Methyl and Total Mercury in Different Media and Associated Fluxes in a Watershed Forest, Southwest China
Abstract
1. Introduction
2. Materials and Methods
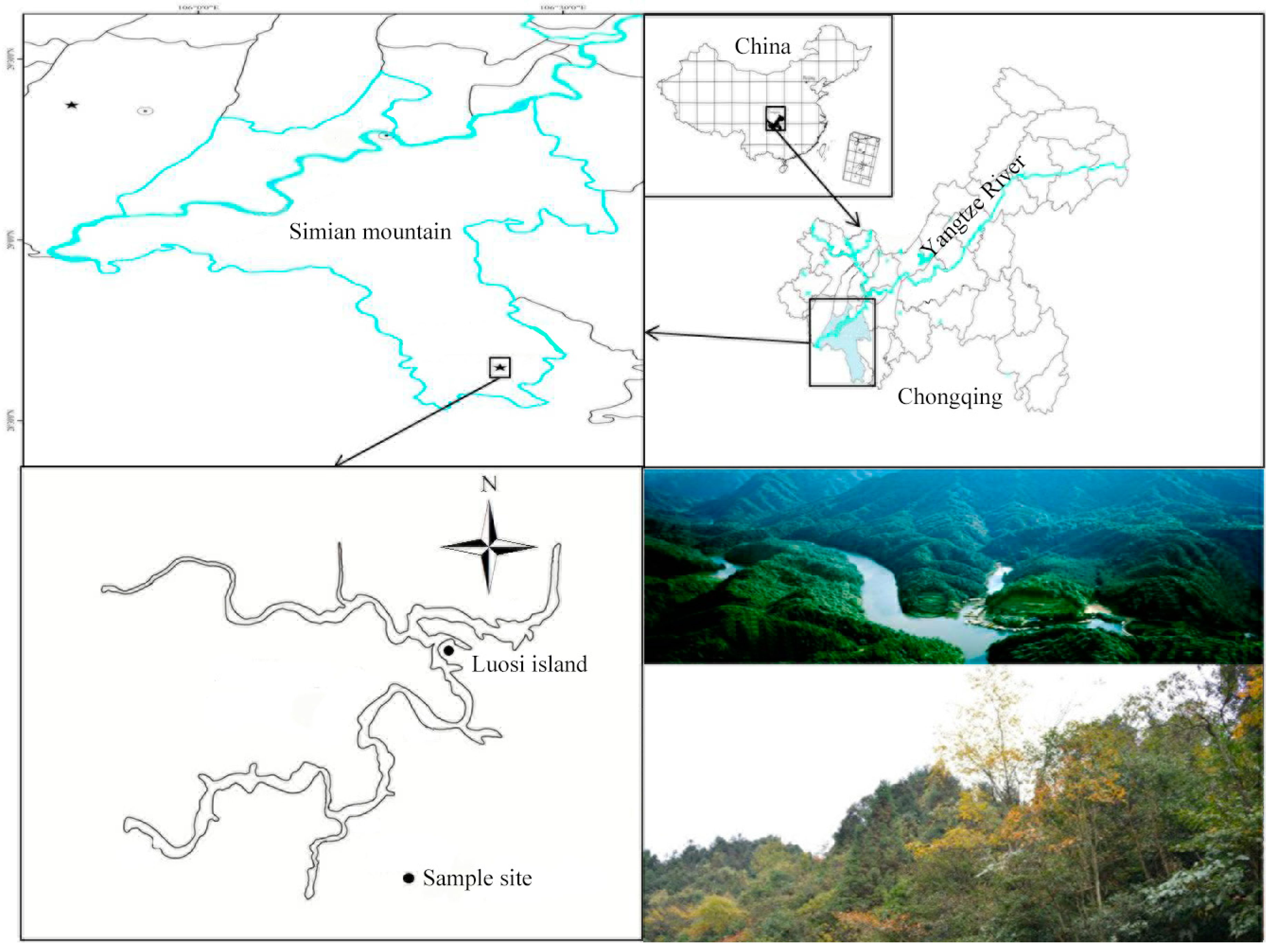
2.1. Site Description
2.2. Sampling Method of Throughfall, Runoff and Soil Percolate
2.3. Sampling Method of Litterfall
2.4. Sample Analysis, Quality Control and Statistical Analysis
3. Results
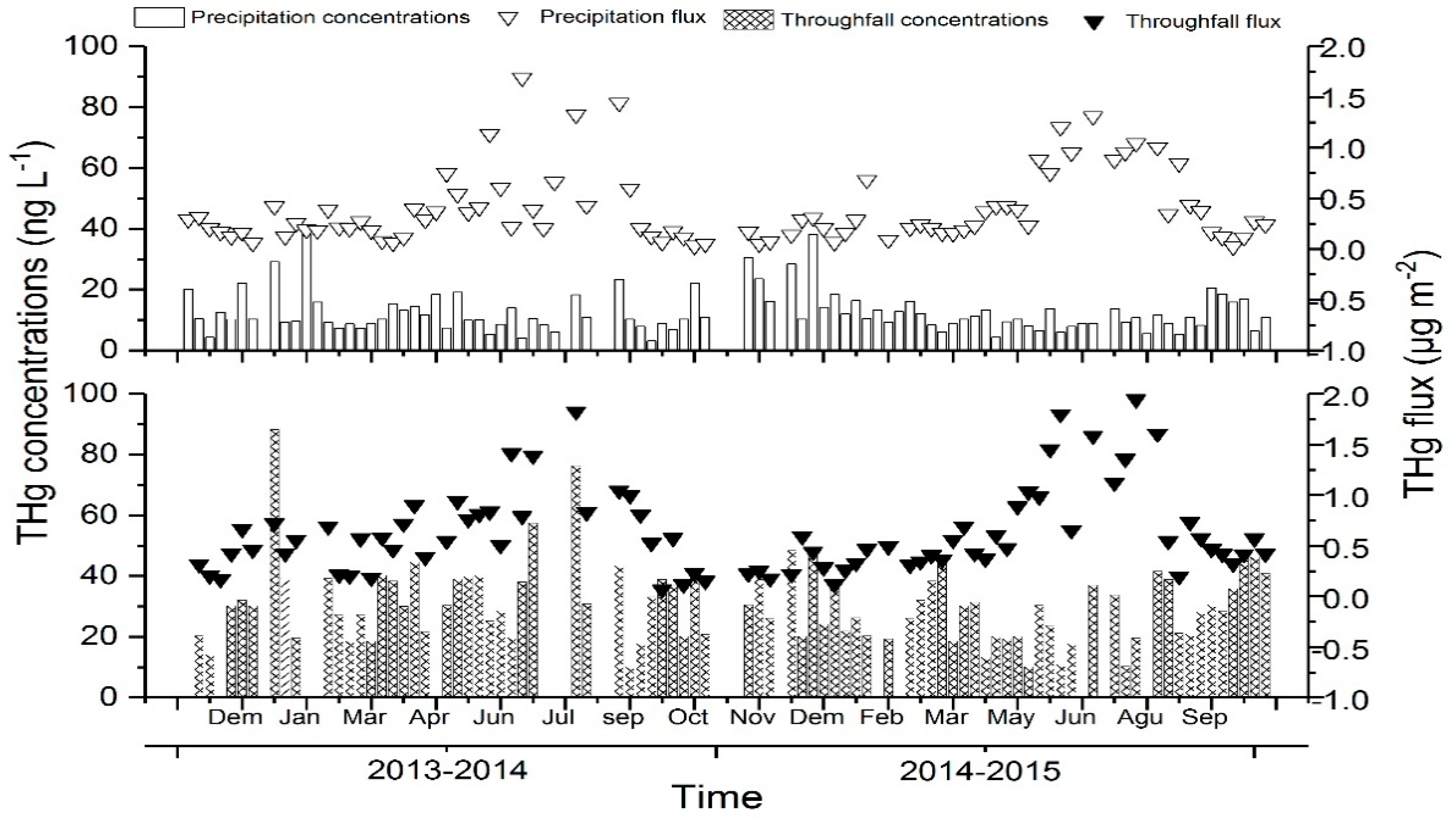
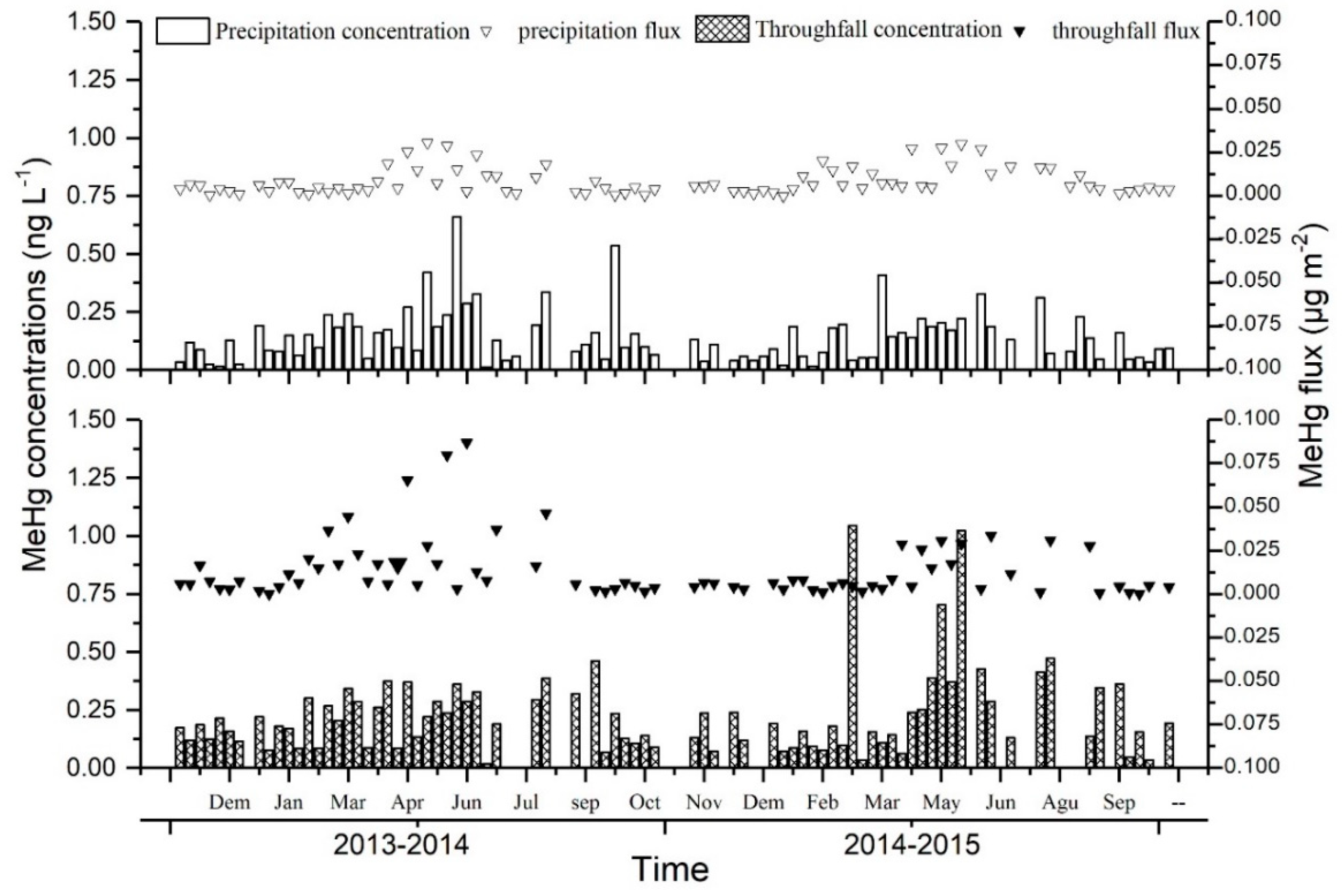
3.1. Influence of the Forest Canopy on Hg Deposition through Throughfall and Litterfall
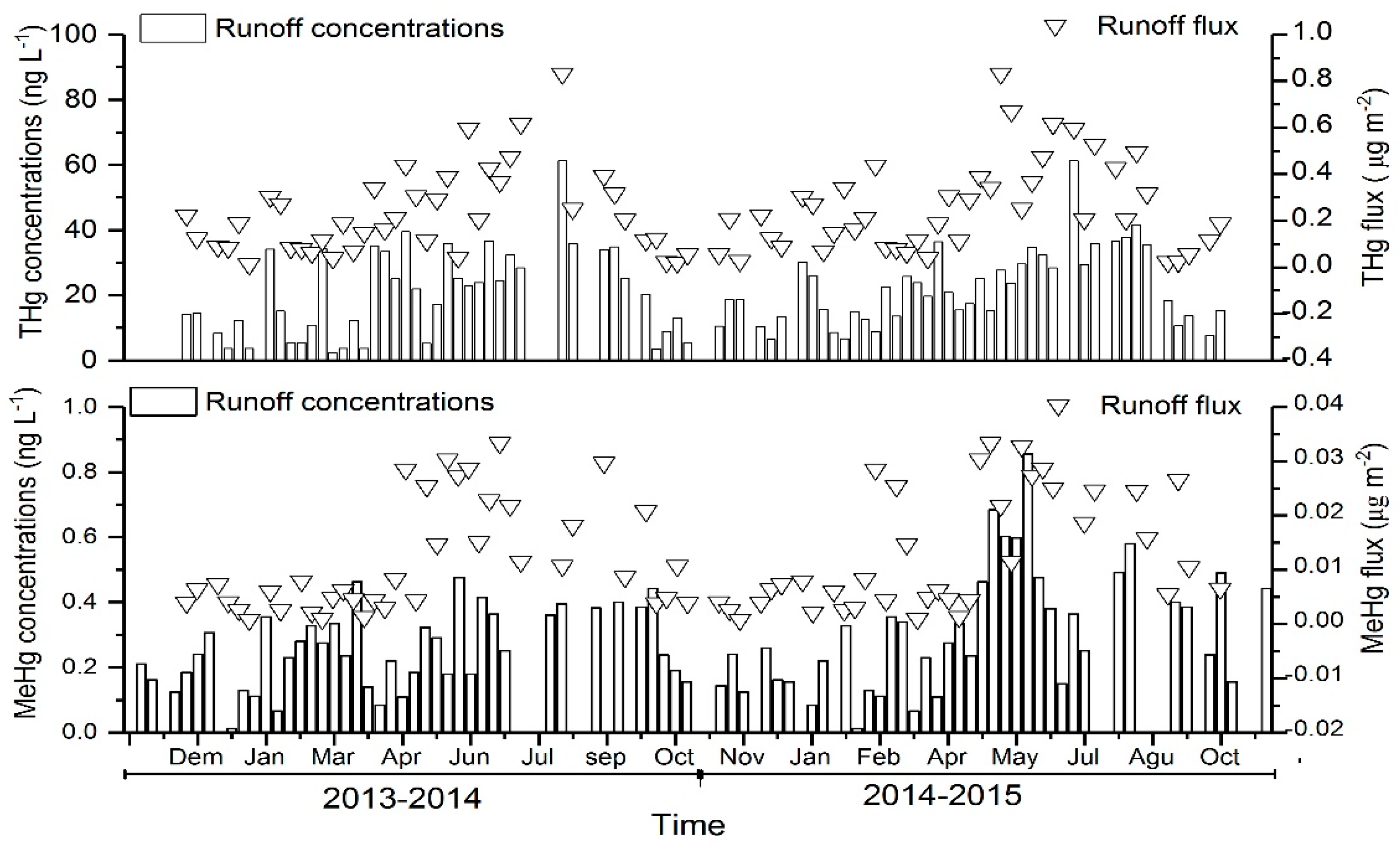
3.2. Dynamics of Hg in the Forest Runoff
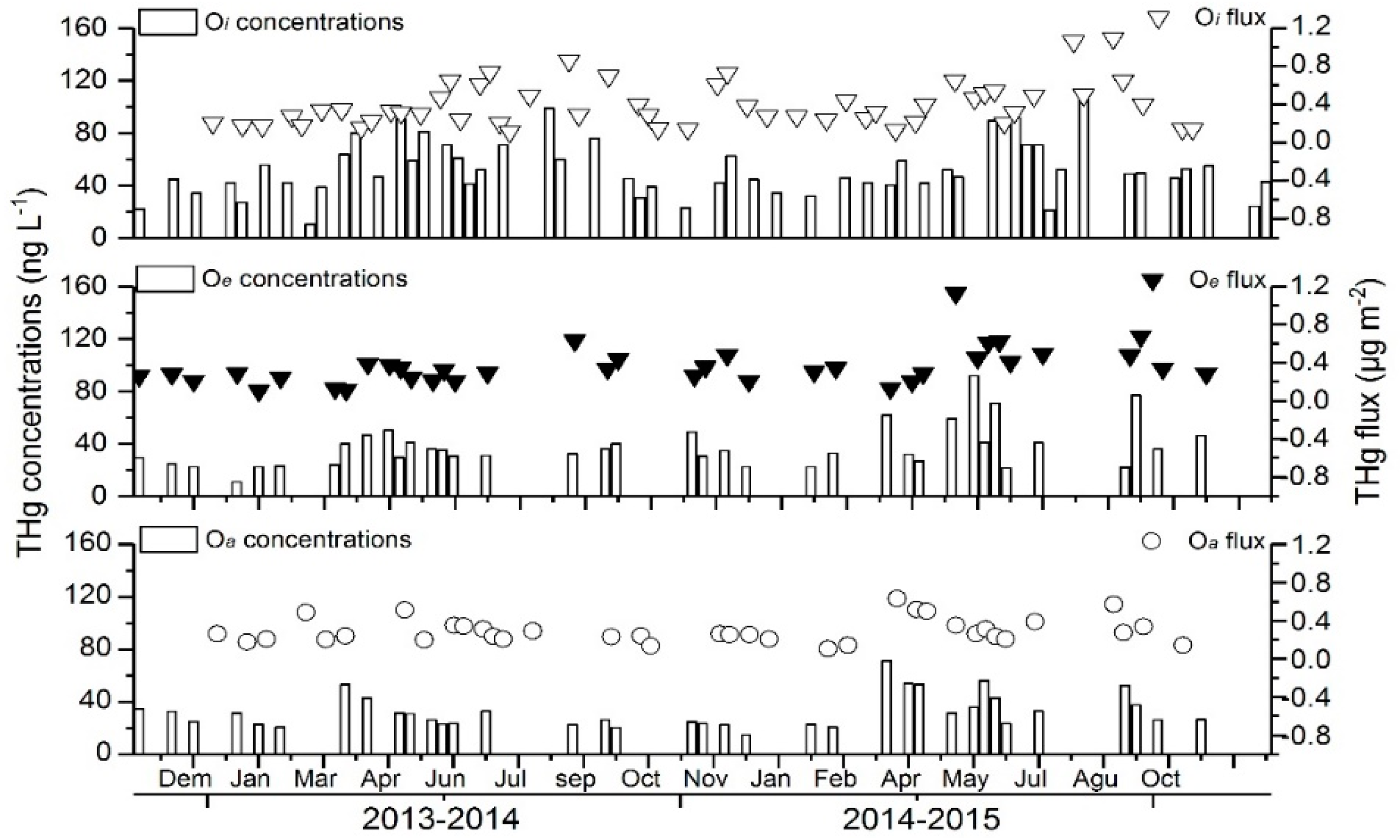
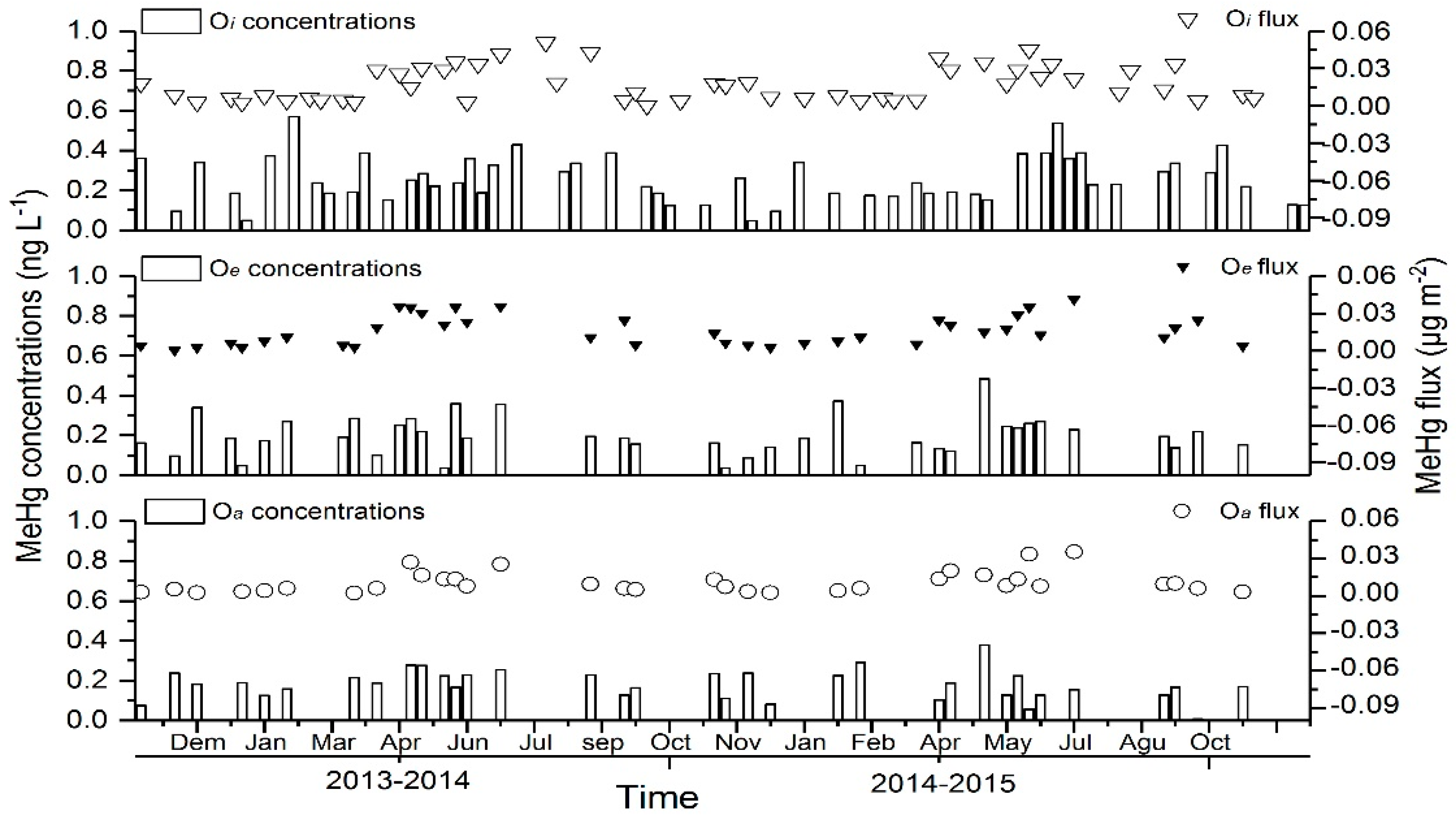
3.3. Dynamics of Hg in the Forest-Floor Percolates
4. Discussion
4.1. Influence of the Forest Canopy on Hg Deposition through Throughfall
4.2. Influence of the Forest Canopy on Hg Deposition through Litterfall
4.3. Dynamics of Hg in the Forest Runoff
4.4. Dynamics of Hg in the Forest-Floor Percolates
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Write, L.P.; Zhang, L.; Cheng, I.; Aherne, J.; Wentworth, G.R. Impacts and effects indicators of atmospheric deposition of major pollutants to various ecosystems—A review. Aerosol Air Qual. Res. 2018, 18, 1953–1992. [Google Scholar] [CrossRef]
