1. Introduction
Emerging and increasing antibiotic microbial resistance (AMR) represents one major threat to human health in Europe and worldwide. Resistance to antibiotics is widely distributed among Gram-positive and Gram-negative bacteria that may cause serious infections in humans, and AMR is increasing in the EU, especially among Gram-negative bacteria. The major drivers behind the occurrence and spread of AMR are the use of antimicrobial agents and the transmission of antibiotic-resistant microorganisms between humans; between animals; and between humans, animals, and the environment [
1].
Bacteria that are resistant to three or more classes of antibiotics are called multidrug-resistant. Infections with these bacteria are associated with increased morbidity, mortality, length of hospitalization, and financial costs [
2]. For a long time, methicillin-resistant
Staphylococcus aureus (MRSA) have been the main point of concern in public health, but during the last couple of years extended-spectrum-ß-lactamase (ESBL)–producing bacteria have become a much more severe problem. While MRSA are predominantly acquired in connection with medical treatments, the picture concerning multidrug-resistant Gram-negative bacteria is much less clear and differs from species to species [
3]. The production of ESBL is the most frequent resistance mechanism among Gram-negative bacteria. The corresponding gene sequences of the ESBLs are mostly on mobile DNA elements and can be transmitted horizontally, which contributes to the spread of these resistance genes [
4]. Due to the resistance of the microorganisms to β-lactam antibiotics and other classes of antibiotics such as the fluoroquinolones (e.g., ciprofloxacin and levofloxacin), the therapeutic spectrum is strongly restricted in the case of infection with ESBL-producing bacteria [
5]. Options for treatment of patients who are infected with multidrug-resistant bacteria are limited to only a few remaining last-line antibiotics, such as carbapenems (e.g., imipenem, ertapenem, meropenem). However, the increasing carbapenem-resistance limits options for the treatment of infected patients [
6,
7]. For the KRINKO (German Commission on Hospital Hygiene and Infection Prevention) definition of multidrug-resistant Gram-negative rods (MRGN), only resistance to antibiotics which are used as primary therapeutics for severe infections (acylaminopenicillins, third and fourth generation cephalosporins, carbapenems, and fluoroquinolones) has been included: 3MRGN (with resistance to three of the four antibiotic groups) and 4MRGN (with resistance to four of the four antibiotic groups). Aminoglycosides, like amikacin, gentamicin, and tobramycin, were not included in the KRINKO classification of multidrug-resistant Gram-negative rods, as they are generally not used as monotherapeutics [
8].
Hydrotherapy is a non-invasive and beneficial treatment for many patients, like patients with chronic diseases, disabilities, or trauma. Very often those patients have a long history of medical treatments, including antibiotic treatments. Thus, the probability that they have a disturbed microflora with an increased rate of carriage of AMR and also a reduced colonization resistance against AMR is relatively high [
9]. Although pool water is usually disinfected, infections are known to occur either due to deficiencies in water treatment or due to colonization of swimming pool equipment [
10,
11,
12,
13]. Therapies performed in a swimming pool cause a large release of bacteria. Bathers transfer approximately 10
5–10
6 CFU per person in 15 min to the surrounding water body [
14]. Bacteria should be inactivated by disinfectants in the pool water; however, this is not always the case, because they can be attached to particles or be protected by an EPS (extrapolymeric substance) and thus not be exposed to the disinfectant. Bacteria can form biofilms on pool surfaces, especially in areas of the pool where the concentration of disinfectant is low [
15,
16,
17]. Additionally, they can be attached to swimming aids, which are made from plastics, often foams, which provide a large surface. They can also be found on tools for cleaning, where they are exposed occasionally but not permanently to disinfectants.
Pseudomonas aeruginosa, a species very often involved in biofilm formation, has been commonly isolated from the pool environment [
18,
19].
