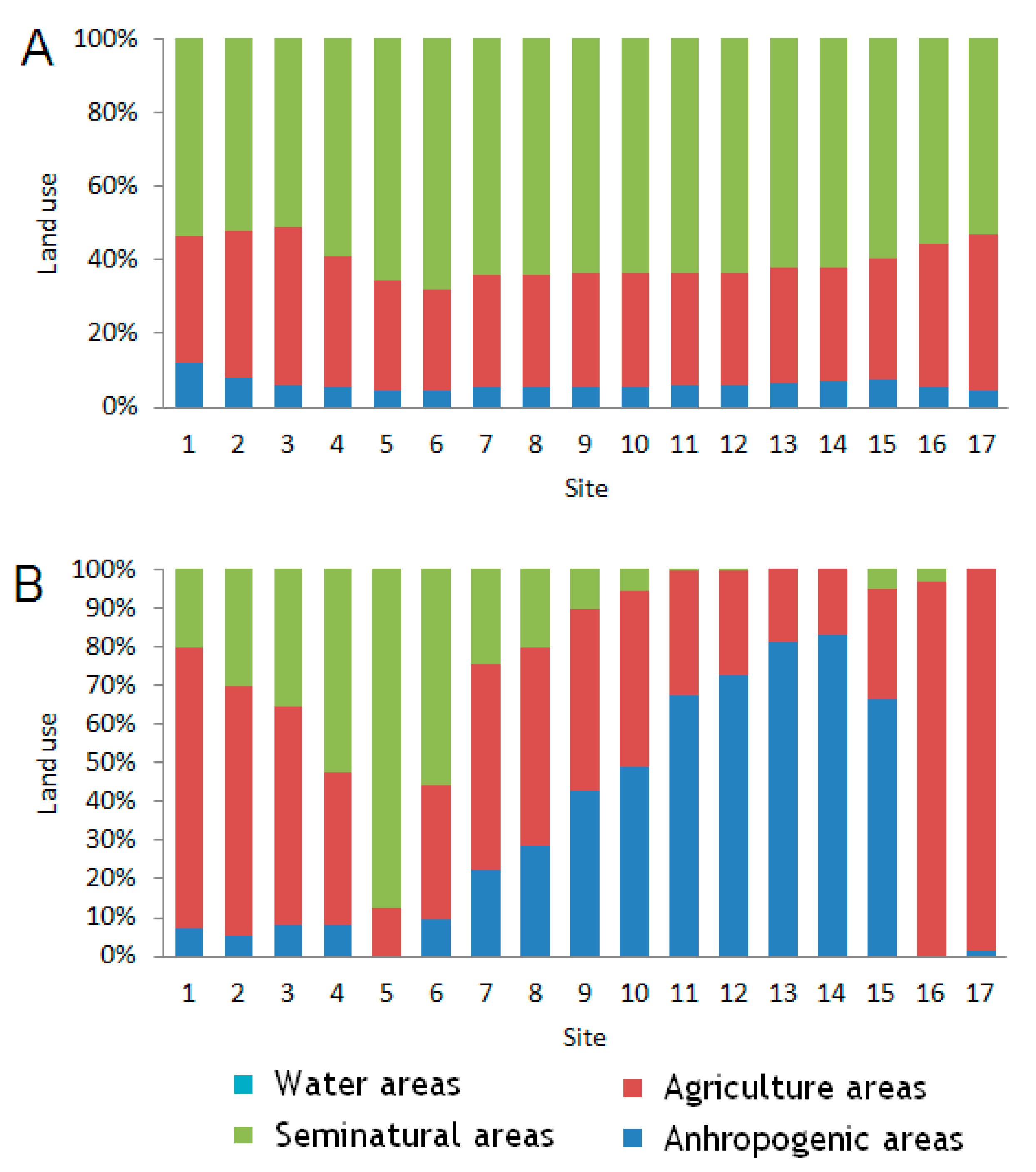
As mentioned above, more extensive agriculture in a catchment area is the factor which confirms the positive influence of anthropic activity on the zooplankton abundance, and especially the rotifers. In the present study, this factor was expressed with a greater number of dams and reservoirs in the vicinity of agricultural and urban settlements, which had an impact on relatively large open-water zones that were much larger than those in the upper section of the Corgo. The presence of impoundments was highly positively correlated with the zooplankton abundance, and specifically small rotifers [
13,
15,
31]. Not only do the impoundments prolong the water retention time, but they increase the river temperature and the amount of nutrient content. Furthermore, this phenomenon was confirmed by the positive correlation between the zooplankton abundance and water temperature and the chlorophyll
a values observed in this study. This relationship is widely known because zooplankton are primarily dependent on primary producers and on bacteria, flagellates, and ciliates [
17,
45,
46,
47].