- Chen, W.K.; Li, T.C.; Sheu, G.R.; Lin, N.H.; Chen, L.Y.; Yuan, C.S. Correlation analysis, transportation mode of atmospheric mercury and criteria air pollutants, with meteorological parameters at two remote sites of mountain and offshore island in Asia. Aerosol Air Qual. Res. 2016, 16, 2692–2705. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, H.; Qiu, G.; Yan, H.; Li, G.; Li, Z. Geochemical processes of mercury in Wujiangdu and Dongfeng reservoirs, Guizhou, China. Environ. Pollut. 2009, 157, 2970–2984. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiang, H.; Qiu, G.; Yan, H.; Li, G.; Li, Z. Mercury mass balance study in Wujiangdu and Dongfeng Reservoirs, Guizhou, China. Environ. Pollut. 2009, 157, 2594–2603. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Feng, X.; Shang, L.; Wang, S.; Zhang, H. Two years of measurements of atmospheric total gaseous mercury (TGM) at a remote site in Mt. Changbai area, Northeastern China. Atmos. Chem. Phys. 2012, 12, 4215–4226. [Google Scholar] [CrossRef]
- Grigal, D.F. Inputs and outputs of mercury from terrestrial watersheds: A review. Environ. Rev. 2002, 10, 1–39. [Google Scholar] [CrossRef]