Immunocompromised patients are particularly susceptible to infections with pathogens (including antibiotic-resistant bacteria) via the mucous membranes and via penetration of bathing water into auditory canals and the nasopharynx. Although some literature exists on antibiotic-resistant bacteria in swimming pools [
16,
17,
20], little is known about the prevalence of AMR. To this day, to our knowledge, no information exists about the public health impact of therapy pools in the dissemination of antibiotic-resistant bacteria. In order to assess the extent of contamination with antibiotic-resistant bacteria, their distribution, and the associated transmission risk in clinical therapy pools, we performed a study on this topic. The main objective of the project was to investigate the occurrence of antibiotic-resistant bacteria in water of therapy pools, in filters, balance tanks, and on surrounding surfaces. Factors contributing to their occurrence will be discussed with regard to details in pool water treatment and disinfection, number of patients entering the pools, and usage of the pools for other purposes. Finally, we derive recommendations for the management of patients colonized with antibiotic-resistant bacteria in hydrotherapy pools.
4. Discussion
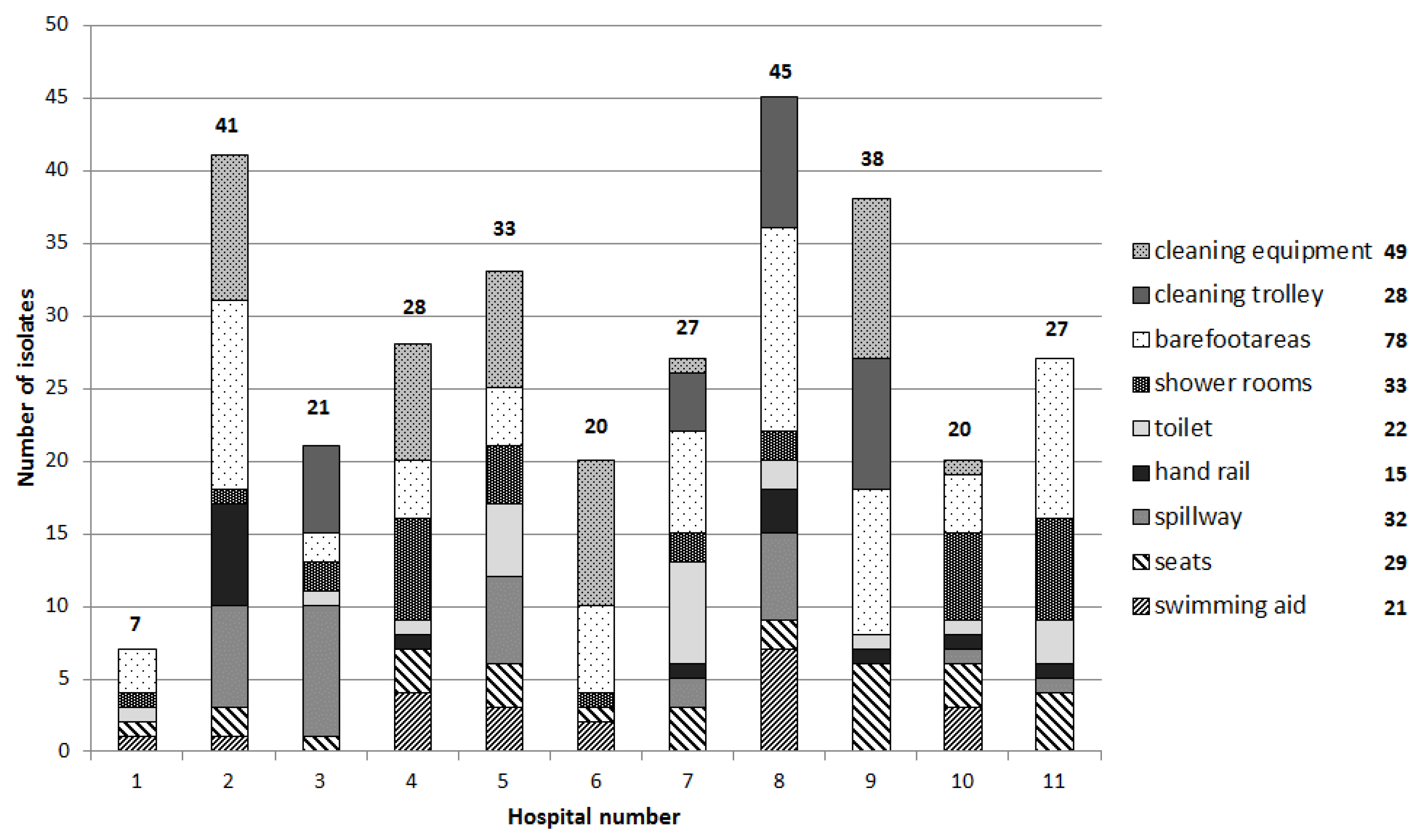
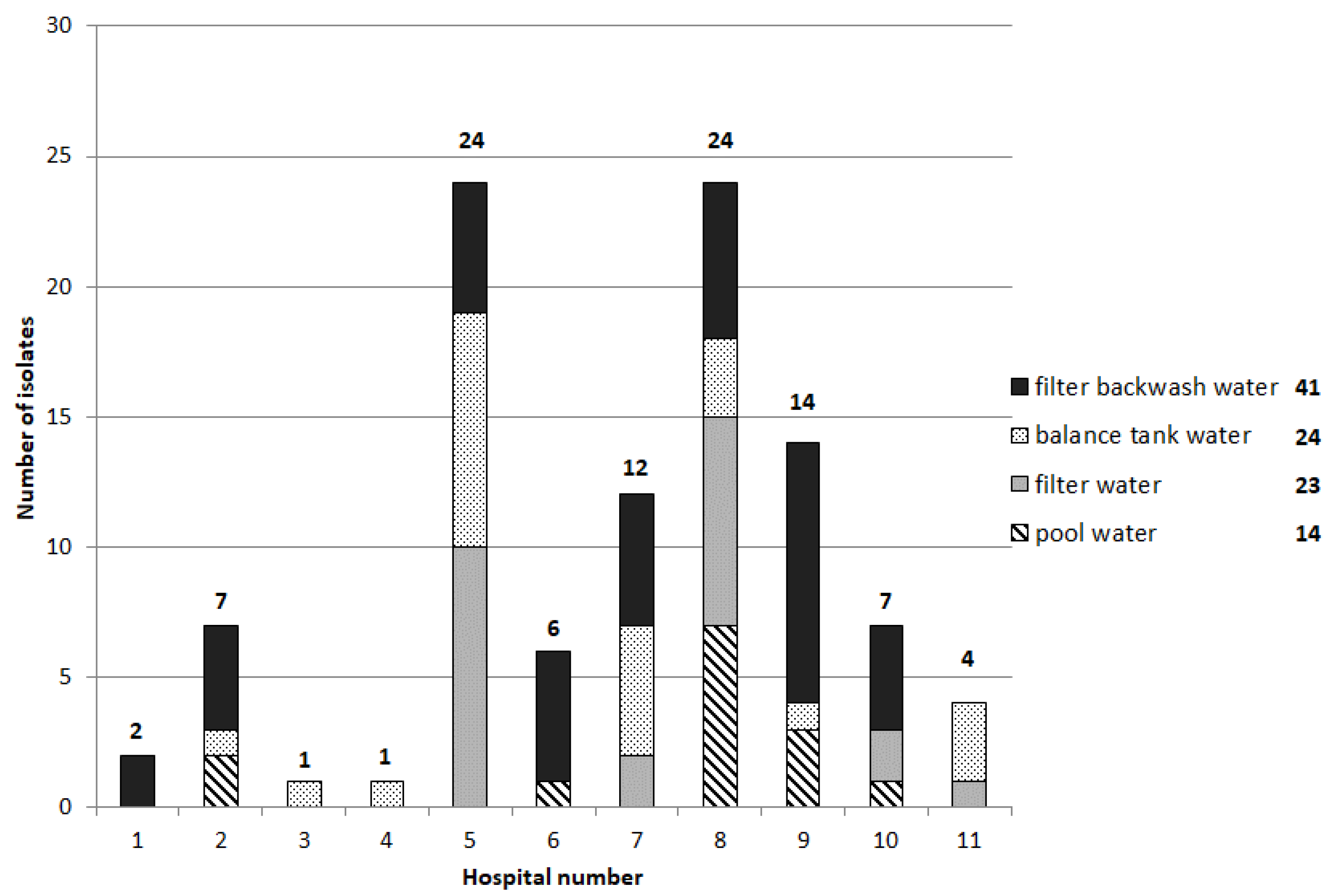
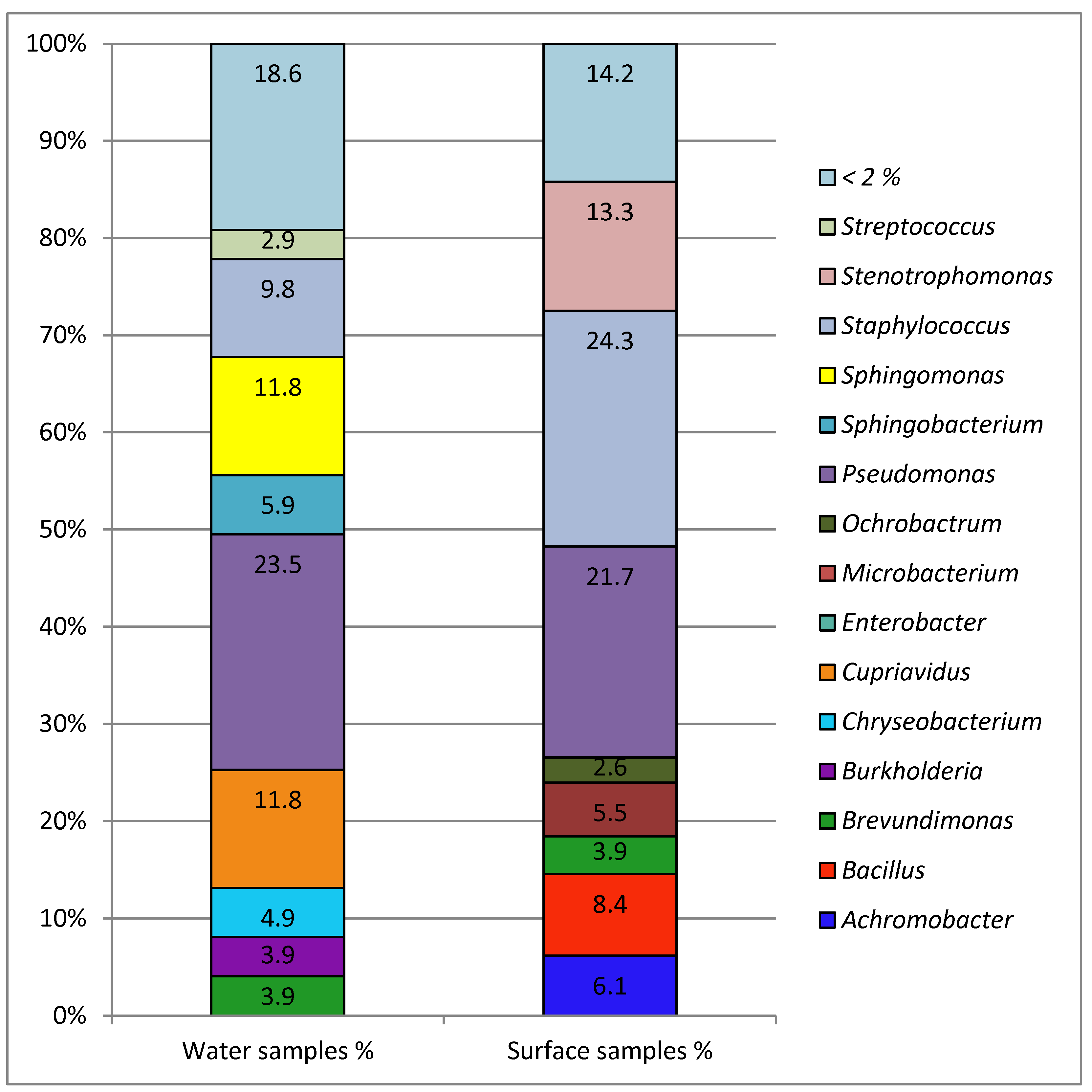
The therapy pool with the highest number of isolates obtained directly from the pool water and from the sampled surfaces had not only the highest number of visitors but also seemed to have problems with the water treatment (high bound chlorine levels). The cleaning intervals of the pool areas were the same between the different health care facilities (once per day, according to the evaluation of the questionnaire); hence there is no detectable correlation between the number of isolates and the cleaning interval. These results indicate a correlation of the incidence of antibiotic-resistant bacterial isolates with the number of patients in combination with deficiencies in water treatment. The isolation of potential human pathogens, particularly
P. aeruginosa, S. maltophilia, and
S. paucimobilis strains, indicates that these inhabitants of the nosocomial environment may have been released by bathers, with contact to surfaces in the surrounding of the pool and the hospital environment, after entering the pool.