No positive correlations between zooplankton abundance and forest areas were found, because in these sections there are no dams or natural water reservoirs where zooplankton could develop and then disperse into the Corgo sections along the forest areas, where the natural bed was not affected by human activity.
As mentioned in the Introduction, it is highly unlikely that anthropogenic changes in the upper section of the Corgo affected the zooplankton communities in the middle and lower sections in any way. The species that dominated upstream were very scarce in the lower sections of the river. Therefore, the influence of river environmental conditions on the zooplankton occurs locally. The abundance of zooplankton, and more specifically the rotifers, may increase due to river hydrological conditions and the presence of impoundments. Certain authors believe that zooplankton can drift over long distances due to high current velocities and turbulence, which prevent sedimentation phenomena and also prevent fish and macroinvertebrates from feeding on plankters [
13,
16,
24,
48,
49]. This is true in the case of the Corgo, and it was confirmed by a positive correlation between current velocity and rotifer abundance, which is not likely to be found in lowland rivers [
34,
35,
39]. Contrary to mountain rivers, lowland rivers are characterized by lower current velocities and weaker turbulences, which in turn may lead to greater reduction in plankton abundance. However, both in lowland and mountain rivers such a phenomenon is shaped by local conditions.
5. Conclusions
It seems that contrary to small lowland rivers, water retention time in reservoirs in small mountain rivers is so short that only small pelagic rotifers, characterized by a short and fast reproductive cycle, can maintain their population. Crustaceans require much longer water retention time. Therefore, crustaceans present in the Corgo can attain relatively high densities in the rural section, which offers them better trophic conditions. Additionally, in this section, current velocity is low, and slackwaters (from which crustaceans are washed out) are connected with river beds and sections densely covered by submerged macrophytes. The dams on the Corgo unequivocally increase the water retention time; however, this period is too short for crustaceans to maintain their populations, but it is sufficient for the rotifers. Therefore, in the Corgo, the urban catchment-use and the presence of dams have a greater impact on the rotifer density and the increase of zooplankton density downstream. Whereas the agricultural catchment-use as local conditions affect the crustacean density, and to a lesser extent the rotifer density. Semi-natural forest catchment had no effect on the increase of zooplankton abundance. Moreover, a reduction in plankton abundance drifting from upstream, that is typical of rivers, occurred in the forest section. Our results show that impounded sections of the Corgo river were characterized by more numerous zooplankton communities than were the unimpounded sections. However, this pattern was observed within a confined geographical area within the same drainage.
In closing, it is worth noting that zooplankton richness and abundance can be a good indicator of trophic status [
40,
50,
51,
52,
53], river-bed transformations, and the use of the riparian zone or the catchment area. Alas, this factor has not been included among the Water Framework Directive’s biological indicators used to assess freshwaters. However, a few years ago, zooplankton were proposed as a good bioindicator of lake conditions [
50,
51,
52]. Moreover, the results of this study as well as the research of others confirm that zooplankton properties allow for the evaluation of the degree of river-bed transformations.