- Schroeder, W.H.; Munthe, J. Atmospheric mercury—An overview. Atmos. Environ. 1998, 32, 809–822. [Google Scholar] [CrossRef]
- Wright, L.P.; Zhang, L.; Marsik, F. Overview of mercury dry deposition, litterfall, and throughfall studies. Atmos. Chem. Phys. 2016, 16, 13399–13416. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Z.; Cheng, I.; Wright, L.P.; Olson, M.L.; Gay, D.A.; Risch, M.R.; Brooks, S.; Castro, M.S.; Conley, G.D.; et al. The estimated six-year mercury dry deposition across North America. Environ. Sci. Technol. 2016, 50, 12864–12873. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yan, H.; Sf Qiu, G.; Tang, S.; Shang, L.; Dai, Q.; Hou, Y. Seasonal variation of gaseous mercury exchange rate between air and water surface over Baihua reservoir, Guizhou, China. Atmos. Environ. 2004, 38, 4721–4732. [Google Scholar] [CrossRef]
- Schlüter, K. Review: Evaporation of mercury from soils. An integration and synthesis of current knowledge. Environ. Geol. 2000, 39, 249–271. [Google Scholar] [CrossRef]
- Rea, A.W.; Lindberg, S.E.; Keeler, G.J. Dry deposition and foliar leaching of mercury and selected trace elements in deciduous forest throughfall. Atmos. Environ. 2001, 35, 3453–3462. [Google Scholar] [CrossRef]