P. aeruginosa can potentially cause disease in healthy humans, but more often it colonizes immunocompromised patients, like those with cystic fibrosis or cancer [
23].
P. aeruginosa is intrinsically resistant to the majority of antimicrobial agents due to the low permeability of its outer membrane and the constitutive expression of various efflux pumps. Any additional acquired resistance severely limits the therapeutic options for treating serious infections. The antimicrobial groups that remain active against the susceptible
P. aeruginosa phenotype include some fluoroquinolones (e.g., ciprofloxacin and levofloxacin), aminoglycosides (e.g., gentamicin, tobramycin, and amikacin), some beta-lactams (piperacillin- tazobactam, ceftazidime, cefepime, imipenem, doripenem, and meropenem), and polymyxins (polymyxin B and colistin). Resistance of
P. aeruginosa to these agents can be acquired through one or more of several mechanisms, like the acquisition of plasmid-mediated resistance genes coding for various
β-lactamases and aminoglycoside-modifying enzymes [
24,
25]. Some strains that have been isolated exhibited resistance to essentially antipseudomonal antibiotics, like fluoroquinolones and carbapenems.
Another problematic nosocomial pathogen is
S. maltophilia, which is also naturally resistant to many broad-spectrum antibiotics (including all carbapenems)
. S. maltophilia is the third most common nosocomial pathogen with multi-drug-resistance [
26].
S. maltophilia is often associated with pulmonary infections, urinary tract infections, bloodstream infections, and colonization of individuals with cystic fibrosis, especially in immunocompromised patients. The treatment of infected patients is very difficult [
27,
28]. It can be considered positive that all
S. maltophilia isolates were susceptible against trimethoprim-sulfamethoxazole, as trimethoprim-sulfamethoxazole is still the treatment of choice for suspected or culture-proven
S. maltophilia infections [
29]. If a patient is infected with one of these strains, polymyxins may also be effective treatment options, though not without frequent adverse effects.
Sphingomonas paucimobilis has been implicated in various types of clinical infection. Although infections by
S. paucimobilis are rarely serious and could be effectively treated with antibiotics,
S. paucimobilis is capable of causing active infections in humans [
30,
31]. However, 93% of the isolates in this study were resistant to aminoglycosides, and one isolate was resistant to ceftazidime. Another study describes carbapenems, against which all isolates were resistant, as the most effective therapy for infections with
S. paucimobilis [
32].
Achromobacter spp. have been identified as opportunistic human pathogens in people with certain immunosuppressive conditions, such as cystic fibrosis, cancer, and kidney failure [
33]. Notably, 80% of the isolates originated from barefoot areas or the floor cleaning equipment, which indicates a transmission due to insufficient management of floor cleaning equipment.
Like
Achromobacter spp.,
Sphingobacterium spiritivorum/multivorum is rarely involved in human infection. Sphingobacterium species are intrinsically resistant to many commonly used antibiotics and can grow in antiseptics and disinfectants [
34]. S. multivorum can produce an extended-spectrum
β-lactamase and a metallo-
β-lactamase, conferring resistance to third-generation cephalosporins and carbapenems, respectively [
35]. Two isolates were both resistant to carbapenems and third-generation cephalosporins.
In addition to these quite important human pathogens, some uncommon antibiotic-resistant Gram-negative bacterial species, like
Chryseobacterium indologenes and
Ochrobactrum anthropi, were isolated. The isolation of
Chryseobacterium indologenes and
Ochrobactrum anthropi from swimming pool water was described previously by Papadopoulou et al. [
17]. In this study,
Ochrobactrum anthropi could only be isolated from the surface samples in the pool surroundings and not from the water samples. For both species, rare clinical significance and resistance to a wide variety of antimicrobial agents has been reported [
36,
37].
However, whether such resistant strains can contaminate bathers and cause infection strongly depends on the immune status of the patient. The study has revealed deficiencies in the operation of the pools, although the extent to which immunocompetent patients may be at risk from multidrug resistant bacteria in the pool water could not be determined. The question of whether patients who are proven carriers of multidrug resistant bacteria may use therapy pools has to be clarified in individual cases, taking the respective bacterial species into account. In the case of unsafe operation or outdated technology and patients colonized with 4MRGN, a ban on use should be considered.