- St Louis, V.L.; Rudd, J.W.; Kelly, C.A.; Hall, B.D.; Rolfhus, K.R.; Scott, K.J.; Lindberg, S.E.; Dong, W. Importance of the forest canopy to fluxes of methyl mercury and total mercury to boreal ecosystems. Environ. Sci. Technol. 2001, 35, 3089–3098. [Google Scholar] [CrossRef] [PubMed]
- Demers, J.D.; Driscoll, C.T.; Fahey, T.J.; Yavitt, J.B. Mercury cycling in soil and litter in different forest types in the Adirondack region, New York, USA. Ecol. Appl. A Publ. Ecol. Soc. Am. 2007, 17, 1341–1351. [Google Scholar] [CrossRef]
- Huang, J.; Kang, S.; Zhang, Q.; Guo, J.; Chen, P.; Zhang, G.; Tripathee, L. Atmospheric deposition of trace elements recorded in snow from the Mt. Nyainqêntanglha region, southern Tibetan Plateau. Chemosphere 2013, 92, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Landis, M.S.; Keeler, G.J. Atmospheric mercury deposition to lake Michigan during the lake Michigan mass balance study. Environ. Sci. Technol. 2002, 36, 4518–4524. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, E.G.; Pacyna, J.M.; Steenhuisen, F.; Wilson, S. Global anthropogenic mercury emission inventory for 2000. Atmos. Environ. 2006, 40, 4048–4063. [Google Scholar] [CrossRef]
- Yu, B.; Fu, X.; Yin, R.; Zhang, H.; Wang, X.; Lin, C.J.; Wu, C.; Zhang, Y.; He, N.; Fu, P. Isotopic composition of atmospheric mercury in China: New evidence for source and transformation processes in air and in vegetation. Environ. Sci. Technol. 2016, 50, 9262–9269. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, H.; Xu, L.; Wang, W.; Wei-Li, L.U.; Meng, L. Reserves and water capacity characteristics of different species of litter in Simian Mountain. J. Soil Water Conserv. 2008, 22, 90–93. (In Chinese) [Google Scholar]
- Branfireun, B.A.; Hilbert, D.; Roulet, N.T. Sinks and sources of methylmercury in a boreal catchment. Biogeochemistry 1998, 41, 277–291. [Google Scholar] [CrossRef]
- Shanley, J.B.; Kamman, N.C.; Clair, T.A.; Chalmers, A. Physical controls on total and methylmercury concentrations in streams and lakes of the northeastern USA. Ecotoxicology 2005, 14, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Watras, C.J.; Morrison, K.A.; Kent, A.; Price, N.; Regnell, O.; Eckley, C.; Hintelmann, H.; Hubacher, T. Sources of methylmercury to a wetland-dominated lake in northern Wisconsin. Environ. Sci. Technol. 2005, 39, 4747–4758. [Google Scholar] [CrossRef] [PubMed]
- Gustin, M.S.; Lindberg, S.E.; Weisberg, P.J. An update on the natural sources and sinks of atmospheric mercury. Appl. Geochem. 2008, 23, 482–493. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The Structure, distribution, and biomass of the world’s forests. Annu. Rev. Ecol. Evol. S. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Ma, M.; Wang, D.; Sun, T.; Zhao, Z.; Du, H. Forest runoff increase mercury output from subtropical forest catchments: An example from an alpine reservoir in a national nature reserve (southwestern China). Environ. Sci. Pollut. Res. 2015, 22, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, D.; Du, H.; Sun, T.; Zhao, Z.; Wei, S. Atmospheric mercury deposition and its contribution of the regional atmospheric transport to mercury pollution at a national forest nature reserve, southwest China. Environ. Sci. Pollut. Res. 2015, 22, 20007–20018. [Google Scholar] [CrossRef] [PubMed]
- Schwesig, D.; Matzner, E. Dynamics of mercury and methylmercury in forest floor and runoff of a forested watershed in central Europe. Biogeochemistry 2001, 53, 181–200. [Google Scholar] [CrossRef]
- USEPA. Method 1631: Revision E: Mercury in Water by Oxidation, Purge and Traps, and Cold Vapor Atomic Fluorescence Spectrometry; USEPA: Washington, DC, USA, 2002.
- USEPA. Method 1630: Methyl Mercury in Water by Distillation, Aqueous Ethylation, Purge and Trap and CVAFS; USEPA: Washington, DC, USA, 2001.
- Larssen, T.; de Wit, H.A.; Wiker, M.; Halse, K. Mercury budget of a small forested boreal catchment in southeast Norway. Sci. Total Environ. 2008, 404, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Feng, X.; Zhu, W.; Rothenberg, S.; Yao, H.; Zhang, H. Elevated atmospheric deposition and dynamics of mercury in a remote upland forest of southwestern China. Environ. Pollut. 2010, 158, 2324–2333. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, D.; Du, H.; Sun, T.; Zhao, Z.; Wang, Y.; Wei, S. Mercury dynamics and mass balance in a subtropical forest, southwestern China. Atmos. Chem. Phys. 2016, 16, 4529–4537. [Google Scholar] [CrossRef]
- Wang, D.; He, L.; Wei, S.; Feng, X. Estimation of mercury emission from different sources to atmosphere in Chongqing, China. Sci. Total Environ. 2006, 366, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xing, D.; Wei, Z.; Jia, Y. Spatial and seasonal variations in soil and river water mercury in a boreal forest, Changbai Mountain, Northeastern China. Geoderma 2013, 206, 123–132. [Google Scholar] [CrossRef]
- Almeida, M.D.; Marins, R.V.; Paraquetti, H.H.M.; Bastos, W.R.; Lacerda, L.D. Mercury degassing from forested and open field soils in Rondônia, Western Amazon, Brazil. Chemosphere 2009, 77, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, E.V.; Machado, W.; Oliveira, R.R.; Sella, S.M.; Lacerda, L.D. Mercury deposition through litterfall in an Atlantic forest at Ilha Grande, Southeast Brazil. Chemosphere 2006, 65, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.S.; Steffen, A.; Eckley, C.S.; Narayan, J.; Pilote, M.; Tordon, R.; Graydon, J.A.; St Louis, V.L.; Xu, X.; Branfireun, B.A. A survey of mercury in air and precipitation across Canada: Patterns and trends. Atmosphere 2014, 5, 635–668. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Monson, B.A.; Staples, D.F.; Bhavsar, S.P.; Holsen, T.M.; Schrank, C.S.; Moses, S.K.; McGoldrick, D.J.; Backus, S.M.; Williams, K.A. Spatiotemporal trends of mercury in walleye and largemouth bass from the Laurentian Great Lakes region. Ecotoxicology 2011, 20, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Artaxo, P.; Campos, R.C.D.; Fernandes, E.T.; Martins, J.V.; Xiao, Z.; Lindqvist, O.; Maenhaut, W. Large scale mercury trace element measurements in the Amazon basin. Atmos. Environ. 2000, 34, 4085–4096. [Google Scholar] [CrossRef]
- Choi, H.D.; Holsen, T.M. Gaseous mercury fluxes from the forest floor of the Adirondacks. Environ. Pollut. 2009, 157, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, R.; Maserti, B.E.; Andersson, M.; Edner, H.; Ragnarson, P.; Svanberg, S. Mercury degassing rate from mineralized areas in the Mediterranean basin. Water Air Soil Pollut. 1997, 93, 59–66. [Google Scholar] [CrossRef]
- Blackwell, B.D.; Driscoll, C.T. Deposition of mercury in forests along a montane elevation gradient. Environ. Sci. Technol. 2015, 49, 5363–5370. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, B.D.; Driscoll, C.T.; Maxwell, J.A.; Holsen, T.M. Changing climate alters inputs and pathways of mercury deposition to forested ecosystems. Biogeochemistry 2014, 119, 215–228. [Google Scholar] [CrossRef]
- Laacouri, A.; Nater, E.A.; Kolka, R.K. Distribution and uptake dynamics of mercury in leaves of common deciduous tree species in Minnesota, U.S.A. Environ. Sci. Technol. 2013, 47, 10462–10470. [Google Scholar] [CrossRef] [PubMed]
- Ci, Z.; Peng, F.; Xue, X.; Zhang, X. Air-surface exchange of gaseous mercury over permafrost soil: An investigation at a high-altitude (4700 m.a.s.l.) and remote site in the central Qinghai-Tibet Plateau. Atmos. Chem. Phys. 2016, 16, 14741–14754. [Google Scholar] [CrossRef]
- Wang, X.; Luo, J.; Yin, R.; Yuan, W.; Lin, C.J.; Sommar, J.; Feng, X.; Wang, H.; Lin, C. Using mercury isotopes to understand mercury accumulation in the montane forest floor of the eastern Tibetan Plateau. Environ. Sci. Technol. 2016, 51, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Ericksen, S.M.; Schorran, D.E.; Johnson, D.W.; Lindberg, S.E.; Coleman, J.S. Accumulation of atmospheric mercury in forest foliage. Atmos. Environ. 2003, 37, 1613–1622. [Google Scholar] [CrossRef]
- Stamenkovic, J.; Gustin, M.S. Nonstomatal versus stomatal uptake of atmospheric mercury. Environ. Sci. Technol. 2009, 43, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
| Layer | THg (ng·g−1) | MeHg (ng·g−1) | Density (g cm−3) | Thickness (m) | THg Content (μg·m−2) | MeHg Content (μg·m−2) | |
|---|---|---|---|---|---|---|---|
| Litterfall | 73.3 ± 14.2 | 0.86 ± 0.4 | 0.38 ± 6.2 | 0.21 | 5849.3 | 8.9 | |
| Soil | Oi | 315.5 ± 27.3 | 1.06 ± 0.3 | 1.37 ± 2.1 | 0.20 | 9509.1 | 31.9 |
| Oe | 408.6 ± 42.2 | 0.61 ± 0.2 | 1.54 ± 5.9 | 0.20 | 20,106.2 | 30.1 | |
| Oa | 368.4 ± 22.7 | 0.26 ± 0.2 | 1.57 ± 16.6 | 0.20 | 25,449.1 | 17.9 | |
| Samples | THg Concentration | MeHg Concentration | Water/Litterfall Flux | THg Flux | Mehg Flux | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ng L−1 | mm | μg m−2 yr−1 | ||||||||
| 2013–2014 | 2014–2015 | 2013–2014 | 2014–2015 | 2013–2014 | 2014–2015 | 2013–2014 | 2014–2015 | 2013–2014 | 2014–2015 | |
| Precipitation | 10.61 ± 5.6 | 9.77 ± 8.3 | 0.13 ± 0.12 | 0.11 ± 0.13 | 1395 | 1480 | 16.19 | 18.89 | 0.18 | 0.16 |
| Throughfall | 22.14 ± 4.9 | 20.92 ± 16.2 | 0.20 ± 0. 08 | 0.19 ± 0.22 | 1073 | 1188 | 22.34 | 24.35 | 0.23 | 0.22 |
| Runoff | 20.32 ± 9.1 | 22.24 ± 19.4 | 0.31 ± 0.19 | 0.29 ± 0.18 | 861 | 884 | 19.93 | 21.66 | 0.27 | 0.26 |
| Percolate (Oi) | 45.59 ± 12.1 | 48.29 ± 34.2 | 0.26 ± 0.12 | 0.25 ± 0.16 | 582 (834) | 602 (850) | 8.03 | 10.20 | 0.22 | 0.21 |
| Percolate (Oe) | 55.84 ± 10.2 | 56.58 ± 28.3 | 0.21 ± 0.16 | 0.22 ± 0.14 | 584 (896) | 610 (867) | 10.54 | 11.95 | 0.15 | 0.16 |
| Percolate (Oa) | 51.05 ± 25.5 | 55.14 ± 19.6 | 0.19 ± 0.11 | 0.17 ± 0.08 | 591 (808) | 604 (829) | 9.27 | 10.04 | 0.15 | 0.14 |
| Litterfall | ng g−1 | g m−2 | μg m−2 yr−1 | |||||||
| 104.5 ± 23.5 | 106.3 ± 18.7 | 0.84 ± 0.12 | 0.83 ± 0.20 | 456 | 461 | 47.65 | 49.01 | 0.38 | 0.39 | |
| Layer | THg Concentration (ng L−1) | MeHg Concentration (ng L−1) | Water/Litterfall Flux (mm) | THg Flux (μg m−2 yr−1) | MeHg Flux (μg m−2 yr−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2013–2014 | 2014–2015 | 2013–2014 | 2014–2015 | 2013–2014 | 2014–2015 | 2013–2014 | 2014–2015 | 2013–2014 | 2014–2015 | |
| Runoff | 20.32 ± 9.1 | 22.24 ± 19.4 | 0.31 ± 0.19 | 0.29 ± 0.18 | 861 | 884 | 19.93 | 21.66 | 0.27 | 0.26 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Ma, M.; Sun, T.; An, S.; Igarashi, Y.; Wang, D. Methyl and Total Mercury in Different Media and Associated Fluxes in a Watershed Forest, Southwest China. Int. J. Environ. Res. Public Health 2018, 15, 2618. https://doi.org/10.3390/ijerph15122618
Du H, Ma M, Sun T, An S, Igarashi Y, Wang D. Methyl and Total Mercury in Different Media and Associated Fluxes in a Watershed Forest, Southwest China. International Journal of Environmental Research and Public Health. 2018; 15(12):2618. https://doi.org/10.3390/ijerph15122618
Chicago/Turabian StyleDu, Hongxia, Ming Ma, Tao Sun, Siwei An, Yasuo Igarashi, and Dingyong Wang. 2018. "Methyl and Total Mercury in Different Media and Associated Fluxes in a Watershed Forest, Southwest China" International Journal of Environmental Research and Public Health 15, no. 12: 2618. https://doi.org/10.3390/ijerph15122618
APA StyleDu, H., Ma, M., Sun, T., An, S., Igarashi, Y., & Wang, D. (2018). Methyl and Total Mercury in Different Media and Associated Fluxes in a Watershed Forest, Southwest China. International Journal of Environmental Research and Public Health, 15(12), 2618. https://doi.org/10.3390/ijerph15122618




